Journal of Diabetes Mellitus
Vol.4 No.2(2014), Article ID:45648,8 pages DOI:10.4236/jdm.2014.42017
Effects of AGE Inhibition with Aminoguanidine in a Diabetic db/db Mouse Wound Model
Margrete Berdal1*, Trond Jenssen1,2
1Metabolic and Renal Research Group, UiT The Arctic University of Norway, Tromsø, Norway
2Department of Organ Transplantation, Oslo University Hospital, Rikshospitalet, Oslo, Norway
Email:*margrete.berdal@uit.no
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 1 April 2014; revised 27 April 2014; accepted 3 May 2014
ABSTRACT
Advanced glycation end products (AGEs) react non-enzymatically with tissue proteins to form irreversible structures involved in atherosclerosis, nephropathy, retinopathy, neuropathy, and wound healing. Studies on AGE-inhibitors have demonstrated possible prevention of diabetes complications. The present open label study was conducted on aminoguanidine (AGu), an inhibitor of AGE-formation, to examine potential effects on wound healing in diabetes type 2-like db/db mice during 5 - 6 weeks. The animals were divided into 4 groups: AGu from the day of wounding (day 0) topically and/or systemically in drinking water (1 g/L; group 1, n = 13); AGu 1 g/L in drinking water from 7 weeks prior to day 0 (group 2, n = 21); AGu 5 g/L in drinking water from 9 - 11 weeks prior to day 0 (group 3, n = 6); placebo controls (group 4, n = 8). Results: Glycated hemoglobin (A1C) was significantly lower in group 3 compared to the other groups (P < 0.05). Percentage change in A1C and body weight from baseline to the end of the experiment were both related to the AGu doses (1 or 5 g/L; A1C-change, P = 0.01; weight-change, P = 0.04, both for linear trend across groups 4, 2, and 3, respectively). Even so, percentage wound closure was not improved in the AGu-treated groups compared to controls (P ≥ 0.8).
Keywords
Diabetes, Wound, Glycation, Inhibition, Animals

1. Introduction
Advanced glycation end products (AGEs) are formed in diabetes by the irreversible glycation of long-lived tissue proteins [1] . Studies have demonstrated that AGE-formation is implicated both in microand macrovascular complications such as atherosclerosis, nephropathy, retinopathy, neuropathy, and wound healing [2] -[7] .
Several studies have been performed on the effects of AGE inhibition since 1986 when a report on the prototype AGE inhibitor—aminoguanidine—was published [1] [8] . This is a scavenger of reactive carbonyl intermediates in the Maillard reaction in which AGEs are formed non-enzymatically [1] . In a phase III clinical trial aminoguanidine (AGu) demonstrated reduced proteinuria and diminished progression of retinopathy [9] .
The AGE inhibitors thiamine and pyridoxine both improved endothelial cell migration in bovine and human cell cultures [10] [11] . Furthermore, benfotiamine, a diacylglycerol-protein kinase C (PKC)- and AGE inhibitor, has been shown to protect against necrosis in ischemic limbs in type 1 diabetic mice [12] .
Interference with the receptor for AGE (RAGE) by using soluble RAGE (sRAGE) or a neutralizing RAGE antibody is another principle of intervening on the action of AGEs [7] [13] . In an experiment with sRAGE wound healing was improved in type 2-like diabetic (db/db) mice [7] . Furthermore, AGE inhibition with aminoguanidine seems to improve wound healing in type 1 diabetic rats [14] [15] .
The effect of AGE inhibition on wound healing has never been assessed in the diabetic db/db mouse model that is the most widely used animal model for type 2 diabetes [16] [17] . The present study was therefore conducted to investigate the potential effects of aminoguanidine on wound healing in this strain of mice.
2. Materials and Methods
2.1. Animals
Diabetic C57Bl/KsBom-db/db mice were studied. All animals were purchased from M & B A/S, Ry, Denmark. The db/db strain is a well-recognized model for type 2 diabetes mellitus in which the diabetic state results from a deficient leptin receptor associated with an autosomal recessive mutation in the db-gene on chromosome four encoding this receptor [18] . The animals become obese, insulin resistant, and hyperinsulinemic. After the age of 2 - 3 months atrophy of pancreatic islets causes severe hyperglycemia [16] .
