Open Journal of Urology
Vol. 3 No. 2 (2013) , Article ID: 31321 , 6 pages DOI:10.4236/oju.2013.32012
Assessment of Warm and Cold Ischemıa on Functions of the Operated Kidney with 99mTc-DMSA in Renal Masses: A Prospective and Randomized Study*
Department of Urology, Medical Faculty, Erciyes University, Kayseri, Turkey
Email: #mesane@gmail.com, ademirtas5135@hotmail.com
Copyright © 2013 Abdullah Demirtas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 5, 2013; revised April 4, 2013; accepted April 13, 2013
Keywords: cold ischemia times; nephron sparing surgery; renal cancer; technetium-99m-dimercaptosuccinic acid; warm ischemia times
ABSTRACT
Objective: To examine the effect of warm and cold ischemia on functions of the operated kidney in cases with a normal contralateral kidney undergoing nephron sparing surgery. Methods: This study enrolled 40 patients with a normal contralateral kidney and without a renal function threatening risk factor, who were operated with NSS. The patients were randomized at admission. They were divided into 2 equal groups as warm and cold ischemia. An ice application for 10 minutes was done to cold ischemia group after clamping renal artery. Renal functions were evaluated with Technesium-99m-Dimercaptosuccinic Acid (DMSA) and serum creatinine at the preoperative and postoperative (day 1, day 15, month 6, and month 12) period. Statistical analysis was done with Mann Whitney U test, Wilcoxon Signed Rank test, and Fredman test. A p value below 0.05 was considered statistically significant. Results: There were no significant differences between the groups in terms of age, body mass index, ischemia time, tumor size, amount of hemorrhage, and procedure time. Both groups had a significantly higher DMSA uptake at the preoperative period compared with the postoperative period (postoperative day 1, day 15, month 6, and month 12) (p < 0.001). However, both groups had similar DMSA uptake results at the postoperative period. Preoperative and postoperative creatinine levels were not significantly different from each other in both groups. Conclusion: Based on tumor localization, nephron sparing surgery without use of superficial cooling appears as a viable option for small renal masses.
1. Introduction
Nephron sparing surgery (NSS) has comparable oncologic outcomes with radical nephrectomy in localized renal cell carcinoma (RCC) [1,2]. Technological advances and a parallel improvement in imaging methods have increased the number of patients detected incidentally at an early stage.
The survival rates for NSS are comparable with radical nephrectomy in tumors of similar stage [2]. Increasing number of studies reporting survival rates over 90% with NSS in small localized tumors in patients with a normal contralateral kidney has transformed NSS into a widespread procedure performed at many centers [3]. Currently, NSS is performed with an open, laparoscopic, and robotic technique [4,5].
Kidney scintigraphy is a noninvasive method to assess kidney functions. Scintigraphic studies performed with Technetium-99m-Dimercaptosuccinic Acid (DMSA) have shown that it is correlated with effective plasma volume, glomerular filtration rate, and creatinine clearance. In addition, DMSA is also a practical indicator of functions of each kidney individually [6].
In this study, we aimed to evaluate the short and longterm effects of warm and cold ischemia after closure of renal artery on the functions of the operated kidney with DMSA scintigraphy in cases with a normal contralateral kidney, which are operated for a renal mass.
