World Journal of Nano Science and Engineering
Vol.3 No.3(2013), Article ID:36675,7 pages DOI:10.4236/wjnse.2013.33009
Size Variation of Gold Nanoparticles Synthesized Using Tannic Acid in Response to Higher Chloroauric Acid Concentrations
1Applied Science and Humanities Section, University Polytechnic, Faculty of Engineering and Technology, Aligarh Muslim University, Aligarh, India
2Department of Applied Physics, Faculty of Engineering and Technology, Aligarh Muslim University, Aligarh, India
Email: *tufail_phys@rediffmail.com
Copyright © 2013 Tufail Ahmad, Wasi Khan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 22, 2013; revised July 23, 2013; accepted July 30, 2013
Keywords: Gold Nanoparticles; Tannic Acid; Chloroauric Acid; Nanoparticle Size; DLS
ABSTRACT
The size evolution of gold nanoparticles synthesized using tannic acid with initial gold chloride concentrations ranging from 0.2 - 2 mM at various tannic acid to chloroauric acid molar ratios (ranging from 2:1 to 12:1) has been analysed. Dynamic light scattering spectroscopic and tramission electron microscopic analyses were performed to assess the size of formed gold nanoparticles. Two different patterns of nanoparticle size evolution were obtained; the size evolution trend below 1 mM chloroauric acid concentration was found to be different from the one obtained at gold chloride concentrations higher than or equal to 1 mM. In case of sizes obtained for less than 1 mM gold chloride concentration, a general decrease in particle size was observed with increase in gold salt concentration. On the contrary, for the particles synthesised using chloroauric acid concentrations higher than or equal to 1 mM, with increase in gold salt concentration, a general increase in nanoparticle diameter was seen. For the molarities 0.2 and 0.5 mM, with increase in tannic acid/ chloroauric acid ratios, first the size decreases and then increases and finally reaches saturation. Particles formed at molarities greater than equal to 1 mM do not exhibit plateaux in their size rather initially decrease and then increase in response to increasing tannic acid/chloroauric acid ratios except for 2 mM concentration at which a small saturation is observed. The findings enumerate that higher gold chloride concentrations leave a significant impact on the sizes of gold nanaparticles obtained using tannic acid as a reducing agent of chloroauric acid solution.
1. Introduction
The last few decades have witnessed the emergence of nanotechnology as a promising field since it deals with nanoparticles displaying unique optical [1], electronic [2], chemical [3], photoelectrochemical [4] or magnetic properties [5] which render them to be applied for diverse functions. The foundation of nanotechnology is based on the size and shape of nanoparticles which play a significant role in tuning these properties. Indeed, the similarity of the size of nanoparticles to that of biological molecules (like proteins and DNA) along with bacteria and viruses has sprouted enormous interest in exploiting nanoparticles for various biomedical applications [6]. Moreover, it has been proposed recently that the uptake of nanoparticles by mammalian cells is size-dependant [6]. This makes the study of the size of nanoparticles of utmost importance.
Owing to the diverse properties exhibited by gold nanoparticles (GNPs) [7], they bear applications in various areas such as cosmetics [8], electronics [9], therapeutics [10,11], imaging [12,13], drug delivery [14,15] and pollution remediation [16,17]. However, applications in such diverse fields often require GNPs to be of a particular size and be in large numbers or higher concentrations [18,19]. Hence, it becomes relevant to study the effect of changes in synthesis procedures on nanoparticle properties including size. The synthesis of GNPs mediated by citrate reduction of gold chloride solution is well documented [20-22]. Citrate molecules act as both reducing and stabilizing agents, allowing for the formation of the colloidal gold [22]. Like citrate, exploitation of tannic acid (a plant derived polyphenol) as a reductant and stabilizer in the synthesis of gold nanoparticles dates back to early 20th century [22,23] but citrate in the past few decades enjoys an extra edge over tannic acid (TA) as an agent for GNPs synthesis. Overwhelming amount of literature is available for the effect of citrate concentration [24], pH [25], temperature [26] and gold chloride concentrations [22] on the characteristic properties of GNPs synthesized using citrate as reductant. However, similar studies on the GNPs synthesized exploiting TA have not been much explored and our knowledge on this subject remains in its infancy. Nevertheless, the realization of this paucity combined with the stability of TA coated nanoparticles in higher particle concentrations prompted the scientific community to study the TA mediated synthesis of GNPs and as a result, reports on this issue had appeared recently. For instance, recently Sivaraman et al. and Zhang et al. have demonstrated the synthesis of GNPs (at room temperature) and nanoplates respectively exploiting TA [27,28]. Moreover, the effect of pH on the size distribution of GNPs synthesized using TA has also been investigated [27]. Aromal and Philip (2012) very recently reported facile one pot synthesis of GNPs using TA and its application in catalysis [28].