The animals were housed under the same conditions as previously reported, including rodent food, SDS RM 1 (E), (Special Diets Services, Essex, England) and water ad libitum [19] . Body weight was measured weekly (Mettler PM 2000, Mettler Instrument Corp., Hightstown, NJ, USA). The Norwegian Ethics Committee for Research on Animals approved the experimental protocols.
Forty-eight animals (26 females) were studied. From the age of 4 - 7 weeks 27 mice were given aminoguanidine (AGu) in the drinking water in concentrations of 1 g/L (n = 21) or 5 g/L (n = 6). The concentrations were based on previous studies [20] .
2.2. Anesthesia and Blood Sampling
General anesthesia was introduced after four hours of fasting (but still with water ad libitum). A mixture of fentanyl/fluanisone and midazolam (final concentrations 0.079 mg/mL fentanyl, 2.50 mg/mL fluanisone, and 1.25 mg/mL midazolam; dose: 0.0075 mL/g body weight) was administered subcutaneously. Blood samples were drawn from the large saphenous vein on anesthetized animals, placed in heparinized tubes, stored in ice for approximately one hour until the measurements of fasting plasma glucose (fPG), plasma lactate (p-lactate), and glycated hemoglobin (A1C).
2.3. Wounding
We used the same excisional model as previously reported, which is a modification of the procedure described by Greenhalgh et al. [19] [21] . In brief, the procedure was performed on anesthetized animals, aged 11 - 18 weeks, having the mid-part of their back shaved, chemically depilated using NairÒ cream (Carter-Wallace Ltd., Folkestone, Kent, England), and washed with tap water. A template was used to mark a 1.5 × 1.5 cm2 area on the skin.The depilated area was disinfected with chlorhexidine 5 mg/mL prepared at the hospital pharmacy and washed with sterile water. A full-thickness skin wound was made on the back of the mice by excising the skin and panniculus carnosus under optimal clean conditions. The wound was thereafter covered with a semi-permeable, transparent polyurethane dressing, OpsiteFlexigridÒ (Smith & Nephew Medical Ltd., Hull, England), that was fixed with the tissue adhesive, enbucrilate (HistoacrylÒ, B. Braun Melsungen AG, Melsungen, Germany), and 5-0 MonosofÔ sutures (Auto Suture Company, Norwalk, CT, USA). The wound margins were finally traced onto glass microscope slides (=area day 0), and buprenorphine was given subcutaneously as analgesia (final concentration 0.030 mg/mL buprenorphine; dose: 0.007 mL/g body wt). Another dose of buprenorphine was given twelve hours after wounding. Furthermore, an isotonic electrolyte solution, Ringer AcetateÒ (Fresenius Kabi Norge AS, Halden, Norway), was given subcutaneously zero and two hours after the surgical procedure. The following four groups were studied, and the observation period was up to 45 days:
1) N = 13 mice were given topical AGu [final concentration 50 mg/mL in NaCl 9 mg/mL (Fresenius Kabi— Norge AS, Halden, Norway)] at the day of wounding (day 0) with (n = 8) or without (n = 5) additional AGu in the drinking water (final concentration 1 g/L) from day 0.
2) In order to examine long-term AGu-effects n = 21 mice received prewounding intervention with AGu in the drinking water (final concentration 1 g/L) from the age of 4 - 6 weeks.
3) Long-termand high-dose effects were studied in n = 6 mice that had prewounding intervention with AGu in the drinking water (final concentration 5 g/L) from the age of 5 - 7 weeks.
4) N = 8 placebo control animals were given tap water and topically applied 100 ml of NaCl 9 mg/mL onto the wound once daily for five consecutive days from surgery.
