Atmospheric and Climate Sciences
Vol.04 No.05(2014), Article ID:51443,8 pages
10.4236/acs.2014.45072
Do Increasing Contents of Methane and Carbon Dioxide in the Atmosphere Cause Global Warming?
G. V. Chilingar, O. G. Sorokhtin, L. F. Khilyuk, M. Liu*
Russian Academy of Natural Sciences, US Section, Los Angeles, USA
Email: *lmfusc2012@gmail.com
Academic Editor: Mohammad Valipour, University of Tehran, Iran
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 September 2014; revised 27 October 2014; accepted 10 November 2014
ABSTRACT
In the Earth atmosphere, methane gradually converts into carbon dioxide which, according to the conventional anthropogenic theory of global warming, is the main driver of global climate change. The authors investigated the greenhouse effect of methane and carbon dioxide in the atmosphere using their tested adiabatic model, which relates the global temperature of troposphere to the atmospheric pressure and solar activity. This model allows one to analyze the global temperature changes due to variations in mass and chemical composition of the atmosphere. Even significant releases of anthropogenic carbon dioxide and methane into the atmosphere do not change aver- age parameters of the Earth’s heat regime and have no essential effect on the Earth’s climate. Thus, petroleum production and other anthropogenic activities resulting in accumulation of additional amounts of methane and carbon dioxide in the atmosphere have practically no effect on the Earth’s climate.
Keywords:
Global Warming, Carbonate Dioxide, Methane

1. Introduction
The content of methane in the atmosphere had been gradually increasing over the last century. In spite of the fact that the methane content constitutes only about 1.8 ppm in the Earth’s atmosphere, the national and interna- tional policy makers declared the methane gas extremely dangerous to the Earth climate because of a high po- tency (about 100 times more potent than CO2 over the time span of 20 years) of its molecules to absorb the infrared radiation. Together with the growing contents of CO2 and other greenhouse gases it supposedly causes drastic changes in the Earth’s climate. According to the conventional anthropogenic theory of global warming as a result of absorption of the infrared radiation by the molecules of the greenhouse gases, these molecules inter- cept infrared photons in the lower layer of troposphere warming the Earth climate. This anthropogenic theory is the “scientific” basis for strong political and economic actions against further expansion of fracking, for exam- ple, in the shale-gas production.
The conventional anthropogenic theory (backed and promoted by IPCC and other national and international organizations over the last 25 years) completely ignores the main physical phenomena of the heat transfer in the atmosphere. In particular, it assumes that the heat transfer in atmosphere occurs exclusively by radiation. Mean- time in the lower dense layer of troposphere it occurs mostly by convection (67% by convection, 8% by radia- tion, and 25% by water vapor condensation) [1] , which is intensified considerably with any additional release of the so-called greenhouse gases. Moreover, analyzing the postulates of the conventional theory one can find out that this theory completely ignores the fact that molecules of methane and other greenhouse gases (H2O, for example) intercept the infrared solar irradiation in the upper layers of stratosphere and, thus, prevent overheating of Earth.
A renowned Swedish scientist, Svante Arrhenius (1896) [2] was the first to forward the idea of Earth’s at- mosphere heating by the greenhouse gases. Since then, the concept was taken for granted as obvious without any verification (Budyko, 1997 [3] ; Global warming, 1993; Greenhouse effect, 1989).
Unfortunately, this view totally dominates conclusions by experts from the Intergovernmental Panel on Cli- mate Change (IPCC) on the climate change, by Greenpeace, UN Environmental Program, World Meteorological Organization (WМО), as well as the conclusions of the European and Russian Environmental organizations.
According to the forecasts based on these ideas, by 2100 climate warming may reach 2.5˚С to 5˚С and the as- sociated rise in the ocean level, of 0.6 to 1 m, which may create problems for densely populated areas of the continental coastal regions as well as for the gas- and oil-industry in some areas of the world. Other deleterious consequences of the global warming are also forecasted (expansion of the deserts, melting of the permafrost, soil erosion, etc.).
The concern about similar catastrophic phenomena and the pressure tactics from the environmental groups, and often mere political and religious speculations on the subject [4] are forcing the governments of developed countries to allocate huge funds for a fight with the consequences of the climate warming ostensibly caused by the man-made releases of “greenhouse” gases into the atmosphere. How justified are these expenses?
