Journal of Sensor Technology
Vol. 2 No. 2 (2012) , Article ID: 19681 , 7 pages DOI:10.4236/jst.2012.22011
Fabrication of Sm-Based Perovskite-Type Oxide Thin-Films and Gas Sensing Properties to Acetylene
Department of Applied Chemistry, Kyushu Institute of Technology, Tobata, Japan
Email: *shims@tobata.isc.kyutech.ac.jp
Received December 7, 2011; revised January 2, 2012; accepted January 10, 2012
Keywords: Perovskite-Type Oxide; Thin-Film; Ac Impedance; Acetylene; Gas Sensor
ABSTRACT
Sm-based perovskite-type oxide (SmMeO3: Me = Cr, Mn, Fe, Co) thin-films could be synthesized by a wet-chemical method using an acetylacetone—Poly(Vinyl Pyrrolidone) (PVP) polymeric precursor method at 750˚C. The perovskitetype oxide thin-films were tried to apply an acetylene gas sensor based on AC impedance spectroscopy. Among the oxides tested, SmFeO3 thin-film sensor showed good sensor responses in which the AC impedance at 20 kHz was depending on acetylene gas concentration between 2 ppm and 80 ppm at 400˚C.
1. Introduction
Lanthanoid-based perovskite-type oxides, such as LnMeO3 (Ln: lanthanoids, Me: transition metals), have been well-known as functional inorganic materials having a wide range of applications for electrode materials of the alkaline fuel cell [1], gas sensor [2-10], ion sensor [11], and for high-performance catalysts for the complete oxidation of hydrocarbons or CO, and NO reduction [12]. Among the lanthanoid-transition metal perovskite-type oxides, Sm-based oxides seem to be interesting materials as they have the largest amount of adsorbed oxygen [13]. For example, the Sm-based perovskite-type oxide sensors have been reported to detect NOx [14], volatile organic compounds [15], ethanol [16] and so on. It is also wellknown that the oxide thin-film devices have good properties as electrochemical devices. So far, oxide thin-film with a perovskite-type structure have been prepared by dry processes such as sputtering and electron-beam deposition methods [17,18], as well as the wet processes of the sol-gel method mainly starting from metal alkoxides or organic acid salts [19,20]. They can field high-quality oxide thin-films; however, they still have some problems, such as relatively low cost performance and lack of handling of the chemicals using the sol-gel method. Consequently, in this work it is focused attention on a wet process to evade such problem, and perovskite-type oxide could be synthesized by a polymer precursor with metal nitrates contained constituent elements [21,22]. By the way, acetylene (C2H2) is widely used as the fuel for cutting and welding metals, so there are also strong needs to detect acetylene as combustible gas. Recently, it has known that small amount of acetylene is to be generated from depleted insulating oils of an oil-immersed transformer. Thus, the acetylene gas sensor could be applicable as a new type of maintenance’s marker of the transformers, especially for the large sized transformers set in remote areas.
The conventional chromatographic method for acetylene detection has high accuracy and is widely used, but it is not suitable for on-site monitoring because of the limited portability as well as the high operating cost. So far, considerable efforts have been directed to develop high performance gas sensors for monitoring acetylene, such as electrochemical sensors [23], and semiconductor type sensors [24,25], however, the sensor for detection acetylene have been seldom reported.
In this study, the Sm-based perovskite-type oxide thinfilm as the material of an acetylene sensor was picked up and systematically evaluated about wet-chemical synthesize of perovskite-type oxide thin-film [26] and the C2H2 sensing properties of the prepared oxide thin-film.