No animals in group 1 had prewounding AGu treatment. Since the two subgroups with or without AGu in the drinking water were similar with respect to all variables measured (for all comparisons, P > 0.1; repeated measurements analysis of variance for percentage wound closure and t-tests for the remaining variables), they were pooled and analyzed together.
Ten out of sixty-five mice (15.4%) died after wounding from unknown reasons and were therefore not included in the analysis. The difference between groups 1 - 4 in terms of post-surgery deaths was not significant, group 1: 5 in 19 (26%); group 2: 2 in 29 (7%); group 3: 3 in 9 (33%); group 4: 0 in 8 (0%); P = 1.0, Kruskal-Wallis test. Another 7 mice (13%) were excluded because of wound infection.
The conditions of the animals during the experiments and the procedures performed at the end of the study period were as previously reported [19] .
The choice of topically applied AGu at a final concentration of 50 mg/mL (406 mM) was significantly higher compared to what was used in a previous study [22] . However, since we only gave one dose (10 mg) this was considered appropriate.
Our previous studies on wound healing gave identical placebo results with or without topical applications of NaCl 9 mg/mL [23] . We therefore chose not to give additional topical placebo wound treatment in the groups with systemic AGu supply (groups 1 - 3).
All experiments were performed within a time frame of 24 months, and results from group 4 have previously been presented [24] .
2.4. Preparation and Application of Aminoguanidine
Topical treatment with AGu was performed by injecting the solution of AGu hemisulfate (Sigma-Aldrich, St. Louis, MO, USA) in NaCl 9 mg/mL onto the wound in the same manner as previously reported on aminated β-1,3-D-glucan [19] .
2.5. Wound Closure Measurement
The measurements were performed at 7 different time points over 17 days as previously reported [19] . Percentage wound closure for day X was calculated using the following formula, where day 0 is the day of surgery:
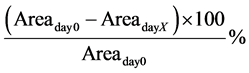
The measurements were performed by computerized planimetry using Adobe Acrobat 7.0 Professional (Adobe Systems Inc., San José, CA, USA) as previously described [24] .
2.6. Metabolic Parameters
Fasting plasma glucose and lactate measurements were performed with the YSI Glucose and L-Lactate Analyzer Model 2300-GL STAT (Yellow Springs Instrument Co., Yellow Springs, OH, USA). A1C was analysed using the DCA 2000Ò + Analyzer Model 5031 C (Bayer Corporation, Elkhart, IN, USA). The analyses were performed according to the manufacturers’ guidelines.
The points in time for the measurements of fPG and baseline body weight (wtday7), day 0 before wounding and the postoperative day 7, respectively, were both chosen to avoid effects of potential stress reactions associated with the wounding.
2.7. Bacteriological Examination and Fungus Cultivation
Samples were harvested from wound bed abradant on anesthetized animals at the end of the experimental period. Animals with signs of a wound infection (green-yellowish secretion and decreased closure rate) and/or growth of wound pathogens (e.g., Staphylococcus aureus) were excluded from the study.
Among 55 mice 7 (13%) were excluded based on signs of wound infection. The occurrence of infections in groups 1 - 4 [1 in 14 (7%), 6 in 27 (22%), 0 in 6 (0%), and 0 in 8 (0%), respectively] was not significantly different between the groups (P = 1.0, Kruskal-Wallis test). Specimens for bacterial growth were taken from 4 of these wounds, and abundant growth of bacteria was detected (samples 1 - 4): 1. Staphylococcus aureus and Enterococci, 2. and 3. Staphylococcus aureus, and 4. Escherichia coli.
2.8. Statistical Analysis
The distributions of all variables were evaluated by visual inspection of frequency histograms. All data were normally distributed and are presented as mean ± standard error of the mean (SE). Statistical significance between groups was tested by t-tests and one-way analysis of variance (oneway ANOVA). Univariate ANOVAs were applied for the testing of potential linear trends across groups. The data on percentage wound closure between days 0 and day 17 were analyzed by repeated measurements ANOVA, and Bonferroni correction was applied for multiple comparisons when appropriate.
