World Journal of Condensed Matter Physics
Vol.06 No.01(2016), Article ID:63312,8 pages
10.4236/wjcmp.2016.61004
Crystal Growth, Structural and Optical Studies of CuGa3Se5 Bulk Compounds
Dayane Habib1, Georges El Haj Moussa1,2
1Physics Department, Faculty of Sciences II, Lebanese University, Jdeidet, Lebanon
2Centre Electronique et Micro-optoélectronique de Montpellier (CEM2), Faculté Sciences et Techniques du Languedoc, Université de Montpellier II, Montpellier, France

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 December 2015; accepted 30 January 2016; published 3 February 2016
ABSTRACT
Bulk materials were synthesized by the Bridgman technique using the elements Cu, Ga, Se. These samples were characterized by Energy Dispersive Spectrometry (EDS) to determine the elemental composition, as well as by X-ray diffraction for structure, hot point probe method for type of conductivity. Optical response (Photoconductivity) and Photoluminescence (PL) and PL-excitation (PLE) at temperatures from 4.2 to 77 K were also used to estimate the band-gap energy of CuGa3Se5. They show a nearly perfect stoechiometry and present p-type conductivity. CuGa3Se5 either have an Ordered Defect Chalcopyrite structure (
Keywords:
Chalcopyrite, Photovoltaic, Bulk Materials Photoluminescence, Optical Response, X-Ray Diffraction, Photoconductivity

1. Introduction
Cu(In1?xGax)Se2 and the related I-III-VI2 chalcopyrite compounds are of great interest due to their potential in photovoltaic and nonlinear optical applications [1] - [3] . Another attractive property is their tolerance to a large range of anion-to-cation off stoechiometry, manifested by the existence of an ordered defect compounds (ODC) with large variations in their Cu/In, Ga/Se ratio [4] . These ODCs, like Cu(In1−xGax)3Se5, generally possess wider gap and the formation of ternary Cu-In-Ga-Se compounds with varying gaps enables the formation of heterojunctions used in the design of high-performance electronic and optoelectronic devices. Ternary semiconductor compound CuGa3Se5 (when x = 1) is a promising material for creation on its basis of a number of semiconductor devices, such as infra-red and visible radiation sources, high-efficient solar cells and other devices of semiconductor and quantum electronics [5] - [7] . The present work prepared the samples of CuGa3Se5 (when x = 1) by the horizontal Bridgman methods [8] [9] using a direct combination of high purity 5 N for Cu, 6 N for Se and Ga. The elements were placed in a quartz tube sealed under a vacuum of 5 ´ 10−6 Torr. Energy Dispersive Spectrometer (EDS) and X-Ray Diffraction (XRD) were used to calculate the compositions of the ingots considered as very important parameters. The hot point probe method is used in order to determine the conduction types of these ingots. Photoconductivity and Photoluminescence allowed us to check their optical properties. The type of transition was determined by varying the gap energy as a function of the temperature and as a function of the excitation power. These studies contribute in the future to improving the efficiency of solar cells formed by heterojunctions made of Cu(InxGa1?x)3Se5 which are promising materials.
2. Experiments
The several crystals used in this work, were synthesized by direct combination of high purity 5 N for Cu and Ga, 6N for Ga and Se in the desired proportions. The elements were placed in a quartz tube sealed under a vacuum of 5 × 10−6 Torr. The latter was placed in a horizontal heater that reached a temperature exceeding the melting point of the compound. It was left in the heater for 72 hours at which point it was allowed to slowly cool down [8] [9] .
Our crystals were characterized by X-Ray Diffraction using a Seifert MZIV powder diffractometer (q, 2q geometry) with Cu (Ka) radiation (l = 1.5406 Ǻ).
The chemical composition of the obtained samples were given by EDS (Link type AN 1000 55/S) coupled to a scanning microscope (Cambridge type S360).
To determine the type of conductivity, the hot point probe method was used.
The Photoluminescence (PL) measurements were performed at different temperatures (from 4.2 K to 85 K) by directly immersing the samples into liquid helium. Excitation was provided by a 632.8 nm He-He laser (20 mW). The illumination of the samples was realized using fiber optic light guides (UV-visible). A 3 mm spot was focused on the sample with a power of 2 mW/cm2. The emission spectrum, collected through another fiber (visible-IR), was analyzed using a grating monochromator (30 cm focal length, 600 lines/mm, blazed at 760 nm).
To get the gap energy value at room temperature, we used the photoconductivity technique. The samples spectral response was measured at a constant light power over the wavelengths range 400 nm - 2000 nm.