In parallel with recent advances in imaging modalities and widespread use of ultrasonography and computerized tomography the rate of detection of early-stage kidney tumors has increased and widespread use of NSS has also started in patients with a normal contralateral kidney [7]. Although the survival rates of tumors at an early stage with a normal contralateral kidney are similar for radical nephrectomy and nephron sparing surgery, patients undergoing RN are at a greater risk of renal failure. Lau et al., in a study examining the clinical and pathological results of 164 patients who underwent NSS and RN with a normal contralateral kidney, reported that the survival rates in both groups were similar and the serum creatinine levels at postoperative period were significantly higher compared to preoperative period in the RN group. They noted that there was no significant difference between preoperative and postoperative periods of the NSS with respect to creatinine levels [8]. Mullerad et al., in a study where they evaluated the functions of the contralateral kidney with DMSA, detected an 11% decrease in the function of the remaining kidney, as compared to preoperative period [9].2. Materials and Methods
The local ethical committee approved the study. Between February 2004 and March 2011, 40 patients with a normal contralateral kidney and no risk factors threatening kidney functions (urinary stone disease, diabetes mellitus, hypertension, congenital anomaly, renal artery stenosis, etc.) that were scheduled for a NSS procedure for a renal mass were included. At admission the patients were randomized into 2 groups as warm and cold ischemia. All patients were informed about the study and all gave their written consent. Preoperative evaluation included urinalysis, complete blood count, serum urea, creatinine, liver function tests, and alkaline phosphatase tests. Imaging tests included abdominal ultrasonography, and computerized tomographic examinations of abdomen and thorax. Clinical and pathological staging were done according to 2002 TNM system [10]. Procedural time, ischemia type and time, and amount of bleeding were recorded in all patients. Superficial kidney temperature was calculated with a sterile thermometer in the cold ischemia group. Renal functions were assessed using preoperative and postoperative (day 1, day 15, month 6, and month 12) DMSA and serum creatinine measurements.
2.1. Surgical Technique
All patients were approached extraperitoneal in a flank position via a flank incision between 11th and 12th ribs. The kidney was freed through gerota fascia without making a contact with the perirenal fatty tissue above the tumor. Renal pedicle was accessed and renal artery and vein were dissected. Intravenous mannitol 100 ml was administered 5 - 10 minutes before the renal artery was closed with a bulldog clamp. Renal vein was not clamped in any patient. In patients in the cold ischemia group the kidney was superficially cooled for 10 minutes using frozen ice cubes of lactated ringer’s solution of 250 - 500 ml through a drape put on the kidney. After the cooling process, the superficial temperature of the kidney was measured immediately after the ice pieces were removed. The tumor was excised together with the perirenal fatty tissue with a 0.5 - 1 cm border from the normal renal parenchyma by using sharp and blunt dissection methods. Surgical border negativity was confirmed by frozen section examination in all patients. A tube drainage catheter was placed around the kidney. The amount of bleeding was calculated by measuring the amount of blood in aspirator.
2.2. DMSA Renal Scintigraphy
In this study, approximately 111 MBq (3mCi) 99mTcDMSA was injected to patients. Before and after injection, the activity of the injector was counted to calculate the correct value. The renal image was acquired using a Gamma camera (Toshiba GCA 901A/SA, Japan) 2 - 4 hours after the injection. Activity of each kidney was counted within a minute using a 256 × 256 matrix. Percent DMSA uptake (%) of each kidney was calculated separately for 40 affected and 40 normal contralateral kidneys.
2.3. Statistical Analysis
The measurable values of the patients were expressed as median (range). The inter-group differences were analyzed using Mann Whitney U test. Wilcoxon Signed Rank test was used to evaluate the difference between two times, while Fredman test was used to evaluate the differences between more than 2 times. Statistical significance was defined as a patient value below 0.05.
3. Results
A total 40 patients were enrolled to this study. The data of the warm and cold ischemia groups are presented on Table 1. 12 male and 8 female patients whose mean age was 49 years old (29 - 62), were included in the warm ischemia group. 7 male and 13 female patients whose mean age was 49 years old (29 - 62) were included in the cold ischemia group. Forty cases with a unilateral renal mass and a normal contralateral kidney were operated with retroperitoneal NSS using an open surgical technique.