Analysis was performed by the statistical package IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA). P < 0.05 (two-tailed) was considered as statistically significant.
3. Results
Wound Healing and Characteristics of the Experimental Animals
Plasma glucose levels in the four intervention groups were similar at wounding (day 0; P > 0.2 for all comparisons, Table 1). Aminoguanidine treatment from day 0 (group 1), or from 7 weeks before day 0 (group 2) did not improve wound healing compared to placebo (group 4), P = 1.0 for both comparisons (Figure 1). Wound closure in the long-term and higher-(AGu) dose group (group 3) tended to be less versus placebo, however mean difference was not significant (−9.1% ± 5.9%, P = 0.8, Figure 1).
At the time of wounding (day 0) all animals were obese and polyuric, both characteristics consistent with diabetes. Furthermore, the characteristics of the four intervention groups were similar at baseline, except for group 3 that was significantly older (Table 1).
At follow-up groups 1, 2, and 4 significantly lost body weight (Table 1, all P £ 0.008, paired-samples t-tests) while group 3 did not (P = 0.7). Moreover, fasting plasma glucose did not change in any group during experiments (P > 0.7), whereas A1C increased slightly in the control group (group 4) (Table 1, P = 0.03). A1C at follow-up was significantly lower in group 3 compared to the three other groups (Table 1).
The percentage changes in body weight and A1C from baseline to the end of the experiment were both AGu dose-related (Figure 2). The corresponding change in fPG however, was not (data not shown).
4. Discussion
The aim of our experiments was to study the potential effects of systemic and/or topical administration of aminoguanidine in a wound model in diabetic db/db mice. AGu did not significantly improve wound healing, regardless of different systemic doses (1 g/L, 5 g/L) and pretreatment up to 11 weeks before wounding (groups 2 and 3).
To our knowledge, there are no other studies in the db/dbor other diabetic mouse models on wound healing associated with the use of AGu. However, in type 1 diabetic rats AGu demonstrated beneficial effects in wound healing and skin flap survival [14] [15] . AGu-treatment in nondiabetic mouse models has been associated with
Table 1. Characteristics of the experimental animals.
Data are mean ± SE. Baseline observations were performed at day 0 (the day of wounding) for all variables except from body weight that was measured at day 7 to avoid effects of potential stress reactions associated with the wounding. Group 3 was significantly older than the three other groups (P < 0.0005). The remaining baseline characteristics were similar across the intervention groups, for all comparisons P > 0.1 (one way analysis of variance (ANOVA), Bonferroni correction for multiple comparisons). In follow-up A1C was significantly lower in group 3 (P = 0.040 versus placebo (group 4), and P = 0.048 versus group 2; repeated measurements ANOVA). †n = 13 for the A1C-measurements, and blood sampling was unsuccessful in follow-up in one animal. AGu, aminoguanidine; fPG, fasting plasma glucose; A1C, glycated hemoglobin; - analysis not performed.
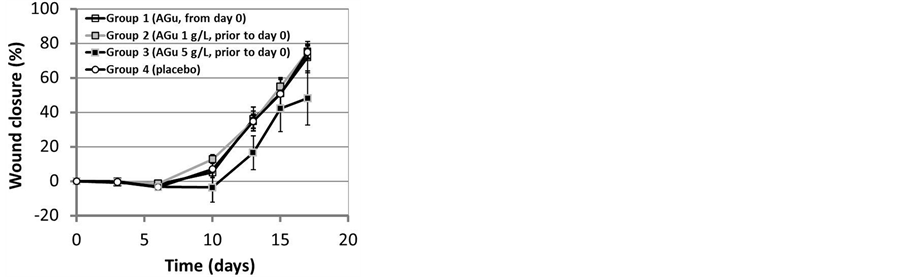
Figure 1. Wound closure in groups 1 (n = 13) and 2 (n = 21) was not significantly different from placebo controls (n = 8; both P = 1.0, repeated measurements analysis of variance). In group 3 (n = 6) wounds tended to close less versus placebo, however not significant (P = 0.8). Data are mean ± SE.