A close review of the problem showed that prior to 1990s there was no physical theory of the greenhouse ef- fect fitted to the data of observations. The first publication on the subject was in 1990 [5] . Before of this, all es- timates of the CO2 and other greenhouse gases effect on Earth’s climate were and, unfortunately, are still per- formed using different subjective computer models and numerous, but not always stable, parameters.
There are direct evidences of the fact that the changes in the partial CO2 pressure in the atmosphere are the effect and not the cause of the climate change [6] . When drilling through the ice sheet at the Antarctic Vostok Station [7] , oceanic average temperature (water evaporation from the oceans formed this very ice sheet) as well as CO2 contents in the air bubbles from the ice were determined. The oceanic water evaporation temperature was determined from isotopic shifts for oxygen and hydrogen (deuterium). Strong direct correlation was ob- served between these parameters over the entire studied Antarctic ice accumulation history of 420 MY (see Figure 1). It was found, based on temporal analyses, that temperature changes occurred first, and then were fol- lowed in 500 to 600 years by the changes in CO2 concentration [8] .
This result is practically a monosemantic statement of the fact that the fluctuations of CO2 concentration in the atmosphere are the effect and not the cause of the climate changes. Besides, the time interval of 500 to 600 years well correlates with the time of complete stirring of the upper, active layer of the World Ocean.
The 20th century warming has the clear natural origin and may soon be replaced by a new cooling-down phase [9] . The instrumental temperature observations were conducted in Southern England since 1749. Solar magnetic activity was recorded in France as the number of Sun spots (the Wolfe numbers) since the 1750’s. The compari- son of these data shows extremely high correlation between the near-surface Earth’s temperature and solar magnetic activity. Thus, the forecasts of the Earth’s climate changes cannot be made without the consideration of the periodic changes in solar activity.
Figure 1. Isotopic air temperature (a) vs. carbon dioxide concentration (b) over the recent 420 MY on the “Vostok” Antarctic station. Field data of the СО2 concentrations and temperature derived from the cores of a well drilled to TD = 3623 m were kindly provided by V. M. Kotlyakov (2000) [7] (the scale of Earth’s average temperatures is our interpretation).
The writers developed a physical model of heat transfer in the atmosphere that includes the most significant parameters of the medium and the definitive characteristics of the climate drivers. This model includes the av- eraged value of the Sun radiation energy impinging the Earth, average atmospheric pressure and atmospheric heat capacity as well as the effect of the negative feedback between the tropospheric albedo and of the average surface temperature. Thus, one can obtain the most reliable parameters of the greenhouse effect although Earth with this approach is just a dimensionless point. This model, in particular, allows analyzing the effect of addi- tional contents of the “greenhouse” gases on the average Earth’s temperature providing numerical estimates of the temperature changes due to any variations in the atmospheric composition. This tested model [1] [10] may be considered one-dimensional as it describes the correlation of the tropospheric temperature with elevation over the planet’s surface.
This approach has definite advantages for the purposes of decision making as it allows for the derivation of the analytical singular result in the solution of global problem, for instance such as the effect of the atmosphere on the total value of its greenhouse effect (for Earth as a whole). The single-dimensional model is amenable to the inclusion of additional and local parameters. These parameters may be, for instance, Earth revolution axis angle with the ecliptics, inflow of the additional heat through the air mass (cyclones) currents, and snow cover albedo [1] .
2. Fundamentals of the Adiabatic Theory of the Heat Transfer in the Earth’s Atmosphere
It is most convenient to select Earth’s average surface temperature Ts as a main variable characterizing the av- eraged global climate (the current value is Ts = 288.2 K » 15˚С). As opposed to the classical approach, we will assume that the main mechanism of heat release from the surface of the planets with a dense atmosphere (the atmospheric pressure р > 0.2 atm) is the convective air mass transfer in the troposphere. In this case, the temper- ature distribution in the troposphere on average must be close to adiabatic, and average planet’s surface temper- ature will depend on the solar constant S, the atmospheric pressure p, the precession angle ψ of the revolving planet, its albedo A and the effective value of the albedo exponent α (determined by the troposphere gaseous composition and humidity).
It is known that the adiabatic temperature distribution is controlled by the atmospheric pressure p and the ef- fective gas heat-capacity [11] : 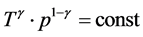 and
and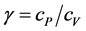 , where cp and cv are the gas heat-capacities under constant pressure and constant volume, respectively. Then the T(p) may be found as:
, where cp and cv are the gas heat-capacities under constant pressure and constant volume, respectively. Then the T(p) may be found as:
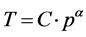 (1)
(1)
where  and
and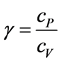 . (1’)
. (1’)
It shows that under adiabatic process the gas temperature in degrees Kelvin (K) is always proportionate to the gas pressure p to the power of the adiabatic exponent α which is a function of the effective heat capacity of the atmospheric gas mixture. The negative feedback pertaining to the solar radiation transformation by the cloud cover must be taken into account: the cloud cover usually plays a major role in the formation of the pla- net’s albedo.