Comparison of body mass index (BMI) of the patients showed a BMI of 28.1 (19 - 34) for warm ischemia group and 26.3 (19 - 40) for the cold ischemia group. These figures were statistically similar but they were higher than the population averages. There were also no significant differences between warm and cold ischemia groups
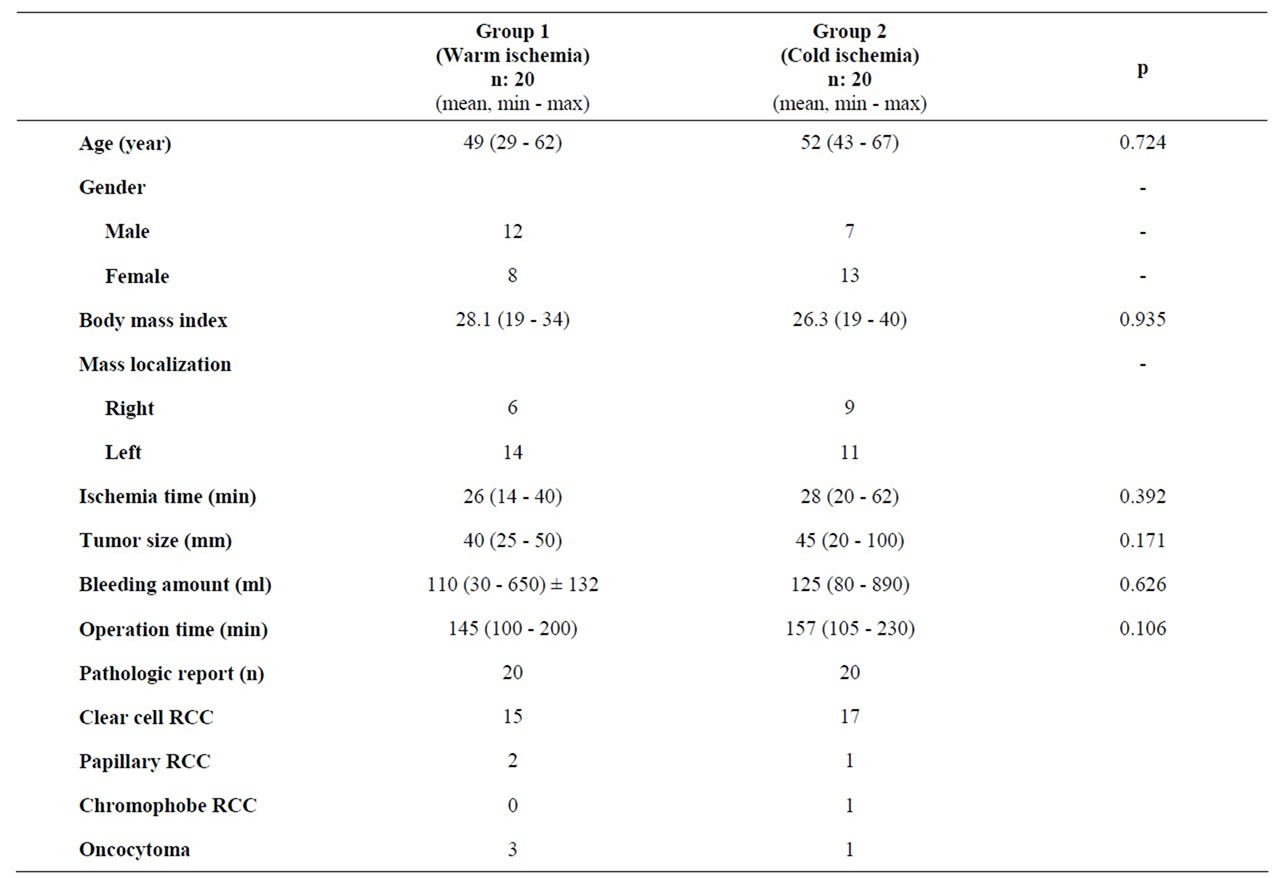
Table 1. Descriptive data in warm and cold ischemia groups.
in terms of age, body mass index, ischemia time, tumor size, amount of bleeding, and procedure time. The 10-minute kidney cooling time was added to calculated cold ischemia time. The intraoperative kidney temperature before the onset of mass excision had a median level of 18 (15 - 25)˚C.
Renal vein injury occurred and a primary repair was performed in one patient. In one patient preoperative tumor perforation was observed. The follow-up of that patient was negative for a recurrent mass. Pleural opening occurred in 4 patients, which had a benign course.
The DMSA uptake rates of the operated kidneys of the warm and cold ischemia groups, measured before and after the surgery are summarized on Table 2. Comparison of the DMSA data revealed that preoperative DMSA uptake levels were significantly higher than those at postoperative period (day 1, day 15, month 6, month 12) (p < 0.001). However, there was no significant difference between DMSA uptake levels at the postoperative period (day 1, day 15, month 6, and month 12).