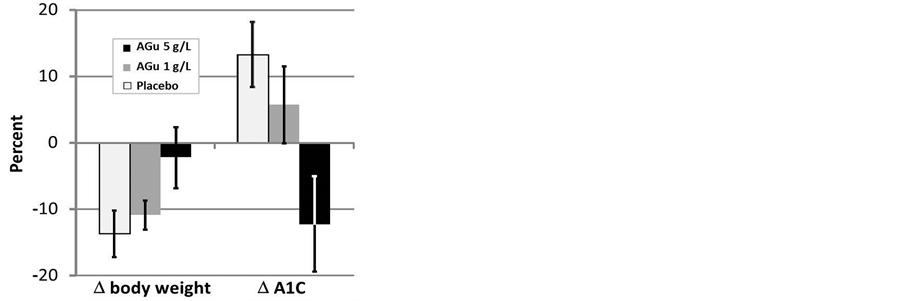
Figure 2. Dose-related percentage change (D) of body weight and glycated hemoglobin (A1C), both from baseline to the end of experiment, after treatment with aminoguanidine (AGu) 1 g/L or 5 g/L for 12 - 17 weeks. Significant linear trends were detected across groups (placebo, n = 8; AGu 1 g/L*, n = 21; AGu 5 g/L, n = 6, respectively) for dose-related percentage change (D body weight, P = 0.04; D A1C, P = 0.01, univariate analysis of variance). *n = 13 for the A1C-measurements. Data are mean ± SE.
effects that are not in accordance with each other. One study on intra-peritoneal AGu administration in young (8 - 9 weeks) animals showed decreased collagen accumulation, and another on topical AGu application in young (10 - 12 weeks) and old (>52 weeks) mice reported increased wound closure [20] [22] . In the present study AGu demonstrated putative metabolic effects since A1C was lower in the high dosage group (group 3) compared to controls (group 4). Even so, wound healing was not improved.
Aminoguanidine is an AGE inhibitor, but also an inhibitor of inducible nitric oxide synthase (iNOS) that is implicated in the NO synthesis from arginine by inflammatory cells and fibroblasts [8] [20] . One could therefore speculate that lack of NO was implicated in our findings since nitric oxide deficiency at the wound site was reported in diabetes, and the intake of a nitric oxide donor in diabetic rats enhanced wound healing [25] .
Our findings are apparently contradictory to some previous studies on AGu in rodent wound models [14] [15] [22] . First, the effects of AGu on wound healing may be different in diabetic and nondiabetic models, and may also rely on strainand/or species differences [14] [15] [20] [22] . Second, the plasma half-life of aminoguanidine is short (~1 hour), and high AGu concentrations are required to continuously react with and trap AGEs [1] . However, a typical high dose is 1 g/L in drinking water as used in the present study (equivalent to ~1 g/kg/d), and we included a five times higher dose as well [1] . In type 1 diabetic rats 1 g/L of AGu in the drinking water improved wound repair, and skin flap necrosis was prevented using intraperitoneal injections of AGu 100 mg/kg/day [14] [15] . In nondiabetic mice the administration of AGu 1 g/L in the drinking water had no effect on the concentrations of the stable end products of NO, nitrite and nitrate, or collagen accumulation in wounds, while AGu 500 mg/kg/day given continuously through intra-peritoneal osmotic pumps decreased the wound fluid nitrite/nitrate concentrations and the accumulation of collagen [20] . Third, the duration of treatment in our model (12 - 17 weeks) may have been inappropriately short to achieve an effect on wound healing. However, beneficial effects and improved wound healing have been achieved after 6-7 days of treatment in different type 1 diabetic rat models, and collagen accumulation decreased in non-diabetic mice after 10 days of intraperitoneal AGu administration [14] [15] [20] .