In the previous publications [1] [6] the authors derived the main equations and showed that temperature dis- tribution in dense (р > 0.2 atm) troposphere is ruled by the following law:
 (2)
(2)
where σ ( = 5.67 × 10–5 erg/cm2·s·deg4) is the Stefan-Boltzmann constant; b is the scaling factor determined by the planet’s given surface temperature Ts in degrees Kelvin (for Earth Ts = 288.2 K), the solar constant S (aver- age value for Earth S = 1.367 × 106 erg/cm2·s), the precession angle ψ (for Earth ψ = 23.44˚), the planet’s albedo А (for Earth А ≈ 0.3) at pt = p0 (р0 = 1 atm), and the adiabatic exponent α = 0.1905. In this case, bα = 1.093. At the Earth precession angle ψ = 23.44, the denominator in Equation (2) is equal to 3.502 (rather than 4 in the classic Boltzmann Equation).
Besides the Equations (1’) for α, there is another way to determine the same parameter. If gas heat capacity is expressed in cal/g·deg, and the gas constant is R = 1.987 cal/mole·deg, then the adiabatic exponent α, as a func- tion of the atmosphere composition and humidity, may be found from the known equations:
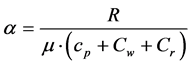 (3)
(3)
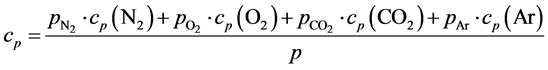 (3’)
(3’)
where μ » 29 is the air molar weight; 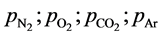 are nitrogen, oxygen, carbon dioxide and argon par- tial pressures; p is total atmospheric pressure; cp(N2) = 0.248; cp(O2) = 0.218; cp(CO2) = 0.197; cp(Ar) = 0.124 cal/g·deg are nitrogen, oxygen, carbon dioxide and argon specific heats at constant pressure [12] ; Сq = Cw + Cr is the correction factor with the dimension of specific heat (it takes into account the total heating effect of the water vapor condensation processes Cw in a humid atmosphere) and the absorption Cr of Sun and Earth heat radiation. For planets with different atmospheres, the Сq parameter is the characteristic of any physical or chemi- cal process resulting in the heat release (or absorption if Cq < 0) within the troposphere. For Earth’s atmosphere, the best fit of the adiabatic theory with the standard troposphere model occurs at α = 0.1905.
are nitrogen, oxygen, carbon dioxide and argon par- tial pressures; p is total atmospheric pressure; cp(N2) = 0.248; cp(O2) = 0.218; cp(CO2) = 0.197; cp(Ar) = 0.124 cal/g·deg are nitrogen, oxygen, carbon dioxide and argon specific heats at constant pressure [12] ; Сq = Cw + Cr is the correction factor with the dimension of specific heat (it takes into account the total heating effect of the water vapor condensation processes Cw in a humid atmosphere) and the absorption Cr of Sun and Earth heat radiation. For planets with different atmospheres, the Сq parameter is the characteristic of any physical or chemi- cal process resulting in the heat release (or absorption if Cq < 0) within the troposphere. For Earth’s atmosphere, the best fit of the adiabatic theory with the standard troposphere model occurs at α = 0.1905.
The atmosphere specific heat may be estimated from its characteristic temperatures. Let us assume that Qa is the atmosphere’s heat content and ma is its mass. Then the atmospheric specific heat radiation component Cr may be found from the effective temperature Те as follows:
 (4)
(4)
Similarly, we may assume that the additional heating of the atmosphere from the planet’s effective tempera- ture to its average surface temperature Ts is described by the total specific heat:
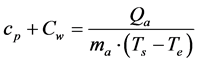 . (4’)
. (4’)
Excluding Qa from the Equations (4) and (4’),
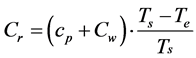 (5)
(5)
and, taking Equation (3) into account, one obtains:
 (5’)
(5’)
 (6)
(6)
On substituting the above Earth atmospheric parameter values α = 0.1905, μ = 29, ср = 0.2394 cal/g·K, Ts = 288 K and Те = 263.5 K into Equations (5’) and (6), one obtains: Сr = 0.0306 cal/g·K, Cw = 0.0897 and Cr + Cw = 0.1203. For the Venus atmosphere with α = 0.1726, μ = 43.2, ср = 0.199 cal/g·K, Ts = 735.3 K and Те = 228 K, the parameters evaluation result in Cr = 0.162, Cw = −0.1164 cal/g·K and Cq = Сr + Cw = 0.0456.