3. Results and Discussion
3.1 Characterization by
The chemical compositions of CuGa3Se5 materials by EDS are presented in Table 1. The samples show a nearly perfect stoechiometry since the magnitude of deviation from stoechiometry, 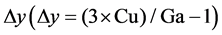 , is very small [10] . The CuGa3Se5 samples present p-type conductivity.
, is very small [10] . The CuGa3Se5 samples present p-type conductivity.
3.2. Characterization by X-Ray Diffraction
The spectra of our samples, obtained by X-Ray Diffraction are identical. Figure 1 presents one spectrum of a CuGa3Se5 sample. They are well-crystallized and all existent peaks are similar to those found in previous work
Table 1. Chemical compositions of CuGa3Se5 bulk samples obtained by EDS.
Figure 1. Spectrum of CuGa3Se5 obtained by X-Ray Diffraction for one sample.
[11] . Thus, our XR spectra show the presence of several preferential orientations according to planes (112), (220) and (312) for all samples. The CuGa3Se5 have a Stannite structure [12] [13] , an Ordered Defect Chalcopyrite structure (ODC), or an Ordered Vacancy Chalcopyrite structure (OVC). Table 2 gives the lattice parameters, a = 5.49 and c = 10.93 of CuGa3Se5, calculated from the spectra. These values are in agreement with literature [14] .
3.3. Characterization by Photoluminescence
The photoluminescence spectrum of CuGa3Se5 is formed of one single emission peak of full width at half maximum of the order of 156 meV peak (Figure 2). The gap energy of our samples at the temperature of liquid helium (4.2 K) is equal to 1.83 eV, these results are in good agreement with literature [14] - [17] .
3.3.1. Influence of Temperature
Figure 3 shows the different photoluminescence spectra of CuGa3Se5, as a function of the temperature at constant excitation intensity (114 mW/cm2). By increasing the temperature, the intensity of the emission peak decreases and a deviation toward low energies is observed. The full width at half maximum increases and the peak intensity decreases, varying the temperature from 4.2 to 77 K.
The activation energy given by the slope of the tangent value to the curve “Intensity of photoluminescence signal as a function of 103/T” shown in Figure 4 was about 185 meV. This value shows that the transition is a D-A type. The defects that appear are probably GaCu, VCu, GaSe [18] [19] . The presence of these types of defects is caused by an excess of Gallium in CuGa3Se5.
Figure 5 shows the positions of emission peak for different values of temperature. The gap energy is determined by adding to the photoluminescence peak value, that of the activation energy, assuming that the latter remains constant. The temperature variation from 4.2 K to 60 K causes a gap energy decrease in the order of 35 meV. This variation can be written as follows:
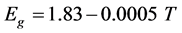
The coefficients of the temperature gap variation for CuIn3Se5 [13] and CuGa3Se5 compounds are in the same order of magnitude. For these samples, the coefficient is negative and slightly higher for CuIn3Se5 [13] than for CuGa3Se5.
3.3.2. Influence of the Excitation Power
Figure 6 shows the variation of CuGa3Se5 photoluminescence spectra as a function of the excitation power at a constant temperature of 4.2 K. In increasing the excitation power, a shift of the peak toward high energies was observed. This result permits us to distinguish the D-A transition from other types of transitions. When this oc-
Figure 2. Photoluminescence spectrum of CuGa3Se5 at 4.2 K.
Figure 3. Variation of CuGa3Se5 photoluminescence spectra with temperature at a constant excitation power (114 mW/cm2).
Figure 4. Photoluminescence signal intensity of CuGa3Se5 as a function of 103/T.
Figure 5. Variation of the peak position (Δ) and the value of the gap (□) along with the temperature of the CuGa3Se5 sample.
Figure 6. Variation of CuGa3Se5 photoluminescence spectra with excitation power at a constant temperature of 4.2 K.
Table 2. Values of a, c and c/a lattice parameters of the different CuGa3Se5 samples.
curs the pairs number becomes increasingly important in this transition and the remote pairs will also begin to take place in the transition. In a D-A type transition, it is known [20] that the signal intensity, I, depends on the power, P, according to a law given by I = C∙Pa, where C and α are constant. From Figure 6, the α value can be calculated.
Figure 7 presents the variation of the light intensity of CuGa3Se5 emission peak as a function of the excitation power at a constant temperature of 4.2 K. The α value found is in the order of 0.925, and thus I = C∙Pa can be rewritten as I = 0.0135P0.925.