A1C is a reversible glycated protein that has shown a significant correlation with hemoglobin-AGE in humans [26] . In our study the largest dose of AGu (group 3) was paralleled by a significantly lower A1C level compared to controls, and the percentage change in A1C from baseline to the end of our experiments was related to the AGu doses (1 g/L or 5 g/L). Accordingly, weight change from baseline to the end of the experiments in these groups was AGu dose-related. These findings are consistent with an antidiabetic effect in the db/db mouse model [27] . Since there is no published evidence that AGu traps AGE precursors in vivo, our results can potentially be explained by less advanced glycation of the islets of Langerhans and less islet atrophy [1] [26] [28] .
Strengths of the present study include first, examination of different AGu doses (1 g/L, 5 g/L) and their effects. Second, the experiments were conducted in a well-recognized animal model of type 2-diabetes and wound healing [7] [21] [24] . Third, metabolic effects (body weight, A1C) associated with the relatively long lasting intervention were detected and accounted for.
Limitations are, first, that the intake of food and water was not measured, and the urine output was not monitored. Second, A1C was not measured in all animals. Third, data on serumand pancreatic insulin are lacking. Fourth, the study lacks measurements in wound tissue, wound fluid, and skin of collagen accumulation (hydroxyproline content), AGE deposition, or nitrite/nitrate concentrations.
5. Conclusion
In conclusion, aminoguanidine did not improve wound healing in the diabetic db/db mouse model. Moreover, since aminoguanidine is both an AGEand an iNOS inhibitor [8] [20] , future experiments could be considered in order to address counteractive mechanisms opposing the beneficial effect of AGE inhibition.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
We appreciate the technical assistance by Hege I. Appelbom, Jorunn H. Eikrem, and Åse Lund, Metabolic and Renal Research Group, UiT The Arctic University of Norway. We would like to thank the technicians at the Department of Comparative Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway for their animal care. This work is supported by a grant from the Norwegian Diabetes Association and The Research Council of Norway.
References
- Nagai, R., Murray, D.B., Metz, T.O. and Baynes, J.W. (2012) Chelation: A Fundamental Mechanism of Action ofAGE Inhibitors, AGE Breakers, and Other Inhibitors of Diabetes Complications. Diabetes, 61, 549-559. http://dx.doi.org/10.2337/db11-1120
- Aso, Y., Inukai, T., Tayama, K. and Takemura, Y. (2000) Serum Concentrations of Advanced Glycation Endproducts Are Associated with the Development of Atherosclerosis as Well as Diabetic Microangiopathy in Patients with Type 2 Diabetes. Acta Diabetologica, 37, 87-92. http://dx.doi.org/10.1007/s005920070025
- Makita, Z., Radoff, S., Rayfield, E.J., Yang, Z., Skolnik, E., Delaney, V., Friedman, E.A., Cerami, A. and Vlassara, H. (1991) Advanced Glycosylation End Products in Patients with Diabetic Nephropathy. The New England Journal of Medicine, 325, 836-842. http://dx.doi.org/10.1056/NEJM199109193251202
- Sebag, J., Buckingham, B., Charles, M.A. and Reiser, K. (1992) Biochemical Abnormalities in Vitreous of Humans with Proliferative Diabetic Retinopathy. Archives of Ophthalmology, 110, 1472-1476. http://dx.doi.org/10.1001/archopht.1992.01080220134035