Comparison of the creatinine levels in both groups revealed no significant difference between preoperative and postoperative periods. The mean preoperative creatinine level was 0.93 mg/dl (0.7 - 1.2) in the warm ischemia group. The creatinine levels at various postoperative time points were 1.02 mg/dl (0.6 - 1.6) at 1st day, 1 mg/dl (0.6 - 1.4) at 15th day, 0.96 mg/dl (0.6 - 1.3) at 6th month, and 1.03 mg/dl (0.6 - 1.4) at 12th month (Friedman test, p = 0.6). The mean preoperative creatinine level was 0.91 mg/dl (0.5 - 1.4) in the cold ischemia group. The postoperative creatinine levels for the cold ischemia group were 0.95 mg/dl (0.5 - 1.5) at 1st day, 1.08 mg/dl (0.80 - 1.6) at 15th day, 1.03 mg/dl (0.5 - 1.3) at 6th month, and 1.03 mg/dl (0.6 - 1.4) at 12th month (Friedman test, p = 0.049).
4. Discussion
Postoperative renal functions are considerably affected by the number of remaining nephrons, and ischemia time and type. Clamping of the renal artery during nephron sparing surgery not only prevents intraoperative bleeding, but it also facilitates access to intrarenal structures by decreasing renal tissue turgor. Addition of renal hypothermia application serves for a more prolonged protection against ischemia [11]. Such operations require operators to know exactly how the kidney will react to ischemia. It takes minutes for renal functions to return to

Table 2. Operated renal DMSA involvement % rates for warm and cold ischemia groups in the preoperative and postoperative periods.
normal in a 10-minute ischemic time, hours in a 20- minute ischemia duration, 3 - 9 days in a 30-minute ischemia, and weeks in a ischemia lasting for 60 minutes whereas an incomplete (30% - 50%) recovery is expected with ischemia times as long as 120 minutes and complete loss of function occurs with ischemia time of 180 minutes. Local hypothermia application is highly effective in protection against ischemic injury when ischemia duration exceeds 30 minutes. With decreasing kidney temperature both adenosine triphosphate and oxygen consumption of cells diminish. Human and animal studies have shown that hypothermia at 20˚C - 25˚C protects a kidney against ischemia for 3 hours [12]. In our study, the median temperature measured from the kidney surface was 18°C (15 - 25). The median ischemia time in this group was 28 (20 - 62) minutes.
Recently, researchers accept 25 minutes as a fair threshold for warm ischemia time. Patients are categorized at long-term based on that figure [13]. In masses requiring a long ischemia time, preferring cold ischemia has been reported to be better with respect to a better preserved renal reserve [14]. In our study the median warm ischemia time was 26 (14 - 40) minutes and the median cold ischemia time was 28 (20 - 62) minutes, with no significant difference between two figures.
While there was a significant difference between preoperative and postoperative periods in terms of DMSA values in both groups, postoperative periods were similar, suggesting similar effects of warm and cold ischemia for about 30 minutes on renal effects. Similar DMSA uptake levels at postoperative periods may be the result of a short ischemia time.
Parallel to technological advances, laparoscopic and robotic techniques have found a place in NSS [4,5]. Recently, it has been possible in experienced centers to make resections for smaller renal masses without accompanying ischemia [15]. The common targets of all these surgical methods are a shortened ischemia time and a better hemostasis. However, open NSS retains its place in small renal masses in comparison to robotic and laparoscopic procedures when the renal mass has a larger size, the mass is located at a difficult-to-reach site, and the center has a limited endourology experience.
Postoperative mild creatinine elevation is also seen in other studies in which NSS is employed. Gill et al. reported a 0.2 mg/dl increase in patients in whom a vascular control was performed [16]. In a similar study, Wolf et al. reported a creatinine level increase of 0.1 mg/dl in a manually assisted partial nephrectomy with no arterial control [17].
In our study the creatinine levels were somewhat higher at postoperative period compared to preoperative period. However, that increase remained within normal limits and did not reach statistical significance.