The objective of the present work was to perform a systematic study on the size evolution of the synthesized GNPs when the molarities of the initial chloroauric acid solutions and their respective TA/chloroauric acid ratios were increased. To investigate the pattern of size evolution of GNPs formed, different starting conditions were taken to induce the synthesis of GNPs: seven chloroauric acid concentrations ranging from 0.2 mM to 2 mM were examined and TA/chloroauric acid molar ratios from 2:1 to 12:1 had been investigated. The sizes of as-synthesized GNPs have been measured by dynamic light scattering (DLS) spectroscopy and transmission electron microscopy (TEM). The patterns of the particle sizes obtained were further analysed and it was found that the concentration of the initial chloroauric acid solution had a marked impact on the size of GNPs obtained. Although reports enumerating the effect of higher gold salt concentrations on the size of GNPs synthesized using citrate are available [21,22], this is the first report where effect of high concentrations of gold chloride has been analysed on GNPs synthesized exploiting TA.
2. Materials and Methods
2.1. Chemicals
Hydrogen tetrachloroaurate (III) hydrate (HAuCl4) and TA (ACS reagent) were purchased from Sigma Aldrich Co. All the chemicals were of highest purity available and used as received. All glasswares used for gold nanoparticle synthesis were washed with freshly prepared aqua regia solution (three parts HCl, one part HNO3) and rinsed with deionized water. The same deionized water was used throughout the experiments.
2.2. Gold Nanoparticle Synthesis
For the gold nanoparticle synthesis, first of all 1% and 5% aqueous stocks of chloroauric acid and TA respectively were prepared. Further, the molarities of HAuCl4 and TA were calculated accurately. The gold chloride concentration was varied between 0.2 to 2 mM by accurately picking gold chloride solution from the 1% stock. The TA to gold (III) molar ratios (MR) ranged from 2:1 to 12:1 by adding fitting amount of TA from the 5% aqueous stock to the 0.2 - 2 mM solutions of boiling gold chloride28. Boiling was continued for 2 minutes. The solutions were kept under continuous stirring for 20 to 30 minutes depending on the appearance of ruby red colour.
2.3. Dynamic Light Scattering (DLS) Measurements
In order to gauge the average hydrodynamic diameter of the as-synthesized GNPs, DLS spectroscopy was performed with the samples of the obtained GNP solutions on a Malvern Zetasizer Nano ZS (Malvern, Southborough, MA). GNP samples were stored at 4˚C and aliquots were taken to repeat the experiment three times for monitoring the size of GNPs by DLS. The average of all DLS measurements was calculated for each GNP sample and the resulting hydrodynamic diameters were recorded.
2.4. Transmission Electron Microscopy (TEM)
In order to validate the results obtained by DLS, microscopic analysis of the various samples of the prepared GNPs was performed using a transmission electron microscope (1200 EX, JOEL Inc, Peabody, MA) following a method described elsewhere [29]. A drop of GNP solution from each of the sample was put on a negative carbon-coated copper grid which was further dried before being transferred to the transmission electron microscope.
3. Results and Discussion
Citrate mediated reduction of chloroauric acid for the generation of GNPs has been well studied with respect to the effect of various concentrations of citrate, effect of pH and temperature, effect of incubation time and more recently reports of the effect of higher gold concentrations on the generation of GNPs by citrate mediated reduction have started appearing. Nevertheless, despite TA mediated synthesis of GNPs being an old approach, studies on the variations in synthesis conditions using TA as the reductant have not been explored much and our understanding of the nature of GNPs synthesized using TA under different conditions remains in its infancy. In the present study, taking a lead from a recent report on the effect of high gold chloride concentrations on the size of GNPs prepared by citrate reduction of gold salt [22], we studied the variation in the size of GNPs produced using TA as the reducing and stabilizing agent with different initial molarities of HAuCl4 solution (ranging from 0.2 to 2mM) and taking TA to HAuCl4 molar ratios in the range of 2:1 to 12:1. DLS and TEM analyses were used to perform the study.
3.1. DLS Studies
DLS studies exhibiting the effect of the initial gold salt concentration on the size of GNPs synthesized are displayed by the Figures 1-4. Figures 1 and 2 show the trends of the hydrodynamic diameter of the generated

Figure 1. Variation of GNP size as a function of tannic acid to chloroauric acid ratios at gold chloride concentrations less than 1 mM.