To check the accuracy of Equation (2) (used for the determination of temperature distribution in Earth’s and Venus’s troposphere based on given composition and pressure) the results were compared with the standard temperature distribution in the Earth’s troposphere [13] and of Venus troposphere [14] . The present-day surface atmospheric pressures are respectively ps = 1 and 90.9 atm with p0 = 1 atm; the precession angles are ψE = 23.44˚ and ΨV ≈ 3.18˚. The results of comparison are presented in Figure 2, which demonstrate perfect fit of the adiabatic model to experimental data.
The quoted comparisons indicate that average temperature distribution in the planet’s troposphere is completely defined by the solar constant, atmospheric pressure (mass), heat capacity of its gas composi- tion and the precession angle. The theoretical temperature on Venus surface turned out to be Ts = 735 K, and on Earth’s surface, 288 K. The empiric values are 735.3 and 288.2 K, respectively. This close fit cannot be ac- cidental and presents the convincing evidence in favor of the adiabatic theory of heat transfer in a dense atmos- phere.
Coming back to the temperature distribution on Earth, the heat release from its surface through convection, radiation and the water vapor condensation in the atmosphere is defined by the total heat content associated with the processes. The dry air specific heat is ср = 0.2394 cal/g·deg, the specific heat of the water vapor is Cw = 0.0897 cal/g·deg, the specific heat associated with radiation is Cr = 0.0306 cal/g·deg, and the Earth’s near-sur- face temperature is Ts = 288.2 K.
Using these data, one can determine the three components of the heat flow in the Earth atmosphere Ep, Ew, and Er: for absolutely dry and transparent atmosphere in which heat transfer may occur only through convection Ep = 68.99 cal/cm2, for the water vapor condensation process Ew = 25.85 cal/cm2, and for the radiation compo-
Figure 2. Experimental temperature distribution in Earth’s troposphere and stratosphere (curve 4) and in Venus’s troposphere (1 and 2) (Planet Venus, 1989) compared with averaged theoretical distributions (5 and 3) based on physical (adiabatic) theory of the greenhouse effect (the temperatures are in absolute degrees Kelvin).
nent Er = 8.82 cal/cm2. Thus, the contribution to the heat transfer from convection component is about 66.55%, from the water vapor condensation processes 24.94%, and from radiation (by “greenhouse gases”) only 8.51% (see Figure 3).
3. Impact of the Increased Content of CO2 on Global Climate Changes
It is important to note that increase in the heat absorption in the troposphere by CO2 and other “greenhouse gases” results only in a decrease of the adiabatic exponent α. Besides, the carbon dioxide atmosphere has greater densi- ty than the nitrogen-oxygen atmosphere and methane has lower density so that the same pressures in these at- mospheres will be reached at different elevations h than those in the nitrogen-oxygen atmosphere:
and
where



Thus, one comes to a seemingly paradoxical conclusion that absorption of the IR radiation in the troposphere does not increase but, conversely, only lowers the temperature of the planet’s troposphere (see Figure 4).
The near surface temperature of the hypothetical methane atmosphere will be 288.1 K which is just 0.1˚C above the usual average Earth’s temperature of 288 K. The temperature distributions within the hypothetical to- tally carbon dioxide and totally methane atmospheres were presented by Chilingar et al. [15] together with the temperature distribution in the existing nitrogen-oxygen atmosphere. For a hypothetical methane atmosphere, the near surface temperature at sea level remains almost unchanged, because the corresponding values of the coefficient α are almost the same (0.1905 for the nitrogen-oxygen atmosphere and 0.1915 for the totally methane atmosphere).
Physically, an explanation of the cooling effect of the atmosphere with the high content of “greenhouse gases” is the high efficiency of the convective heat transfer from the planet’s surface to the lower stratosphere, from which this heat is rapidly dissipating into the outer space through radiation. As the greenhouse gases absorb the
Figure 3. Heat transfer balance in Earth troposphere: 66.6% of the heat is lost through the air convection, 24.9%, with humidity condensation, and only 8.5%, with radiation.