Studies examining renal functions with a scintigraphic test after a nephron sparing surgery are limited; moreover, we did not find any study evaluating the postoperative first day. Bakirtas et al. compared preoperative and postoperative DMSA uptakes, creatinine clearances, and creatinine levels and found no significant differences in a study of cold ischemia where the mean arterial clamping time was 53.7 minutes and the mean tumor size was 37 mm. No statistically significant difference was observed between preoperative and postoperative periods [18]. Kondo et al. compared renal functions after partial nephrectomy in 2 groups in which renal artery control and cold ischemia was applied and no ischemia was applied. The operated kidney had a DMSA uptake of 39.9% in the cold ischemia group and 34.8% in the group in which no ischemia was applied. There was no significant difference between both groups and arterial closure had no unfavorable effects on the functions of the involved kidney at the postoperative period [19].
Preoperative knowledge about renal functions is an important tool in assessment of the postoperative residual functions. In the present study, the preoperative median DMSA uptake of the involved kidney was 49.1% (36 - 66) in the warm ischemia group and 48.7% (34 - 57) in the cold ischemia group. There was a significant difference between preoperative and postoperative periods. However, no significant differences were detected between postoperative DMSA uptakes at postoperative day 1 (very short-term) and month 12 (long-term). In this study, decrease in renal functions compared to preoperative levels may be due to the amount of the resected parenchyma, rather than ischemia time and ischemia type. There is a need for studies in NSS patients, which will assess the ratio of the parenchymal tissue resected together with tumor tissue to all renal parenchyma and the effect of that ratio on kidney functions. We randomized patients based on their presentation rank. However, since the mass of intact kidney tissue resected together with the tumor may change depending on the tumor size, a randomization based on tumor size may lead to a more accurate postoperative DMSA assessment. Nevertheless, there is a need for studies with a larger sample size. When it is taken account that superficial cooling prolongs procedure time, NSS may be performed without superficial cooling in selected cases. Furthermore, instead of using classical partial nephrectomy, use of enucleation methods as the preferred technique in which less intact parenchymal tissue is resected without conceding oncological principles may be an effective method in preserving functions of the operated kidney.
5. Conclusion
Based on these results, NSS without the application of superficial cooling appears as a preferable method depending on the tumor localization in small renal masses. Future prospective, large-scale studies are needed in this subject.
6. Acknowledgement
We are grateful to Prof. Dr. Mustafa KULA for his information about DMSA technique.
REFERENCES
- R. G. Uzzo and A. C. Novick, “Nephron Sparing Surgery for Renal Tumors: İndications, Techniques and Outcomes,” The Journal of Urology, Vol. 166, No. 1, 2001, pp. 6-18. doi:10.1016/S0022-5347(05)66066-1
- S. E. Lerner, C. A. Hawkins, M. L. Blute, et al., “Disease Outcome in Patients with Low Stage Renal Cell Carcinoma Treated with Nephron Sparing or Radical Surgery,” The Journal of Urology, Vol. 155, No. 6, 1996, pp. 1868-1873. doi:10.1016/S0022-5347(01)66032-4
- S. Pahernik, F. Roos, C. Hampel, et al., “Nephron Sparing Surgery for Renal Cell Carcinoma With Normal Contralateral Kidney: 25 Years of Experience,” The Journal of Urology, Vol. 175, No. 6, 2006, pp. 2027-2031. doi:10.1016/S0022-5347(06)00271-0
- A. Finelli and I. Gill, “Laparoscopic Partial Nephrectomy, in Rosette JMCH and Gill I: Laparoscopic Urologic Surgery in Malignancies,” Springer, Berlin, 2005, pp. 49-57. doi:10.1007/3-540-27606-8_6
- S. Kaul, R. Laungani, R. Sarle, et al., “Da Vinci-Assisted Robotic Partial Nephrectomy: Technique and Results at a Mean of 15 Months of Follow-Up,” European Urology, Vol. 51, No. 1, 2007, pp. 186-191. doi:10.1016/j.eururo.2006.06.002
- A. Taylor Jr., “Quantitation of Renal Function with Static İmaging Agents,” Seminars in Nuclear Medicine, Vol. 12, No. 4, 1982, pp. 330-344. doi:10.1016/S0001-2998(82)80014-7
- H. W. Herr, “A History of Partial Nephrectomy for Renal Tumors,” The Journal of Urology, Vol. 173, No. 3, 2005, pp. 705-708. doi:10.1097/01.ju.0000146270.65101.1d
- W. K. Lau, M. L. Blute, A. L. Weaver, et al., “Matched Comparison of Radical Nephrectomy vs Nephron-Sparing Surgery in Patients with Unilateral Renal Cell Carcinoma and a Normal Contralateral Kidney,” Mayo Clinic Proceedings, Vol. 75, No. 12, 2000, pp. 1236-1242. doi:10.4065/75.12.1236
- M. Mullerad, A. Kastin, E. Issaq, et al., “The Value of Quantitative 99m Technetium Dimercaptosuccinic Acid Renal Scintigraphy for Predicting Postoperative Renal İnsufficiency in Patients Undergoing Nephrectomy,” The Journal of Urology, Vol. 169, No. 1, 2003, pp. 24-27. doi:10.1016/S0022-5347(05)64026-8
- F. L. Grene and I. D. Fleming, “AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual,” 6nd Edition, Lippinchott, Philadelphia, 2002, pp. 355-360.