2. Experimental
2.1. Synthesis of Perovskite-Type Oxide Thin-Films
Perovskite-type oxide (SmMeO3: Me = Cr, Mn, Fe, Co) thin-films were synthesized by a polymer precursor method [26] as shown in Figure 1. Metal nitrates were dissolved in Ethylene Glycol (EG) solvent with Polyvinylpyrrolidone (PVP) (3.75 wt%) and acetylacetone (AcAc), as a polymer additive and a coordination agent,
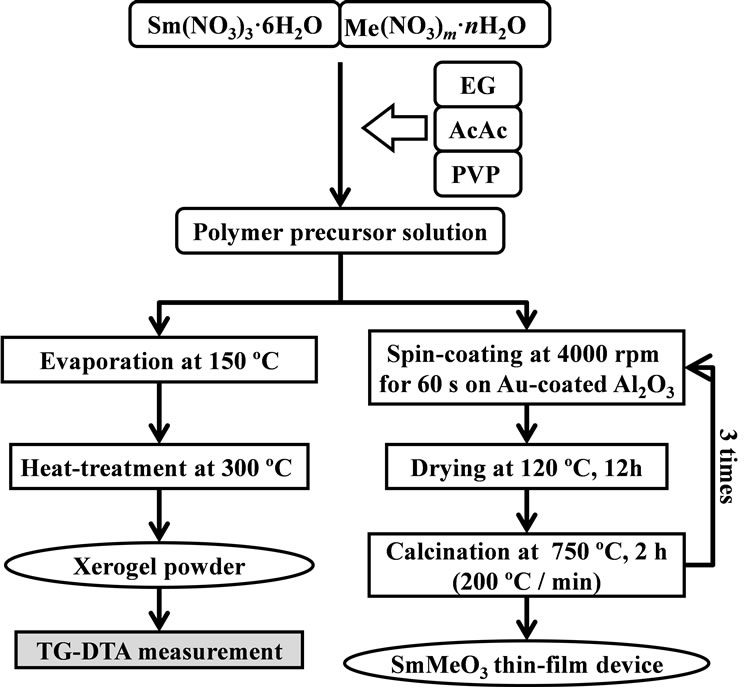
Figure 1. Experimental procedure for preparation of perovskitetype oxide SmMeO3 (Me = Cr, Mn, Fe, Co) thin-films and Sm-Co xerogel powder.
respectively. The solution thus prepared was spin-coated on an alumina substrate with Au interdigitated electrodes at 4000 rpm, and finally sintered at 750˚C in air. The spin-coating and sintering processes were repeated several times to adjust the thickness.
The samples were analyzed by X-ray diffraction using CuKα radiation (XRD: JEOL JDX3500K), field emission type scanning electron microscope (FE-SEM: JEOL JSM- 6500F/III), and thermo gravimetric-differential thermal analysis (TG-DTA: Rigaku 8120H). Electrical conductivities of the thin-films were measured in air (PO2 = 0.21 atm) at the temperature range between 200˚C and 500˚C in the frequency range from 50 Hz to 5 MHz with applied voltage of 0.5 V by AC impedance method (LCR meter: HIOKI 3532-50).
2.2. Fabrication of Sensor Devices
Figure 2 shows schematic diagram of the measurement apparatus. The perovskite-type oxide thin-film sensor device was connected to LCR meter with Au lead wires attached with a silver paste covered with an inorganic adhesive. Gas sensing properties were investigated by AC impedance method using the LCR meter at 400˚C - 500˚C. Sample gases, containing C2H2 were prepared from a parent gas, i.e., 2 - 80 ppm C2H2 diluted with nitrogen, by mixing with nitrogen and/or oxygen, were flowed at a total flow rate of 100 cm3/min. The oxygen partial pressure of the sample gases was fixed at 0.21 atm. Sensitivities of the responses of the sensors were defined as Equation (1);
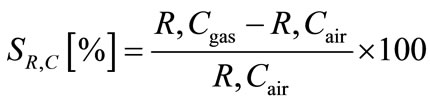 (1)
(1)

Figure 2. Schematic diagrams of the measurement apparatus and the oxide thin-film device.
where Rgas (Cgas) and Rair (Cair) denote Resistance (Capacitance) in gas and Resistance (Capacitance) in air, respectively.
3. Results and Discussion
3.1. Preparation of Perovskite-Type Oxide Thin-Films
To determine the thermal heat-treatment temperature, TG-DTA curves of the precursor powder were investigated. As shown in Figure 3, the both curves indicated a weight loss (ca. 5%) between room temperature and 200˚C, which corresponds to the thermal decomposition of adsorbed moisture and remained solvent EG in the xerogelpowder. And then, small and large exothermic peaks at around 200˚C and 300˚C with both mass decreases were observed. The former seems to correspond to the combustion of AcAc which is not able to connect the network with metal ion, EG and PVP like a polymer and the later corresponds the combustion of the network consisted of them, respectively. The reason why the two mass decreases with exothermic were almost the same might be come from the same amount of the AcAc with or without the polymer connect. An exothermic peak at 650˚C with mass decrease was probably due to the oxidation and desorption of the remained carbon oxide and crystallization. As stabilization of TG-DTA curves after 700˚C was observed, the sintering temperature was fixed at 750˚C.
Figure 4 shows XRD patterns of the SmMeO3 (Me = Cr, Mn, Fe, Co) thin-films prepared at 750˚C by the polymer precursor method on an Al2O3 substrate with Au electrode. XRD patterns of the thin-films consisted of perovskite-type oxide phase, Al2O3 and Au peaks from REVISED

Figure 3. TG-DTA curves of the Sm-Co xerogel powder in air until 1000˚C (10˚C/min).

