Journal of Biomaterials and Nanobiotechnology
Vol. 4 No. 3A (2013) , Article ID: 33626 , 7 pages DOI:10.4236/jbnb.2013.43A004
Effects of Gabaergic Phenols on Phospholipid Bilayers as Evaluated by 1H-NMR
![]()
1Instituto de Investigaciones Biológicas y Tecnológicas (IIBYT), CONICET—Universidad Nacional de Córdoba, Córdoba, Argentina; 2Departamento de Engenharia Ambiental, Universidade Estadual Paulista Júlio de Mesquita Filho, Sorocaba, Brasil; 3Departamento de Bioquímica, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, Brasil.
Email: *dagarcia@efn.uncor.edu
Copyright © 2013 Gabriela N. Reiner et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 22nd, 2013; revised May 25th, 2013; accepted June 5th, 2013
Keywords: Phenols; Propofol; GABA-A Receptor; Membrane Interaction; 1H-RMN
ABSTRACT
The phenols propofol and thymol, and lately carvacrol, eugenol and chlorothymol, have been shown to act as positive allosteric modulators on GABAA receptor, which is the main inhibitory receptor of the central nervous system. GABAA receptor is an intrinsic membrane protein which activity may be affected by surface-active compounds and by physical changes in the membrane. Recently, we demonstrated that these phenols interacted with the lipid membrane phase, suggesting their anesthetic activity could be the combined result of their specific (with receptor proteins) as well as nonspecific (with surrounding lipid molecules) interaction modulating the supramolecular organization of the receptor environment. In the current study, by using 1H-NMR spectroscopy, we have investigated the effects of the insertion and the possible preferential location of the five phenol derivatives with GABAergic activity on EPC membranes. The results indicate that all compounds are able to insert in EPC phospholipid vesicles and to locate in the region between the polar group (choline molecule), the glycerol and the first atoms of the acyl chains, being the more lipophilic compounds (propofol and chlorothymol) that seem to prefer a deeper bilayer insertion. The location of the phenol molecules would reduce the repulsive forces among phospholipids head groups allowing closer molecular packing and finally diminishing the mobility of the hydrocarbon chains, as revealed by 1H spin relaxation times.
1. Introduction
General anesthetics are substances that induce a reversible state of unconsciousness, characterized by amnesia and analgesia. Their exact action mechanism remains incompletely elucidated. They were originally believed to act via nonspecific interactions with the lipid bilayers, affecting membrane fluidity. More recently, general anesthetics have been shown to act by modulating ligandgated ion channels such as the GABAA receptor (GABAR) (see references in [1]).
The GABA-R, a ligand-gated ion channel, constitutes the main inhibitory receptor of the central nervous system. GABA-Rs, besides being activated by the GABA neurotransmitter, are modulated by numerous therapeutically important drugs, including barbiturates, anesthetics, benzodiazepines, neurosteroids and ethanol. These compounds are GABA-Rs allosteric modulators as they bind to distinct sites to potentiate GABA-evoked currents [1-5]. The phenols propofol and thymol, and lately carvacrol, eugenol and chlorothymol, have been shown to act as positive allosteric modulators on this receptor [6-8].
GABA-R is an intrinsic membrane protein which activity may be affected by surface-active compounds and by physical changes in the membrane [4,9-12]. Taking into account the lipophilicity of the above described phenols, their interaction with the lipid membrane phase, especially the lipids surrounding the receptor and a consequent non-specific receptor modulation cannot be discarded, justifying a detailed study of drug-membrane interaction.
Recently we determined several lipophilic parameters for these five gabaergic phenols. The results obtained, based on the octanol-water partition coefficient (logP o/w), retention data in high performance liquid chromatography (HPLC) using C18 and immobilized artificial membrane (IAM) columns at different temperatures, and partition coefficients determined with phospholipid liposomes, demonstrated the high capacity of all the compounds to interact with membrane phases [13]. In addition, by using Langmuir films and epifluorescence images, we described that all the compounds were able to diffuse into the membrane, placing themselves between phospholipid molecules probably at the head-group region [14]. Finally, by means of fluorescence anisotropy studies we have recently found that all five compounds were able to decrease the microviscosity of artificial membranes [8].
Altogether these results indicate that the phenols compounds interact with the lipid membrane phase, suggesting their anesthetic activity could be the combined result of their specific (with receptor proteins) as well as nonspecific interaction (with surrounding lipid molecules) modulating the supramolecular organization of the receptor environment.