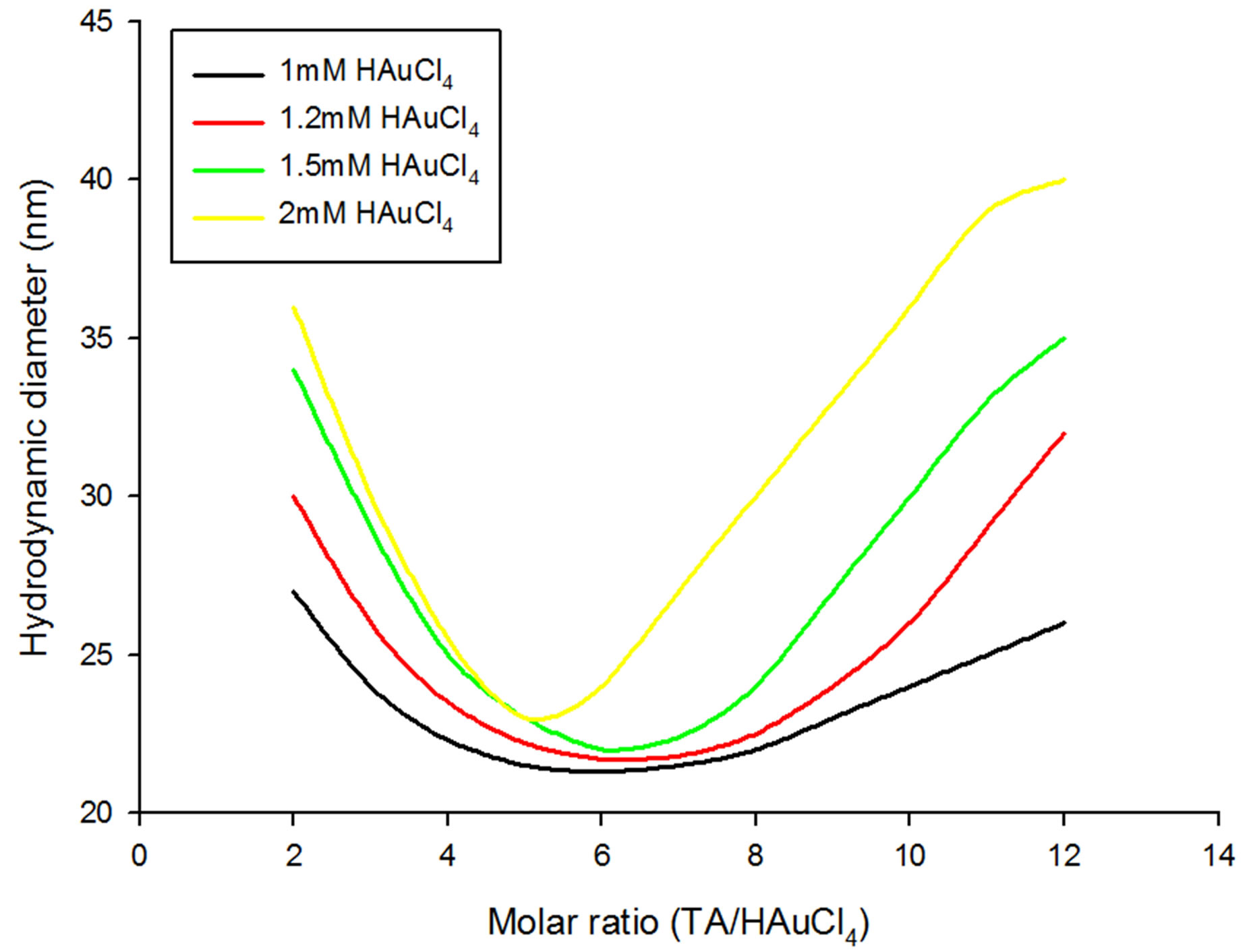
Figure 2. Size variation of GNPs with respect to increasing molar ratios of tannic acid/gold chloride at various concentrations of chloroauric acid below and equal to 1 mM.

Figure 3. Diameter of GNPs as a function of various concentrations of chloroauric acid at different tannic acid to HAuCl4 ratios ranging from 2:1 to 6:1.