- Sugimoto, K., Nishizawa, Y., Horiuchi, S. and Yagihashi, S. (1997) Localization in Human Diabetic Peripheral Nerve of N(epsilon)-Carboxymethyllysine-Protein Adducts, an Advanced Glycation Endproduct. Diabetologia, 40, 1380- 1387. http://dx.doi.org/10.1007/s001250050839
- Loughlin, D.T. and Artlett, C.M. (2009) 3-Deoxyglucosone-Collagen Alters Human Dermal Fibroblast Migration and Adhesion: Implications for Impaired Wound Healing in Patients with Diabetes. Wound Repair and Regeneration, 17, 739-749. http://dx.doi.org/10.1111/j.1524-475X.2009.00532.x
- Goova, M.T., Li, J., Kislinger, T., Qu, W., Lu, Y., Bucciarelli, L.G., Nowygrod, S., Wolf, B.M., Caliste, X., Yan, S.F., Stern, D.M. and Schmidt, A.M. (2001) Blockade of Receptor for Advanced Glycation End-Products Restores Effective Wound Healing in Diabetic Mice. The American Journal of Pathology, 159, 513-525. http://dx.doi.org/10.1016/S0002-9440(10)61723-3
- Brownlee, M., Vlassara, H., Kooney, A., Ulrich, P. and Cerami, A. (1986) Aminoguanidine Prevents Diabetes-Induced Arterial Wall Protein Cross-Linking. Science, 232, 1629-1632. http://dx.doi.org/10.1126/science.3487117
- Bolton, W.K., Cattran, D. C., Williams, M.E., Adler, S.G., Appel, G.B., Cartwright, K., Foiles, P.G., Freedman, B.I., Raskin, P., Ratner, R.E., Spinowitz, B.S., Whittier, F.C. and Wuerth, J.P. (2004) Randomized Trial of an Inhibitor of Formation of Advanced Glycation End Products in Diabetic Nephropathy. American Journal of Nephrology, 24, 32-40. http://dx.doi.org/10.1159/000075627
- Ascher, E., Gade, P.V., Hingorani, A., Puthukkeril, S., Kallakuri, S., Scheinman, M. and Jacob, T. (2001) Thiamine Reverses Hyperglycemia-Induced Dysfunction in Cultured Endothelial Cells. Surgery, 130, 851-858. http://dx.doi.org/10.1067/msy.2001.117194
- Kelso, B.G., Brower, J.B., Targovnik, J.H. and Caplan, M.R. (2011) Pyridoxine Restores Endothelial Cell Function in High Glucose. Metabolic Syndrome and Related Disorders, 9, 63-68. http://dx.doi.org/10.1089/met.2010.0085
- Gadau, S., Emanueli, C., Van Linthout S., Graiani, G., Todaro, M., Meloni, M., Campesi, I., Invernici, G., Spillmann, F., Ward, K. and Madeddu, P. (2006) Benfotiamine Accelerates the Healing of Ischaemic Diabetic Limbs in Mice through Protein Kinase B/Akt-Mediated Potentiation of Angiogenesis and Inhibition of Apoptosis. Diabetologia, 49, 405-420. http://dx.doi.org/10.1007/s00125-005-0103-5
- Flyvbjerg, A., Denner, L., Schrijvers, B.F., Tilton, R.G., Mogensen, T.H., Paludan, S.R. and Rasch, R. (2004) Long- Term Renal Effects of a Neutralizing RAGE Antibody in Obese Type 2 Diabetic Mice. Diabetes, 53, 166-172. http://dx.doi.org/10.2337/diabetes.53.1.166
- Teixeira, A.S., Caliari, M.V., Rocha, O.A., Machado, R.D. and Andrade, S.P. (1999) Aminoguanidine Prevents Impaired Healing and Deficient Angiogenesis in Diabetic Rats. Inflammation, 23, 569-581. http://dx.doi.org/10.1023/A:1020246624605
- Ozturk, A., Firat, C., Parlakpinar, H., Bay-Karabulut, A., Kirimlioglu, H. and Gurlek, A. (2012) Beneficial Effects of Aminoguanidine on Skin Flap Survival in Diabetic Rats. Experimental Diabetes Research, 2012, Article ID: 721256. http://dx.doi.org/10.1155/2012/721256
- Coleman, D.L. and Hummel, K.P. (1967) Studies with the Mutation, Diabetes, in the Mouse. Diabetologia, 3, 238-248. http://dx.doi.org/10.1007/BF01222201