Figure 4. XRD patterns of the perovskite-type oxide SmMeO3 (Me = Cr, Mn, Fe, Co) and LaFeO3 thin-films. (a) SmCoO3 (25-1071); (b) SmFeO3 (39-1490); (c) SmMnO3 (25- 0747); (d) SmCrO3 (08-0169); (e) LaFeO3 (37-1493).
the substrate.
SEM images of the SmMeO3 (Me = Cr, Mn, Fe, Co) thin-films with 3 times spin-coatings were shown in Figure 5. The surface of the SmFeO3 thin-film prepared at 750˚C was relatively smooth and consisted of homogeneous fine grains of dimension approximately 30 nm, and thickness of the film was ca. 220 nm. The SmMnO3 and SmCoO3 thin-films showed similar characteristics, although the grain size was as large as 50 - 100 nm. The SmCrO3 thin-film however showed more large grain-size and thickness of the film was ca. 440 nm.
3.2. Gas Sensing Properties
For investigate the impedance responses of the sensor devices, the dependence of frequency response on C2H2 concentration in air (PO2 = 0.21 atm) of the sensor devices were firstly measured from 50 Hz to 5 MHz at the temperature range between 400˚C and 500˚C. Figure 6 shows Nyquist’s plots of the SmFeO3 thin-film device in air and 80 ppm C2H2 at 400˚C. Although the few disarray plots at lower frequency were observed, the Nyquist’s plots showed good-looking semicircles. The increase in resistance change with increasing C2H2 concentration was observed especially at around 20 kHz which seems to be come from the grain boundary characteristics as shown by equivalent circuits. Moreover, the frequency responses from boundaries between intraparticle and grain boundary were lower by frequency-shifted with
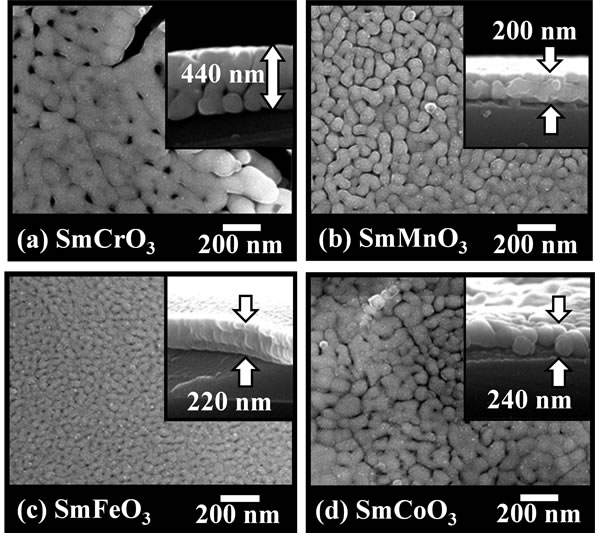
Figure 5. SEM images of the perovskite oxide thin-films on an Al2O3 substrate with 3 times spin-coatings; (a) SmCrO3; (b) SmMnO3; (c) SmFeO3; (d) SmCoO3.
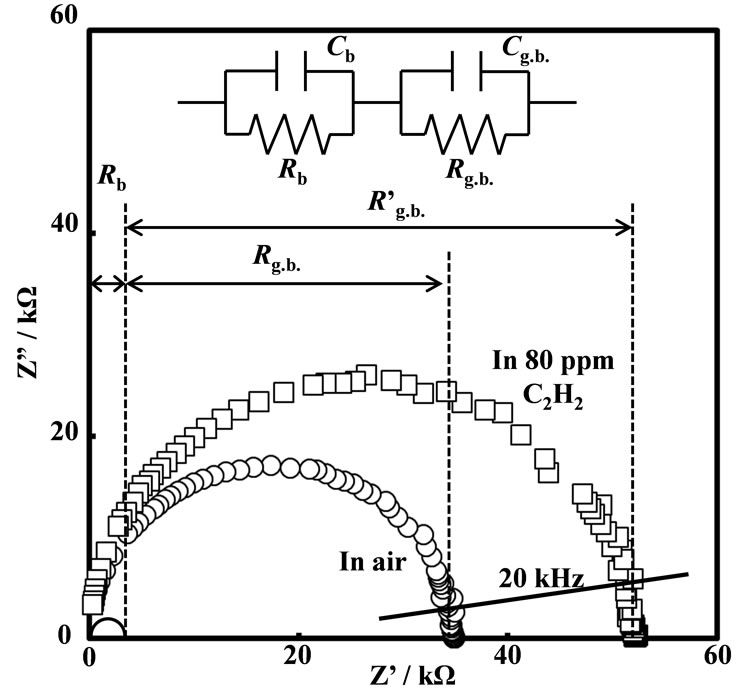
Figure 6. Nyquist’s plots and the equivalent circuits for the SmFeO3 thin-film device in air and 80 ppm at 400˚C.