Earth’s heat radiation in the lower layers of troposphere, its energy transforms into the heat oscillations of the gas molecules. This, in turn, leads to expansion of the gas mixture and its rapid ascent to the stratosphere where the heat excess is lost through radiation into the outer space.
To replace these volumes of the warm air, the already cooled air descends from the upper troposphere. As a result, the global average atmospheric temperature slightly decreases. One particular consequence of it is that with an increase in the carbon dioxide and methane contents in troposphere the convective mass exchange of the atmospheric gases must substantially accelerate. Thus, it is not out of the question that the intensification of synoptic processes in Earth troposphere (but not temperature increase) may be a result of the carbon dioxide and other “greenhouse gases” accumulation.
Similarly, if one mentally replaces Venus’s carbon dioxide atmosphere by the nitrogen-oxygen atmosphere at the same pressure of 90.9 atm, its surface temperature would increase from 735.3 K tо 793.4 K (or from 462 tо 520˚С, see Figure 5).
Figure 4. Averaged temperature distributions in Earth’s troposphere [6] : 1. For actual model of Earth’s atmosphere with the nitrogen-oxygen air mix; 2. For Earth’s atmosphere model with the carbon dioxide air composition (all other parameters are the same as in the standard model 1). Comparison of the graphs shows that CO2 accumulation results in climate cooling.
Figure 5. Averaged temperature distributions in Venus’s troposphere based on Equation (2) (After Sorokhtin, Chilingar, and Khilyuk, 2007) [6] : 1. Tem- perature distribution for the real carbon-dioxide Venus troposphere (above 60 km); 2. Temperature distribution for a hypothetical model of nitrogen-oxygen Venus troposphere, all other conditions being constant.
4. Impact of Increased Content of CH4 on Global Climate Changes
Methane molecules have a high capacity of absorption of the infrared photons. According to estimates of EPA, the GWP (Global Warming Potential) of methane is 21 times higher than GWP of carbon dioxide over the 100 year time span. Light methane released in the atmosphere ascends into its upper layers where it initially reacts with ozone, ultimately producing (through the chain of reactions) water and carbon dioxide.
This process of chemical reactions can be summarized in a single equation as follows:

The “greenhouse gases” involved in the reaction absorb infrared radiation at different parts of the infrared spectrum. The rate of oxidation primarily depends on availability of the free OH radicals. The estimated by IPCC and adapted by EPA [16] , the methane life-time in the atmosphere is in the range of 8 - 12 years.
Based on these considerations one can consider the global warming (cooling) effect of the methane releases as the effect of supplying additional amount of CO2 corresponding to the amount (and corresponding absorption capacity) of CO2. (For every molecule of CH4 one can substitute 21 molecules of CO2). Thus, one can analyze the radiation warming (cooling) effect of methane as the effect of additional concentration 21 times 1.8 ppm of CO2. This additional concentration of CO2 (37.8 ppm) leads to cooling of the atmosphere as a result of increased convection in the lower layers of the troposphere. Oxidation of methane is a main source of water vapor in the upper stratosphere. The methane gas together with the water vapor shields the Earth’s surface from the solar ir- radiation, thus lowering the average Earth surface temperature.
Therefore, it turns out that the common concept of Earth’s climate warming due to accumulation of the anth- ropogenic CO2 and other “greenhouse gases” is a myth. On the contrary, the atmospheric accumulation of CO2 and CH4, with all other conditions constant, can result only in the global climate cooling and in somewhat in- creased synoptic activity in the Earth’s troposphere. There is currently not a single proven fact of the “green- house gases” effect on Earth’s climate.
A detailed correlation of the Arctic near-surface temperatures with the pulsations of solar radiation is pro- vided in Figure 6 [17] . Using these data and the graphs of the anthropogenic CO2 accumulation, Robinson et al. [18] showed without any doubt that the carbon dioxide concentration in Earth’s atmosphere has no effect on Earth climate. Moreover, in view of 60-year fluctuations of the solar activity and the fact that the last cycle of the fluctuation began around 1970, it may be expected that the next cool down will occur within 20 to 30 years.
In summary, the authors would like to draw attention of the scientists and politicians to the fact that numerous traditional and “classical” model forecasts of the climate changes caused by the atmospheric releases of the so- called “greenhouse” gases are as a rule based on intuitive considerations. Besides, there is currently not a sin- gle proven fact of the “greenhouse gases” effect on Earth’s climate.