3.4. Characterization by Photoconductivity
We have determined the band gap energy value by analyzing our samples using spectral Photoconductivity [21] . Figure 8 illustrates the Photoconductivity spectrum ((αhν)2 as a function of hν) of CuGa3Se5. This spectrum denotes high speeds of surface recombination. A saturation level at high energy was not observed. In these cases, the gap value is given by an approximate value which was found by taking the abscissa of each curve at PCmax/2. The gap value at room temperature is 1.80 eV, which match those found by Photoluminescence and in literature [14] -[17] .
4. Conclusion
The CuGa3Se5 samples have been prepared by the Bridgman method. The different samples have then been characterized by several techniques (EDS, XR, hot point probe, photoconductivity and photoluminescence). All samples present good stoechiometry and are well crystallized. Their lattice parameters a and c are similar to those in previous publications, specifically c/a ≈ 2. The CuGa3Se5 samples present p-type conductivity. The characterization by photoluminescence allowed the gap value of 1.83 eV to be determined for these compounds.
Figure 7. Variation of light intensity of the CuGa3Se5 emission peak as a function of the excitation power at a constant temperature of 4.2 K.
Figure 8. Photoconductivity spectrum ((αhν)2 as a function of hν) of CuGa3Se5 at room temperature.
Studying the variation of the gap as a function of the temperature and of the excitation power showed that the transition is a D-A type and that the appeared defects are probably GaCu, VCu, GaSe. The gap value at room temperature determined by Photoconductivity is equal to 1.8 eV. These results will allow the fabrication in the near future of solar cells formed by heterojunctions made of Cu(InxGa1?x)3Se5 to get a good efficiency at a low cost.
Cite this paper
DayaneHabib,Georges El HajMoussa, (2016) Crystal Growth, Structural and Optical Studies of CuGa3Se5 Bulk Compounds. World Journal of Condensed Matter Physics,06,27-34. doi: 10.4236/wjcmp.2016.61004
References
- 1. Habib, D., Al Asmar, R., El Helou, Z. and El Haj Moussa, G. (2013) Influence of Iodine Pressure on the Growth of CuIn1-xGaxSe2 Thin Films Obtained by Close-Spaced Vapor Transport “CSVT”. World Journal of Condensed Matter Physics, 3, 164-168.
http://dx.doi.org/10.4236/wjcmp.2013.34026 - 2. Lee, J.Y., Seong, W.K., Kim, J.-H., Cho, S.-H., Park, J.-K., Lee, K.-R., Moonand, M.-W. and Yang, C.-W. (2015) Synthesis and Characterization of Single-Crystal Cu(In, Ga)Se2 Nanowires: High Ga Contents and Growth Behavior. CrystEngComm, 17, 4950-4957.
http://dx.doi.org/10.1039/C5CE00752F - 3. Souilah, M., Rocquefelte, X., Lafond, A., Guillot-Deudon, C., Morniroli, J.-P. and Kessler, J. (2009) Crystal Structure Re-Investigation in Wide Band Gap CIGSe Compounds. Thin Solid Films, 517, 2145-2148.
http://dx.doi.org/10.1016/j.tsf.2008.10.077 - 4. Zhang, S.B., Wei, S.-H., Zunger, A. and Katayama-Yoshida, H. (1998) Defect Physics of the CuInSe2 Chalcopyrite Semiconductor. Physical Review B, 57, 9642.
http://dx.doi.org/10.1103/PhysRevB.57.9642 - 5. Wasim, S.M., Rincon, C. and Marin, G. (2002) Electrical Properties of the Ordered Defect Compound CuIn3Se5. Physica Status Solidi (A), 194, 244.
http://dx.doi.org/10.1002/1521-396X(200211)194:1<244::AID-PSSA244>3.0.CO;2-T - 6. Marin, G., Marguez, R., Guewara, R., Wasim, S.M., Delgado, J.M., Rincon, C., Perez, G.S., Molina, I. and Bocaranda, P. (2000) Crystal Growth, Structural and Optical Characterization of the Ordered Vacancy Compounds of the I-III3-VI5 and I-III5-VI8 Families. Japanese Journal of Applied Physics, 39, 44-45.
http://dx.doi.org/10.7567/JJAPS.39S1.44 - 7. Rincon, C., Wasim, S.M., Marin, G. and Molina, I. (2003) Temperature Dependence of the Optical Energy Band in CuIn3Se5 and CuGa3Se5. Journal of Applied Physics, 93, 780.
http://dx.doi.org/10.1063/1.1528305 - 8. El Haj Moussa, G.W., Ariswan, Khoury, A., Guastavino, F. and Llinarés, C. (2002) Fabrication and Study of Photovoltaic Material CuIn1–xGaxSe2 Bulk and Thin Films Obtained by the Technique of Close-Spaced Vapor Transport. Solid State Communications, 122, 391-396.