- Tesch, G.H. and Lim, A.K. (2011) Recent Insights into Diabetic Renal Injury from the db/db Mouse Model of Type 2 Diabetic Nephropathy. American Journal of Physiology—Renal Physiology, 300, F301-F310. http://dx.doi.org/10.1152/ajprenal.00607.2010
- Lee, G.H., Proenca, R., Montez, J.M., Carroll, K.M., Darvishzadeh, J.G., Lee, J.I. and Friedman, J.M. (1996) Abnormal Splicing of the Leptin Receptor in Diabetic Mice. Nature, 379, 632-635. http://dx.doi.org/10.1038/379632a0
- Berdal, M., Appelbom, H.I., Eikrem, J. H., Lund, A., Zykova, S., Busund, L.T., Seljelid, R. and Jenssen, T. (2007) Aminated b-1,3-D-Glucan Improves Wound Healing in Diabetic db/db Mice. Wound Repair and Regeneration, 15, 825-832. http://dx.doi.org/10.1111/j.1524-475X.2007.00286.x
- Schaffer, M.R., Tantry, U., Thornton, F.J. and Barbul, A. (1999) Inhibition of Nitric Oxide Synthesis in Wounds: Pharmacology and Effect on Accumulation of Collagen in Wounds in Mice. European Journal of Surgery, 165, 262-267. http://onlinelibrary.wiley.com/doi/10.1080/110241599750007153/abstract
- Greenhalgh, D.G., Sprugel, K.H., Murray, M.J. and Ross, R. (1990) PDGF and FGF Stimulate Wound Healing in the Genetically Diabetic Mouse. American Journal of Pathology, 136, 1235-1246. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1877595/?page=1
- Fleming, T.H., Theilen, T.M., Masania, J., Wunderle, M., Karimi, J., Vittas, S., Bernauer, R., Bierhaus, A., Rabbani, N., Thornalley, P.J., Kroll, J., Tyedmers, J., Nawrotzki, R., Herzig, S., Brownlee, M. and Nawroth, P.P. (2013) Aging- Dependent Reduction in Glyoxalase 1 Delays Wound Healing. Gerontology, 59, 427-437. http://dx.doi.org/10.1159/000351628
- Berdal, M., Appelbom, H.I., Eikrem, J.H., Lund, A., Busund, L. T., Hanes, R., Seljelid, R. and Jenssen, T. (2011) Aminated β-1,3-D-Glucan Has a Dose-Dependent Effect on Wound Healing in Diabetic db/db Mice. Wound Repair and Regeneration, 19, 579-587. http://dx.doi.org/10.1111/j.1524-475X.2011.00715.x
- Berdal, M. and Jenssen, T. (2013) No Association between Glycemia and Wound Healing in an Experimental db/db Mouse Model. ISRN Endocrinology, 2013, 1-6. http://dx.doi.org/10.1155/2013/307925
- Witte, M.B., Kiyama, T. and Barbul, A. (2002) Nitric Oxide Enhances Experimental Wound Healing in Diabetes. British Journal of Surgery, 89, 1594-1601. http://dx.doi.org/10.1046/j.1365-2168.2002.02263.x
- Makita, Z., Vlassara, H., Rayfield, E., Cartwright, K., Friedman, E., Rodby, R., Cerami, A. and Bucala, R. (1992) Hemoglobin-AGE: A Circulating Marker of Advanced Glycosylation. Science, 258, 651-653. http://dx.doi.org/10.1126/science.1411574
- Arakawa, K., Ishihara, T., Oku, A., Nawano, M., Ueta, K., Kitamura, K., Matsumoto, M. and Saito, A. (2001) Improved Diabetic Syndrome in C57BL/KsJ-db/db Mice by Oral Administration of the Na(+)-Glucose Cotransporter Inhibitor T-1095. British Journal of Pharmacology, 132, 578-586. http://dx.doi.org/10.1038/sj.bjp.0703829
- Piercy, V., Toseland, C.D. and Turner, N.C. (1998) Potential Benefit of Inhibitors of Advanced Glycation End Products in the Progression of Type II Diabetes: A Study with Aminoguanidine in C57/BLKsJ Diabetic Mice. Metabolism, 47, 1477-1480. http://www.metabolismjournal.com/article/S0026-0495(98)90073-7/abstract
Abbreviations
AGE: advanced glycation end products AGu: aminoguanidine A1C: glycated hemoglobin A1c
NOTES

*Corresponding author.