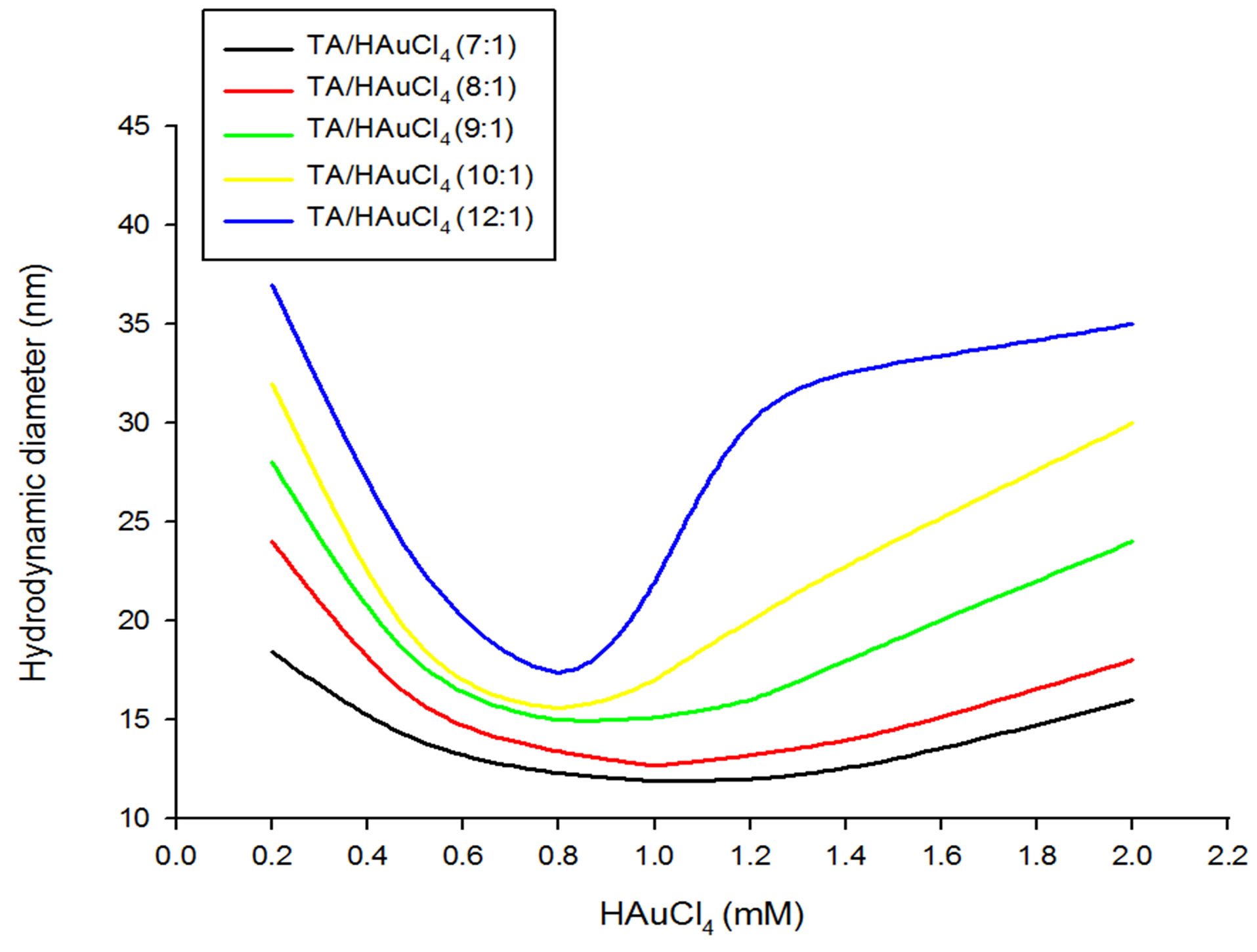
Figure 4. Size of GNPs with respect to HAuCl4 concentrations at various tannic acid/HAuCl4 ratios ranging from 7:1 to 12:1.
GNPs as a function of TA to HAuCl4 molar ratios at various molarities of HAuCl4 solution taken. Under varying initial gold chloride concentration, the size evolution of the generated GNPs exhibited two different patterns; one for HAuCl4 molarities less than 1 mM (Figure 1) and the other for the HAuCl4 molarities greater than or equal to 1mM (Figure 2).