increasing C2H2 concentration. Response characteristics of the SmFeO3 and SmCoO3 thin-film devices to C2H2 at 20 kHz, 400˚C are shown in Figures 7 and 8, respectively. The resistance and the capacitance components were divided from the complex impedancemetric measurement. The capacitance responses of both devices showed no response. Although the SmCoO3 thin-film device showed no resistance response at all, it was found that SmFeO3 thin-film device showed excellent resistance sensor response with good response and recovery
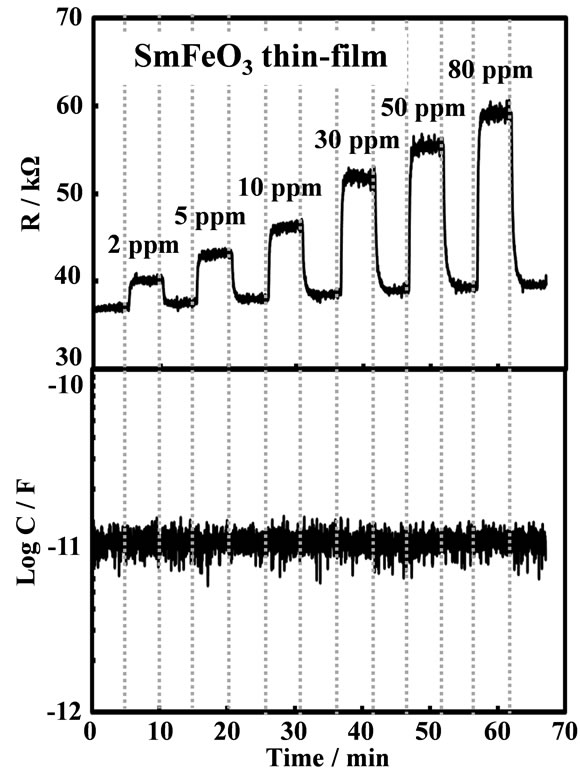
Figure 7. Response transients of SmFeO3 thin-film device to various C2H2 concentrations at 20 kHz, 400˚C.
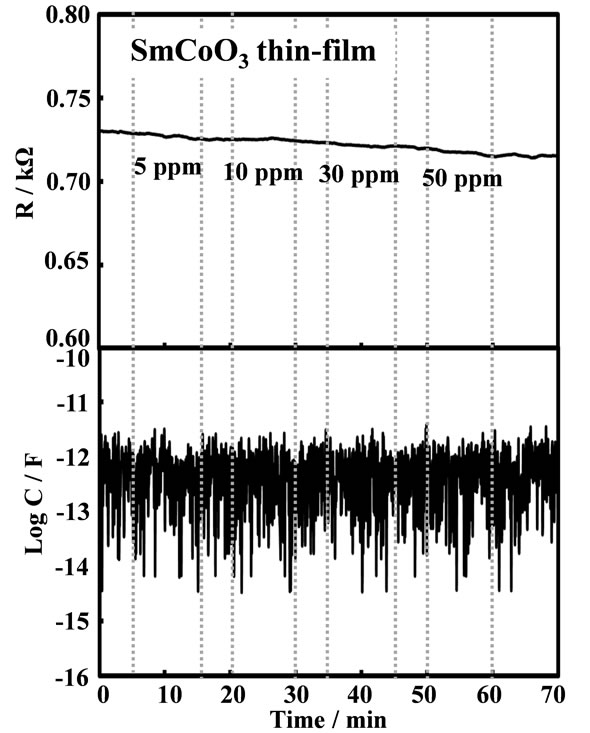
Figure 8. Response transients of SmCoO3 thin-film device to various C2H2 concentrations at 20 kHz, 400˚C.