- B. A. Laven, M. A. Orvieto, M. S. Chuang, et al., “Renal Tolerance to Prolonged Warm Ischemia Time in a Laparoscopic versus Open Surgery Porcine Model,” The Journal of Urology, Vol. 172, No. 6, 2004, pp. 2471-2474. doi:10.1097/01.ju.0000138158.16968.8d
- A. C. Novick, “Renal Hypothermia: In Vivo and ex Vivo,” The Urologic Clinics of North America, Vol. 10, 1983, pp. 637-644.
- R. H. Thompson, B. R. Lane, C. M. Lohse, et al., “Every Minute Counts When the Renal Hilum Is Clamped during Partial Nephrectomy,” European Urology, Vol. 58, 2010, pp. 340-345. doi:10.1016/j.eururo.2010.05.047
- B. R. Lane, P. Russo, R. G. Uzzo, et al., “Comparison of Cold and Warm Ischemia during Partial Nephrectomy in 660 Solitary Kidneys Reveals Predominant Role of Nonmodifiable Factors in Determining Ultimate Renal Function,” The Journal of Urology, Vol. 185, No. 3, 2011, pp. 421-427. doi:10.1016/j.juro.2010.09.131
- G. S. Sandhu, E. H. Kim, Y. S. Tanagho, et al., “RobotAssisted Partial Nephrectomy: Off-Clamp Technique,” Journal of Endourology, Vol. 27, No. 1, 2013, pp. 4-7. doi:10.1089/end.2012.0170
- I. S. Gill, M. M. Desai, J. H. Kaouk, et al., “Laparoscopic partial Nephrectomy for Renal Tumor: Duplicating Open Surgical Techniques,” The Journal of Urology, Vol. 167, No. 2, 2002, pp. 475-476. doi:10.1016/S0022-5347(01)69066-9
- J. S. Wolf, Jr., B. D. Seifman and J. E. Montie, “Nephron Sparing Surgery for Suspected Malignancy: Open Surgery Compared to Laparoscopy with Selective Use of Hand Assistance,” The Journal of Urology, Vol. 163, No. 6, 2000, pp. 1659-1664. doi:10.1016/S0022-5347(05)67515-5
- H. Bakirtas, M. Eroglu, S. Naldoken, et al., “NephronSparing Surgery: The Effect of Surface Cooling and Temporary Renal Artery Occlusion on Renal Function,” Urology International, Vol. 82, 2009, pp. 24-27. doi:10.1159/000176020
- T. Kondo, H. Nakazawa, F. Ito, et al., “Impact of Arterial Occlusion during Partial Nephrectomy on Residual Renal Function: An Evaluation with (99m) Technetium-Dimercaptosuccinic Acid Scintigraphy,” International Journal of Urology, Vol. 9, No. 8, 2002, pp. 435-440. doi:10.1046/j.1442-2042.2002.00498.x
NOTES
*There is no financial disclosure of any of the authors.
#Corresponding author.