Variation of hydrodynamic diameters of GNPs as a function of various TA to chloroauric acid ratios at different initial concentrations of HAuCl4
The GNPs synthesized from 0.2 and 0.5 mM chloroauric acid solutions exhibit large hydrodynamic diameters (around 38 and 33 nm respectively) when a TA/ HAuCl4 ratio of 2:1 is used (Figure 1). The size of GNPs was found to abruptly decrease and reach a minimum diameter at TA/HAuCl4 ratio of 4:1 for both 0.2 and 0.5 mM chloroauric acid solution, however, the sizes were found to increase again as the TA/HAuCl4 molar ratios increased and finally saturation was achieved beyond the molar ratio 8:1. While the evolution of the GNP sizes followed similar fashions for 0.2 and 0.5 mM chloroauric acid solutions, for the same TA/HAuCl4 ratios, the GNPs generated from 0.5 mM chloroauric acid solutions were found to be smaller in size than those prepared using 0.2 mM chloroauric acid solutions. The differential sizes become more apparent while moving towards higher ratios as exhibited by the wide gap in the size profile of GNPs produced by 0.2 mM and 0.5 mM chloroauric acid (Figure 1). As seen in the Figure 2, the size of GNPs synthesized using 0.2 mM chloroauric acid reaches saturation at diameters of around 39 nm whereas the hydrodynamic diameter profile for 0.5 mM HAuCl4 plateaus around 29 nm. These results overlap with those observed by Zabetakis et al. for GNPs formed using citrate and chloroauric acid less than 0.8 mM. They too observed that for chloroauric concentrations below 0.8 mM, higher HAuCl4 concentrations generate saturation with smaller sized particles. However, in their case the saturation was achieved at higher molar ratios (>14:1) but in the present study, tannic to chloroauric acid ratio of 9:1 induced plateau formation. Moreover, the particle size at a particular TA/HAuCl4 ratio was found to be around 10nm larger than induced by the same citrate/HAuCl4 ratio. Larger particle size and smaller saturation ratio can be explained as TA is a more aggressive reducing agent that consumes rapidly all the chloroauric acid to form GNPs.
GNPs prepared from 0.8mM chloroauric acid solution exhibit a mid-way pattern as shown with 0.2 and 0.5 mM chloroauric acid solutions and those displayed with chloroauric acid concentrations beyond or equal to 1 mM. It is observed that 0.8 mM gold solutions form smaller GNPs than the GNPs formed with 0.2 and 0.5 mM gold solutions. However, at 12:1 ratio, the particle size for 0.8 mM solution was found to be greater than 0.6 mM choloroauric acid solution. Till ratio 4:1, the particle size continuously decreases, forms plateau from 5:1 to 7:1 and again rises and the particles attain 30nm diameter at the TA/HAuCl4 ratio of 12:1 (Figure 1).
Formation of GNPs from 1 - 2 mM chloroauric acid solutions with respect to TA/HAuCl4 ratios is displayed by Figure 2. In a manner similar to lower HAuCl4 concentrations, first with increasing TA/HAuCl4 ratios, hydrodynamic diameter of GNPs formed from 1 - 2 mM chloroauric acid solution decrease until they reach a minimum size at around 6:1 ratio and then again increase as the ratio is increased. Interestingly, in contrast to chloroauric acid concentration less than 0.8 mM, in none of the ratios saturation is observed except a short plateau for 2 mM gold solution along 11:1 and 12:1 ratios. Moreover, the slopes for decrease and increase in diameter become steeper with increase in concentration of choloroauric acid that renders formations of large sized GNPs. Furthermore, the least hydrodynamic diameter obtained for 1, 1.2 and 1.5 mM HAuCl4 ranged between 21 - 22 nm but with 2mM concentration, it was found to be 24 nm. Also, the highest diameter of 40 nm was observed for 2 mM chloroauric acid solution (Figure 2).
Size variation of GNPs in response to various initial molarities of chloroauric acid at different TA to HAuCl4 ratios
Variation of hydrodynamic diameter of as-synthesized GNPs with respect to initial molarity of HAuCl4 has also been studied at different TA to HAuCl4 molar ratios (Figures 3 and 4). As exhibited by Figure 4, with increase in chloroauric acid concentration, the hydrodynamic diameters for ratios 2:1 and 3:1 first decrease, reach a minima at 0.8 mM concentration and again increase steeply till 1.2 mM concentration but further the rise in diameter is not sharp rather a slow growing slope is observed from 1.2 to 2 mM concentration. For each of the slope from 4:1 to 6:1 ratios, as the HAuCl4 concentration increases, first the hydrodynamic diameter decreases to reach a minimum value at 1 mM concentration and further the slope rises slowly with increasing concentration of HAuCl4. Moreover, when the TA/HAuCl4 ratio is increased from 2:1 to 6:1, a universal decrease in the hydrodynamic diameter of GNPs is seen across the choloroauric acid concentrations ranging from 0.2 to 2 mM.