rates. As the Fe-doped perovskite-type oxide thin-film device showed the resistance response, the surface adsorption and/or reaction of C2H2 on the oxide seems to be unique and important. C2H2 adsorption on oxides should cause the change-transfer in the oxides, which affect the change in resistance as well as capacitance of the oxides. Little capacitance change of the sensor device might be come from the sensor structure, such as the thickness of the oxide and the physical structure of the Au-electrodes. The resistance responses of the SmFeO3 device to various concentration of C2H2 at 400˚C - 500˚C were shown in Figure 9. The responses of SmFeO3 device was decreased with increasing operating temperature from 400˚C to 500˚C. This seems come from the p-type semi-conductive property and the coverage of oxygen ions of the perovskite-type oxides, because the adsorbed oxygen ions are reduced by reduction reaction on the surface with causing the release of electrons in the perovskites [27-29]. Table 1 summaries the sensitivities of the SmMeO3 (Me = Cr, Mn, Fe, Co) thin-film devices to 10 ppm C2H2 at 400˚C - 500˚C. Unlike the response of the SmFeO3 device, the SmCoO3, SmCrO3 and SmMnO3
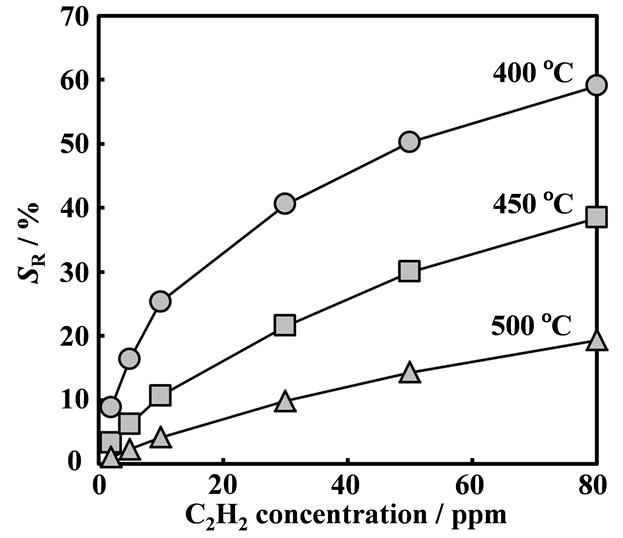
Figure 9. Dependence on C2H2 concentrations of the SmFeO3 devices at the temperature range between 400˚C and 500˚C at 20 kHz.
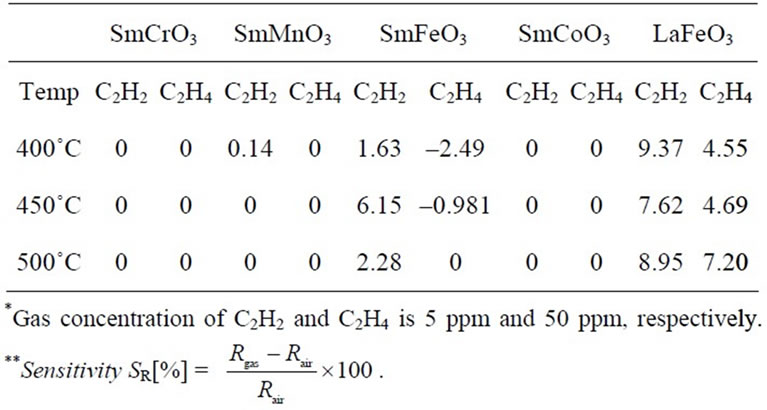
Table 1. Sensitivities of SmMeO3 (Me = Cr, Mn, Fe, Co) and LaFeO3 devices to 5ppm C2H2 and 50 ppm C2H4 at 400˚C - 500˚C at 20 kHz.
devices seldom showed apparent response to low concentration of C2H2 at the operating temperatures. One of the results would be expressed that surface-controlled gas sensor based oxide semiconductor is to be high conductivity by increasing working temperature. In details, the sensitivities were decreased because the change of surface conductivity with chemical reaction on oxide surface is relatively small for high bulk conductivity even if the oxide device is reacted to same C2H2 concentration.
For investigating the electrical properties of the prepared oxide thin-films, the conductivities was determined from Arrhenius equation in air at the temperature range between 200˚C and 500˚C in the frequency range from 50 Hz to 5 MHz with applied voltage of 0.5 V by AC impedance method as shown in Figure 10. The conductivities σ [S∙cm–1] of all thin-films were calculated from their real part impedance (Z') corresponding to the minimum of their imaginary part (Z") as shown in Figure 6.
The prepared thin-films represented an increased conductivity with increasing operating temperature, which has a typical semiconductor behavior. Although the SmCrO3 and SmFeO3 thin-films seldom showed low conductivity below 300˚C, the SmMnO3 and SmCoO3 thin-films showed still high conductivity below 300˚C. And also, large difference of the conductivities between SmFeO3 and SmCoO3 over the operating temperature range. Additionally, the activation energies of the films exhibited smaller than 1 except for the SmFeO3. Consequently, the SmMnO3 and SmCoO3 with a high conductivity and low activation energy would make it cause to react with a detection gas on their quite surface. As the variant condition of film formation and film thickness, the results showed a similar leaning with the La-based

Figure 10. Arrhenius plots and activation energies of SmMeO3 (Me = Cr, Mn, Fe, Co) and LaFeO3 thin-films.