5. Conclusion
The writers investigated the greenhouse effect using their adiabatic model, which relates the global temperature of troposphere to the atmospheric pressure and solar radiation. This model allows one to analyze the global-
Figure 6. Near-surface temperature in the Arctic vs. solar activity (after Robinson et al., 2007) [17] .
temperature changes due to variations in mass and chemical composition of the atmosphere. Even significant releases of anthropogenic carbon dioxide and methane into the atmosphere do not change average parameters of the Earth’s heat regime and have no essential effect on the Earth’s climate warming. Moreover, based on the adiabatic model of heat transfer, the writers showed that additional releases of CO2 and CH4 lead to cooling (and not to warming as the proponents of the conventional theory of global warming state) of the Earth’s atmosphere. The additional methane releases possess a double cooling effect: First, they intensify convection in the lower layers of troposphere; Second, the methane together with associated water vapor intercept part of the infrared solar irradiation reaching the Earth. Thus, petroleum production and other anthropogenic activities resulting in accumulation of additional amounts of methane and carbon dioxide in the atmosphere have practically no effect on the Earth’s climate.
References
- Sorokhtin, O.G., Chilingar, G.V., Khilyuk, L.F. and Gorfunkel, M.V. (2007) Evolution of the Earth’s Global Climate. Energy Sources, Part A, 29, 1-19.
- Arrhenius, S. (1896) On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground. Philosophical Magazine, 41, 237-276. http://dx.doi.org/10.1080/14786449608620846
- Budyko, М.H. (1997) The Carbon Dioxide Problem. Gidrometeoizdat, Sankt Petersburg, 60 p.
- Gore, А. (2006) Inconvenient Truth. Rodale Books, 328 p.
- Sorokhtin, О.G. (1990) Greenhouse Effect of Atmosphere in the Geological History of EARTH. Doklady Akademii Nauk SSSR, 315, 587-592.
- Sorokhtin, O.G., Chilingar, G.V. and Khilyuk, L.F. (2007) Global Warming and Global Cooling: Evolution of Climate on Earth. Elsevier, Amsterdam, 313 p.
- Kotlyakov, V.M. (2000) Glaciology of Antarctica. Nauka, Moscow, 384 p.
- Fischer, H., Wahlen, M., Smith, J., Mastroianni, D. and Deck, B. (1999) Ice Core Records of Atmospheric CO2 around the Last Three Glacial Terminations. Science, 283, 1712-1714. http://dx.doi.org/10.1126/science.283.5408.1712
- Landscheidt, T. (2003) New Little Ice Age Instead of Global Warming? Energy and Environment, 14, 327-350. http://dx.doi.org/10.1260/095830503765184646
- Sorokhtin, O.G., Chilingar, G.V., Sorokhtin, N.O. and Gorfunkel, M.V. (2011) Evolution of Earth and Its Climate: Birth, Life and Death of Earth. Elsevier, Amsterdam, 763 p.
- Landau, L.D. and Lifshits, E.M. (1979) Statistical Physics, Part 1. Nauka, Moscow, 559 p.
- Naumov, G.B., Ryzchenko, B.N. and Khodakovskiy, I.L. (1971) Reference Book of Thermodynamic Characteristics. Atomizdat, Moscow, 240 p.
- Bachinskiy, A.I., Putilov, V.V. and Suvorov, N.P. (1951) Handbook of Physics. Uchpedgiz, Moscow, 380 p.
- (1989) Venus (Atmosphere, Surface and Structure). Nedra, Moscow, 482 p.
- Chilingar, G.V., Sorokhtin, O.G., Khilyuk, L. and Gorfunkel, M.V. (2009) Greenhouse Gases and Greenhouse Effect. Environmental Geology, 58, 1207-1213. http://dx.doi.org/10.1007/s00254-008-1615-3
- EPA (2010) Methane and Nitrous Oxide Emissions from Natural Sources. US Environmental Protection Agency, Washington DC.
- Robinson, A.B., Robinson, N.E. and Soon, W. (2007) Environmental Effects of Increased Atmospheric Carbon Dio- xide. Journal of the American Physicians and Surgeons, 12, 79-90.
- Robinson, A.B., Baliunas, S.L., Soon, W. and Robinson, Z.W. (1998) Environmental Effects of Increased Atmospheric Carbon Dioxide. Journal of the American Physicians and Surgeons, 3, 171-178.
NOTES
*Corresponding author.