As the TA/HAuCl4 ratios were further increased from 7:1 to 12:1, a common increase in the size of GNPs was observed across the range of chloroauric acid concentrations taken. For the ratios from 7:1 to 10:1, with increase in HAuCl4 concentration, the particle size was observed to decrease unless it reached minima at 0.8 or 1mM concentration and then slowly increased with increasing chloroauric acid concentration. Interestingly, at 12:1 ratio, as the HAuCl4 concentration increased although the size decreased steeply but it did not increase in sharply with further increase in chloroauric acid concentration rather increase slowly till 1.2 mM concentration and then levelled off beyond this (Figure 4).
Of note, for the same TA/HAuCl4 ratio, with different initial HAuCl4 concentrations GNPs of different diameter are produced as observed in the Figures 4 and 5.
For example, for the ratio 7:1, at the concentrations 1.2, 1.5 and 2.0, GNPs with diameter 12, 13 and 16nm are formed respectively. The results discussed above are more or less similar to the findings obtained for effect of higher gold concentrations on the synthesis of citrate mediated GNPs, although with slight variations at some instances. This may happen since TA behaves similarly to citrate as a reducing and stabilizing agent in GNP synthesis.

Figure 5. Representative TEM images of GNPs synthesized using initial chloroauric acid concentrations of 0.2, 0.5, 1 and 2 mM at tannic acid to HAuCl4 ratios of 4:1, 6:1, 9:1 and 12:1.
3.2. TEM Analysis
In order to ascertain the size pattern of GNPs obtained by DLS, TEM analysis was also done. GNP samples prepared with initial chloroauric acid concentrations of 0.2, 0.5, 1.0, and 2 mM were chosen. TEM images were captured for each of these concentrations for TA/HAuCl4 ratios of 4:1, 6:1, 9:1 and 10:1. Particle sizes were measured for each of the 16 samples (Figure 5) and a comparative study was performed with the measurements observed by DLS. The sizes measured by TEM were time and again found to be smaller than the hydrodynamic diameter measured by DLS (in solution) which was quite expected. Nevertheless, the pattern of sizes observed by TEM analysis was in well concordance with the sizes obtained by DLS.
As observed in Figure 5, samples with TA/HAuCl4 ratios 4:1, 6:1, 9:1 and 12:1 gave almost similar sizes for 0.2mM concentration with respect to corresponding 2.0 mM concentration HAuCl4 which very well match the hydrodynamic diameters exhibited by DLS. The size obtained for 0.2 mM HAuCl4 at 12:1 ratio was found to largest (36 nm) followed by the size measured for 2 mM concentration at 12:1 ratio (34.4 nm) amongst all the samples tested. The ratio 9:1 at 1mM HAuCl4 concentration exhibited the smallest size (14 nm) followed by the size obtained for 0.5 mM concentration at the same ratio and for 1 mM concentration at the ratio 6:1(17.2 nm). The sizes measured for the ratios 4:1, 6:1 and 12:1 at the concentrations 0.5, 0.2 and 1 mM respectively are observed to be almost same i.e. of 21 nm. The results obtained by TEM are in complete agreement with the sizes measured by DLS.
4. Conclusion
The data of the present study elaborate that HAuCl4 solutions exhibit two different patterns, one at a concentration below 1 mM and other with molarities higher than or equal to 1 mM with respect to TA/HAuCl4. With increasing concentration of HAuCl4, a general decrease in the size of GNPs is observed whereas the sizes obtained for HAuCl4 molarities greater than 1 mM present a different pattern; as the concentration of chloroauric acid increased, a general increase in the size of as-synthesized GNPs is observed. GNPs synthesized from HAuCl4 concentrations below 0.8 mM exhibit a minimum in their size at the ratio 4:1 followed by an increase in size which further levels off beyond the ratio 8:1. However, this trend is not followed by the size of GNPs synthesized above 1 mM concentration wherein except for a minimal saturation from ratios 10:1 to 12:1 for 2 mM concentration, all other gold solutions with molarities less than 2 mM exhibit a general pattern of first decrease in size, reaching a minimum and then increase in diameters as a function of TA/HAuCl4 ratios. So, from the results obtained in this study, it can be concluded that the higher ionic strength as a result of high molarities of HAuCl4 leave a significant impact on the size of gold nanoparticles obtained. Moreover, the findings demonstrated in the present study are very much similar to those reported earlier for gold nanoparticles synthesized using citrate under higher concentrations of gold chloride [22]. Hence, it can be said that TA behaves similar to citrate in controlling the GNP size when higher concentrations of HAuCl4 are used. TA may render a “similar” effect since it behaves as a reducing and stabilizing agent in GNP synthesis as citrate does but the effect might not be “same” owing to the greater aggressiveness of TA as a reductant than citrate.