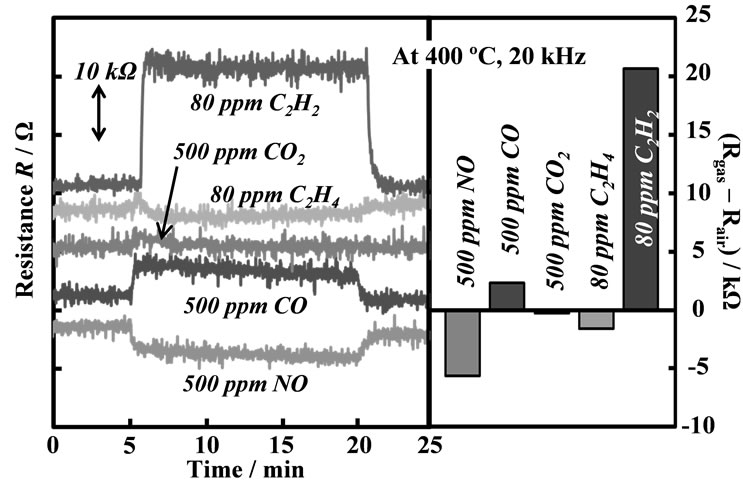
Figure 11. Selectivity of the SmFeO3 thin-film device to various gases, such as 80 ppm C2H2, 80 ppm C2H4, 500 ppm CO2, 500 ppm CO and 500 ppm NO at 400˚C, 20 kHz.
perovskite thin-film reported Ngamou et al. and occurred the charge transfer (Me4+ ↔ Me3+) via the Me-O-Me bonds [30].
Figure 11 shows the selectivity of SmFeO3 thin-film device to interference gases such as 80 ppm C2H4, 500 ppm CO and 500 ppm CO2 at 20 kHz at 400˚C. The SmFeO3-based device showed little response to C2H4, CO and CO2, although this device showed good response to C2H2. This is the face that the obtained behavior arises from various mechanisms. In case of the device, one could express a general surface reaction as shown in Equation (2), where the pre-adsorbed reducing gas R with a surface oxygen species ( ,
, or
or ) and releases electrons to the conduction band of the sensor [27,29].
) and releases electrons to the conduction band of the sensor [27,29].
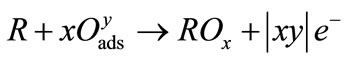 (2)
(2)
4. Conclusion
The perovskite-type oxide SmMeO3 (Me = Cr, Mn, Fe, Co) thin-film sensors could be prepared by a polymer precursor method. The SmFeO3-based thin-film device showed good sensing characteristics to C2H2 between 2 and 80 ppm at 400˚C with 90% response time of ca. 15 sec. Moreover, the sensor device had relatively high sensitivity which showed increasing resistance response to C2H2.
REFERENCES
- D. B. Meadowcroft, “Low-Cost Oxygen Electrode Material,” Nature, Vol. 226, No. 5248, 1970, pp. 847-848. doi:10.1038/226847a0
- M. Yang, L. Huo, H. Zhao, S. Gao and Z. Rong, “Electrical Properties and Acetone-Sensing Characteristics of LaNi1–xTixO3 Perovskite System Prepared by Amorphous Citrate Decomposition,” Sensors and Actuators B: Chemical, Vol. 143, No. 1, 2009, pp. 111-118. doi:10.1016/j.snb.2009.09.003
- L. Zhang, H. Qin, P. Song, J. Hu and M. Jiang, “Electric Properties and Acetone-Sensing Characteristics of La1–xPbxFeO3 Perovskite System,” Materials Chemistry and Physics, Vol. 98, No. 2-3, 2006, pp. 358-362. doi:10.1016/j.matchemphys.2005.09.041
- E. L. Brosha, R. Mukundan, R. Lujan and F. H. Garzon, “Mixed Potential NOx Sensors Using Thin Film Electrodes and Electrolytes for Stationary Reciprocating Engine Type Applications,” Sensors and Actuators B: Chemical, Vol. 119, No. 2, 2006, pp. 398-408. doi:10.1016/j.snb.2005.12.044
- G. N. Chaudhari, S. V. Jagtap, N. N. Gedam, M. J. Pawar and V. S. Sangawar, “Sol-Gel Synthesized Semiconducting LaCo0.8Fe0.2O3-Based Powder for Thick Film NH3 Gas Sensor,” Talanta, Vol. 78, No. 3, 2009, pp. 1136-1140. doi:10.1016/j.talanta.2009.01.030
- K. Sahner, D. Schönauer, R. Moos, M. Matam and M. L. Post, “Effect of Electrodes and Zeolite Cover Layer on Hydrocarbon Sensing with p-Type Perovskite SrTi0.8Fe0.2O3–δ Thick and Thin Films,” Journal of Materials Science, Vol. 41, No. 18, 2006, pp. 5828-5835. doi:10.1007/s10853-006-0299-x