http://dx.doi.org/10.1016/S0038-1098(02)00100-X - 9. El Haj Moussa, G., Ajaka, M., El Tahchi, M., E. Eid, and Llinares, C. (2005) Ellipsometric Spectroscopy on Polycrystalline CuIn1–xGaxSe2: Identification of Optical Transitions. Llinaresphysica Status Solidi (A), 202, 469-475.
http://dx.doi.org/10.1002/pssa.200406934 - 10. Contreras, M.A., Wiesner, H., Mtson, R., Tuttle, J., Ramanathan, K. and Noufi, R. (1996) Defect Chalcopyrite Cu(In1-xGax)3Se5 Polycrystalline Thin-Film Materials. Materials Research Society Symposium Proceedings, 426, 243-254.
http://dx.doi.org/10.1557/PROC-426-243 - 11. Marin, G., Tauleigne, S., Wasim, S.M., Rincon, C., Guervara, R., Delgado, J.M., Mora, A.E. and Sanchez Perez, G. (1998) X-Ray Powder Diffraction and Optical Characterizations of the Cu(In1-xGax)3Se5 Semiconducting Systems. Materials Research Bulletin, 33, 1057-1068.
- 12. Suzuki, M., Uenoyama, T., Wada, T., Hanada, T. and Nakamura, Y. (1997) Effect of Crystal Symmetry on Electronic Structures of CuInSe2 and Related Compounds. Japanese Journal of Applied Physics, 36, L1139-L1141.
http://dx.doi.org/10.1143/JJAP.36.L1139 - 13. Habib, D., Aoudé, O., Karishy, S. and Moussa, G. (2015) Fabrication, Characterization and Optical Properties of CuIn3Se5 Bulk Compounds. World Journal of Condensed Matter Physics, 5, 201-208.
http://dx.doi.org/10.4236/wjcmp.2015.53021 - 14. Negami, T., Kohara, N., Nikihiko, M., Wada, T. and Hirao, T. (1995) Preparation and Characterization of Cu(In1-xGax)3Se5 Thin Films. Applied Physics Letters, 67, 825-827.
http://dx.doi.org/10.1063/1.115456 - 15. Rincón, C., Wasim, S.M., Marínand, G. and Molina, I. (2003) Temperature Dependence of the Optical Energy Band Gap in CuIn3Se5 and CuGa3Se5. Journal of Applied Physics, 93, 780-782.
http://dx.doi.org/10.1063/1.1528305 - 16. León, M., Levcenko, S., Syrbu, N.N., Nateprov, A., Tezlevan, V., Merinoand, J.M. and Arushanov, E. (2006) Fundamental Absorption Edge in CuIn5Se8 and CuGa3Se5 Single Crystals. Physica Status Solidi(a), 203, 2904-2908.
http://dx.doi.org/10.1002/pssa.200669506 - 17. Levcenko, S., Syrbu, N.N., Nateprov, A., Arushanov, E., Merinoand, J.M. and León, M. (2006) Optical Properties of CuGa3Se5 Single Crystals. Journal of Physics D: Applied Physics, 2006, Article ID: 391515.
http://dx.doi.org/10.1088/0022-3727/39/8/010 - 18. Schön, J.H., Schenker, O., Riazi-Nejad, H., Freimelt, K., Kloc, C. and Bucher, E. (1997) Characterization of Defect Levels in Doped and Undoped CuGaSe2 by Means of Photoluminescence Measurements. Physica Status Solidi(a), 161, 301-313.
http://dx.doi.org/10.1002/1521-396X(199705)161:1<301::AID-PSSA301>3.0.CO;2-Q - 19. Matsushita, H., Jitsukawa, H., Sugano, H., Takizawa, T. and Endo, S. (1997) Electrical and Optical Properties of CuGaSe2 Single Crystals Grown by Solution Method Using CuSe Solvent. Proceedings of the 11th International Conference on Ternary and Multinary Compounds, Salford, 8-12 September 1997, 535.
- 20. Meada, K. (1965) Temperature Dependence of Pair Band Luminescence in GaP. Journal of Physics and Chemistry of Solids, 26, 595-605.
http://dx.doi.org/10.1016/0022-3697(65)90135-6 - 21. Ariswan, El Haj Moussa, G., Abdelali, M., Guastavino, F. and Llinares, C. (2002) Structural, Optical and Electrical Properties of the Ordered Vacancy Compound CuIn3Se5 Thin Films Fabricated by Flash Evaporation. Solid State Communications, 124, 391-396.
http://dx.doi.org/10.1016/S0038-1098(02)00603-8