REFERENCES
- A. Krolikowska, A. Kudelski, A. Michota and J. Bukowska, “SERS Studies on the Structure of Thioglycolic Acid Monolayers on Silver and Gold,” Surface Science, Vol. 532-535, 2003, pp. 227-232. doi:10.1016/S0039-6028(03)00094-3
- G. Peto, G. L. Molnar, Z. Paszti, O. Geszti, A. Beck and L. Guczi, “Electronic Structure of Gold Nanoparticles Deposited on SiOx/Si(100),” Materials Science and Engineering: C, Vol. 19, No. 1-2, 2002, pp. 95-99. doi:10.1016/S0928-4931(01)00449-0
- A. Kumar, S. Mandal, P. R. Selvakannan, R. Parischa, A. B. Mandale and M. Sastry, “Investigation into the Interaction between Surface-Bound Alkylamines and Gold Nanoparticles,” Langmuir, Vol. 19, No. 15, 2003, pp. 6277-6282. doi:10.1021/la034209c
- N. Chandrasekharan and P. V. Kamat, “Improving the Photoelectrochemical Performance of Nanostructured TiO2 Films by Adsorption of Gold Nanoparticles,” Journal of Physical Chemistry B, Vol. 104, No. 46, 2000, pp. 10851- 10857. doi:10.1021/jp0010029
- Y. Hong, R. J. Honda, N. V. Myung and S. L. Walker, “Transport of Iron-Based Nanoparticles: Role of Magnetic Properties,” Environmental Science & Technology, Vol. 43, No. 23, 2009, pp. 8834-8839. doi:10.1021/es9015525
- B. D. Chithrani, A. A. Gnazani and W. C. W Chan, “Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells,” Nano Letters, Vol. 6, No. 4, 2006, pp. 662-668. doi:10.1021/nl052396o
- M. C. Daniel and D. Astruc, “Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology,” Chemical Reviews, Vol. 104, No. 1, 2004, pp. 293-346. doi:10.1021/cr030698+
- A. Fathi-Azarbayjani, L. Qun, Y. W. Chan and S. Y. Chan, “Novel Vitamin and Gold-Loaded Nanofiber Facial Mask for Topical Delivery,” AAPS PharmSciTech, Vol. 11, No. 3, 2010, pp. 1164-1170. doi:10.1208/s12249-010-9475-z
- J. S. Lee, J. Cho, C. Lee, I. Kim, J. Park, Y. M. Kim, H. Shin, J. Lee and F. Caruso, “Layer-by-Layer Assembled Charge-Trap Memory Devices with Adjustable Electronic Properties,” Nature Nanotechnology, Vol. 2, 2007, 790- 795. doi:10.1038/nnano.2007.380
- M.-C. Bowman, T. E. Ballard, C. J. Ackerson, D. L. Feldheim, D. M. Margolis and C. Melander, “Inhibition of HIV Fusion with Multivalent Gold Nanoparticles,” Journal of the American Chemical Society, Vol. 130, No. 22, 2008, pp. 6896-6897. doi:10.1021/ja710321g
- J. Bresee, K. E. Maier, A. E. Boncella, C. Melander and D. L. Feldheim, “Growth Inhibition of Staphylococcus aureus by Mixed Monolayer Gold Nanoparticles,” Small, Vol. 7, No. 14, 2011, pp. 2027-2031. doi:10.1002/smll.201100420
- J. F. Hainfeld, D. N. Slatkin, T. M. Focella and H. M. Smilowitz, “Gold Nanoparticles: A New X-Ray Contrast Agent,” British Journal of Radiology, Vol. 79, No. 939, 2006, pp. 248-253. doi:10.1259/bjr/13169882
- W. E. Ghann, O. Aras, T. Fleiter and M. C. Daniel, “Syntheses and Characterization of Lisinopril-Coated Gold Nanoparticles as Highly Stable Targeted CT Contrast Agents in Cardiovascular Diseases,” Langmuir, Vol. 28, No. 28, 2012, pp. 10398-10408. doi:10.1021/la301694q
- E. Boisselier and D. Astruc, “Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity,” Chemical Society Reviews, Vol. 38, No. 6, 2009, pp. 1759-1782. doi:10.1039/b806051g
- J. Bresee , K. E. Maier, C. Melander and D. L. Feldheim, “Identification of Antibiotics Using Small Molecule Variable Ligand Display on Gold Nanoparticles,” Chemical Communications, Vol. 46, No. 40, 2010, pp. 7516-7518. doi:10.1039/c0cc02663h
- T. Xia, M. Kovochich, J. Brant, M. Hotze, J. Sempf, T. Oberley, C. Sioutas, J. I. Yeh, M. R. Wiesner and A. E. Nel, “Comparison of the Abilities of Ambient and Manufactured Nanoparticles to Induce Cellular Toxicity According to an Oxidative Stress Paradigm,” Nano Letters, Vol. 6, No. 8, 2006, pp. 1794-1807.