- E. Delgado and C. R. Michel, “CO2 and O2 Sensing Behavior of Nanostructured Barium-Doped SmCoO3,” Materials Letters, Vol. 60, No. 13-14, 2006, pp. 1613-1616. doi:10.1016/j.matlet.2005.11.080
- Y. Shimizu and N. Yamashita, “Solid Electrolyte CO2 Sensor Using NASICON and Perovskite-Type Oxide Electrode,” Sensors Actuators B: Chemical, Vol. 64, No. 1-3, 2000, pp. 102-106. doi:10.1016/S0925-4005(99)00491-8
- Y. L. Chai, D. T. Ray, G. J. Chen and Y. H. Chang, “Synthesis La0.8Sr0.2Co0.5Ni0.5O3–δ Thin Films for High Sensitivity CO Sensing Material Using the Pechini Process,” Journal of Alloys and Compounds, Vol. 333, No. 1-2, 2002, pp. 147-153. doi:10.1016/S0925-8388(01)01688-7
- L. Malavasi, C. Tealdi, A. Montenero, J. M. Tulliani, P. Moggi, M. Guglielmi, G. Flor, A. Lorenzi, A. Martucci, L. Montanaro and G. Chiodelli, “Materials Development for CO-Detection with Improved Selectivity through Catalytic Activation,” Sensors Actuators B: Chemical, Vol. 118, No. 1-2, 2006, pp. 121-128. doi:10.1016/j.snb.2006.04.008
- Y. Shimizu, A. Ishikawa, K. Iseki and S. Takase, “Perovskite-Type Oxide-Based Electrode: A New Sensor for Hydrogen-Phosphate Ion,” Journal of the Eletrochemical Society, Vol. 147, No. 10, 2000, pp. 3931-3934. doi:10.1149/1.1393998
- H. M. Zhang, Y. Shimizu, Y. Teraoka, N. Miura and N. Yamazoe, “Oxygen Sorption and Catalytic Properties of La1–xSrxCo1–yFeyO3 Perovskite-Type Oxides,” Journal of Catalysis, Vol. 121, No. 2, 1990, pp. 432-440. doi:10.1016/0021-9517(90)90251-E
- H. Aono, E. Traversa, M. Sakamoto and Y. Sadaoka, “Crystallographic Characterization and NO2 Gas Sensing Property of LnFeO3 Prepared by Thermal Decomposition of Ln-Fe Hexacyanocomplexes, Ln[Fe(CN)6]∙nH2O, Ln = La, Nd, Sm, Gd, and Dy,” Sensors Actuators B: Chemical, Vol. 94, No. 2, 2003, pp. 132-139. doi:10.1016/S0925-4005(03)00328-9
- M. C. Carotta, G. Martinelli, Y. Sadaoka, P. Nunziante and E. Traversa, “Gas-Sensitive Electrical Properties of Perovskite-Type SmFeO3 Thick Films,” Sensors Actuators B: Chemical, Vol. 48, No. 1-3, 1998, pp. 270-276. doi:10.1016/S0925-4005(98)00011-2
- M. Tomoda, S. Okano, Y. Itagaki, H. Aono and Y. Sadaoka, “Air Quality Prediction by Using Semiconducting Gas Sensor with Newly Fabricated SmFeO3 Film,” Sensors Actuators B: Chemical, Vol. 97, No. 2-3, 2004, pp. 190-197. doi:10.1016/j.snb.2003.08.013
- M. Zhao, H. Peng, S. Fang and J. Hu, “Microstructure, Electrical and Ethanol-Sensing Properties of PerovskiteType SmFe0.7Co0.3O3,” Sensors Actuators B: Chemical, Vol. 130, No. 2, 2008, pp. 609-613. doi:10.1016/j.snb.2007.10.017
- C. M. Chiu and Y. H. Chang, “The Influence of Microstructure and Deposition Methods on CO Gas Sensing Properties of La0.8Sr0.2Co1–xNixO3−δ Perovskite Films,” Sensors Actuators B: Chemical, Vol. 54, No. 3, 1999, pp. 236- 242. doi:10.1016/S0925-4005(99)00117-3
- M. J. Montenegro, T. Lippert, S. Müller, A. Weidenkaff, P. R. Willmott and A. Wokaun, “Pulsed Laser Deposition of Electrochemically Active Perovskite Films,” Applied Surface Science, Vol. 197-198, 2002, pp. 505-511. doi:10.1016/S0169-4332(02)00326-4
- T. Kumagai, H. Yokota, K. Kawaguchi, W. Kondo and S. Mizuta, “Preparation of Superconducting YBa2Cu3O7–δ Thin Films by the Dipping-Pyrolysis Process Using Organic Acid Salts,” Chemistry Letters, Vol. 16, No. 8, 1987, pp. 1645-1646. doi:10.1246/cl.1987.1645
- H. J. Hwang and M. Awano, “Preparation of LaCoO3 Catalytic Thin Film by the Sol-Gel Process and Its NO Decomposition Characteristics,” Journal of the European Ceramic Society, Vol. 21, No. 10-11, 2001, pp. 2103-2107. doi:10.1016/S0955-2219(01)00181-9
- K. Tsuchida, S. Takase and Y. Shimizu, “Sol-Gel Synthesis of Perovskite-Type Oxide Thin-Film with Metal Organic-Acid and Its Application to Amperometric Hydrogen-Phosphate Ion Sensor,” Sensors and Materials, Vol. 16, No. 3, 2004, pp. 171-180.
- Y. Pimtong-Ngam, S. Jiemsirilers and S. Supothina, “Preparation of Tungsten Oxide-Tin Oxide Nanocomposites and Their Ethylene Sensing Characteristics,” Sensors and Actuators A: Physical, Vol. 139, No. 1-2, 2007, pp. 7-11. doi:10.1016/j.sna.2006.10.032
- L. R. Jordan and P. C. Hauser, “Electrochemical Sensor for Acetylene,” Analytical Chemistry, Vol. 69, No. 14, 1997, pp. 2669-2672. doi:10.1021/ac9700585
- Q. Qi, T. Zhang, X. Zheng, H. Fan, L. Liu, R. Wang and Y. Zeng, “Electrical Response of Sm2O3-Doped SnO2 to C2H2 and Effect of Humidity Interference,” Sensors Actuators B: Chemical, Vol. 134, No. 1, 2008, pp. 36-42. doi:10.1016/j.snb.2008.04.011
- S. T. Marshall, D. K. Schwartz and J. W. Medlin, “Selective Acetylene Detection through Surface Modification of Metal-Insulator-Semiconductor Sensors with Alkanethiolate Monolayers,” Sensors Actuators B: Chemical, Vol. 136, No. 2, 2009, pp. 315-319. doi:10.1016/j.snb.2008.11.026
- Y. Shimizu and T. Murata, “Sol-Gel Synthesis of Perovskite-Type Lanthanum Manganite Thin Films and Fine Powders Using Metal Acetylacetonate and Poly(Vinyl Alcohol),” Journal of the American Ceramis Society, Vol. 80, No. 10, 1997, pp. 2702-2704. doi:10.1111/j.1151-2916.1997.tb03178.x
- S. M. Bukhari and J. B. Giorgi, “Cobaot Doped Sm0.95Ce0.05FeO3–δ for Detection of Reducing Gases,” Journal of the Electrochemical Society, Vol. 158, No. 6, 2011, pp. J159-J164. doi:10.1149/1.3570674
- S. M. Bukhari and J. B. Giorgi, “Tuneability of Sm(1–x)CexFeO3±λ Perovskites: Thermal Stability and Electrical Conductivity,” Solid State Ionics, Vol. 180, No. 2-3, 2009, pp. 198-204. doi:10.1016/j.ssi.2008.12.002
- S. Luo, G. Fu, H. Chen, Z. Liu and Q. Hong, “Gas-Sensing Properties and Complex Impedance Analysis of CeAdded WO3 Nanoparticles to VOC Gases,” Solid-State Electronics, Vol. 51, No. 6, 2007, pp. 913-919. doi:10.1016/j.sse.2007.04.010
- P. H. T. Ngamou and N. Bahlawane, “Chemical Vapor Deposition and Electric Characterization of Perovskite Oxides LaMO3 (M = Co, Fe, Cr and Mn) Thin Films,” Journal of Solid State Chemistry, Vol. 182, No. 4, 2009, pp. 849-854. doi:10.1016/j.jssc.2008.12.017
NOTES
*Corresponding author.