- K. L. Dreher, “Health and Environmental Impact of Nanotechnology: Toxicological Assessment of Manufactured Nanoparticles,” Toxicological Sciences, Vol. 77, No. 1, 2004, pp. 3-5. doi:10.1093/toxsci/kfh041
- J. M. Bergen, H. A. von Recum, T. T. Goodman, A. P. Massey and S. Pun, “Gold Nanoparticles as a Versatile Platform for Optimizing Physicochemical Parameters for Targeted Drug Delivery,” Macromolecular Bioscience, Vol. 6, No. 7, 2006, pp. 506-516. doi:10.1002/mabi.200600075
- M. Gaumet, A. Vargas, R. Gurny and F. Delie, “Nanoparticles for Drug Delivery: The Need for Precision in Reporting Particle Size Parameters,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 69, No. 1, 2008, pp. 1-9. doi:10.1016/j.ejpb.2007.08.001
- X. Ji, X. Song, J. Li, Y. Bai, W. Yang and X. J. Peng, “Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate,” Journal of the American Chemical Society, Vol. 129, No. 45, 2007, pp. 13939- 13948. doi:10.1021/ja074447k
- J. Kimling, M. Maier, B. Okenve, V. Kotaidis, H. Ballot and A. J. Plech, “Turkevich Method for Gold Nanoparticle Synthesis Revisited,” Journal of Physical Chemistry B, Vol. 110, No. 32, 2006, pp. 15700-15707. doi:10.1021/jp061667w
- K. Zabetakis, W. E. Ghann, S. Kumar and M. Daniel, “Effect of High Gold Salt Concentrations on the Size and Polydispersity of Gold Nanoparticles Prepared by an Extended Turkevich-Frens Method,” Gold Bulletin, Vol. 45, No. 4, 2012, pp. 203-211. doi:10.1007/s13404-012-0069-2
- W. Ostwald, “An Introduction to Theoretical and Applied Colloid Chemistry,” John Wiley and Sons, New York, 1917, p. 23.
- A. A. Volkert, V. Subramaniam and A. J. Haes, “Implications of Citrate Concentration during the Seeded Growth Synthesis of Gold Nanoparticles,” Chemical Communications, Vol. 47, No. 1, 2011, pp. 478-480. doi:10.1039/c0cc02075c
- W. Patungwasa and J. H. Hadak, “pH Tunable Morphology of the Gold Nanoparticles Produced by Citrate Reduction,” Materials Chemistry and Physics, Vol. 108, No. 1, 2008, pp. 45-54. doi:10.1016/j.matchemphys.2007.09.001
- S. Link and M. A. El-Sayed, “Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles,” Journal of Physical Chemistry B, Vol. 103, No. 21, 1999, pp. 4212-4217. doi:10.1021/jp984796o
- S. K. Sivaraman, S. Kumar and V. Santhanam, “RoomTemperature Synthesis of Gold Nanoparticles-Size-Control by Slow Addition,” Gold Bulletin, Vol. 43, No. 4, 2010, pp. 275-286. doi:10.1007/BF03214997
- S. A. Aromal and D. Philip, “Facile One-Pot Synthesis of Gold Nanoparticles Using Tannic Acid and Its Application in Catalysis,” Physica E: Low Dimensional Systems and Nanostructures, Vol. 44, No. 7-8, 2012, pp. 1692- 1696. doi:10.1016/j.physe.2012.04.022
- V. Germain, J. Li, D. Ingert, Z. Wang and M. P. Pileni, “Stacking Faults in Formation of Silver Nanodisks,” Journal of Physical Chemistry B, Vol. 107, No. 34, 2003, pp. 8717-8720. doi:10.1021/jp0303826
NOTES
*Corresponding author.