One approach to investigate the interactions between drugs and lipid molecules is the use of 1H-NMR which could give information about changes on the membrane dynamics by the mapping of the different bilayer regions [15]. In the current study, by using 1H-NMR spectroscopy, we have investigated the effects of the insertion and the possible preferential location of the five phenol derivatives with GABAergic activity (propofol, thymol, carvacrol, eugenol and chlorothymol) on EPC membranes.
2. Experimental
2.1. Materials
Propofol (2,6-bis(isopropyl)-phenol), thymol (5-methyl- 2-isopropyl-phenol), carvacrol (2-methyl-5-isopropylphenol), eugenol (2-methoxy-4-prop-2-enyl-phenol) and chlorothymol (5-methyl-4-chloro-2-isopropyl-phenol) were obtained from Sigma Chemical Co. (St Louis, MO, USA), and used without further purification. Egg phosphatidyl choline (EPC) was from Avanti Polar Lipids (Alabaster, USA). Water was bidistilled in an all-glass apparatus (pH 6.5 ± 0.3). Other drugs and solvents used were of analytical grade.
2.2. Membrane Preparation
Liposomes were obtained by evaporating stock chloroform solutions of EPC under a stream of N2. The samples were left under vacuum for no less than 2 h to remove residual solvent. The lipids were then suspended in 0.05 M phosphate buffer solution, pH 7.4 and vortexed for 5 min to form large multilamellar vesicles (MLVs).
For NMR experiments, small unilamellar vesicles (SUV) were used. Briefly, MLVs, obtained as described above, but suspended in D2O, were sonicated until clear (ca. 15 min) in a Sonics and Materials equipment (Newtown, CT). During sonication the temperature was kept at 0˚C - 4˚C by intermittent (1 min) agitation cycles, in an ice-water bath.
2.3. Partition Coefficient Determination
Phenols concentrations inside the membrane, expressed as molar ratios with respect to EPC, were calculated from the membrane—buffer partition coefficient, P, of each compound. In turn P was determined by phase-separation between MLVs and buffer at pH 7.4, according to the Equation (1) [16]:
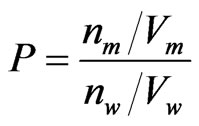 (1)
(1)
where n denotes the number of phenol moles, V is the volume, and the subscripts m and w refer to the membrane and aqueous phase, respectively. The volume of the membrane phase, Vm, was calculated assuming a lipid density of 1 g/mL [16]. The amount of each phenolic compound bound to the lipid phase was optically determined at their corresponding wavelengths of maximal absorption between 270 and 282 nm [13] after ultra-centrifugation at 120,000 × g for 2 h, by subtracting the supernatant concentration from the total drug concentration measured before phase mixing.
2.4. Nuclear Magnetic Resonance (NMR) Experiments
Spectra were collected in a Varian Innova 600 MHz (LNBio, Campinas, Brazil) equipment. The samples were degassed to avoid the interference of dissolved O2 with longitudinal relaxation times (T1) measurements. For 1H-NMR, a 90˚ pulse was typically 10 - 15 μs and the recycling time was set to 5 times the largest T1 (those of the aromatic protons of phenols), typically 6 s. T1 were obtained by the conventional inversion-recovery technique, at 37˚C. Using the determined partition coefficient values—see Section 2.3, all phenols were added to the sonicated vesicles up to 1:3 phenol:EPC molar ratio within the membrane.
3. Results and Discussion
Membrane-buffer partition coefficients were determined previously to the NMR experiments in order one could calculate the proper phenol amount to guarantee a 1:3 drug:lipid molar ratio in the membrane. PEPC/w (between egg-phosphatidylcholine liposomes and phosphate buffer, pH 7.4) values, follow a similar behavior to the partition coefficient determined in other comparable systems reported before [13] with a hydrophobic profile of: chlorotymol ≥ propofol > carvacrol ≥ thymol > eugenol.
1H-NMR spectra (600 MHz) of each compound, of EPC unilamellar vesicles and of samples containing vesicles in the presence of each compound, at a 1:3 (phenol:lipid) molar ratio, were collected. Typical spectra of phenol, EPC small vesicles and phenol in EPC vesicles (1:3 mole%) are shown in Figure 1, for propofol.
The assignments of EPC and phenols hydrogen signals are indicated in Figure 2, where capital letters refer to the phospholipid, and lower-case letters identify the nonequivalent resonance peaks of each phenol compound These assignments were in good agreement with those reported in the literature for EPC [15-17] and carvacrol, thymol and eugenol [18,19].
From the chemical shifts (C.S.) corresponding to the phenolic hydrogens in water or in EPC vesicles, changes in the chemical shifts between both systems (DC.S.) were calculated. The same procedure was applied to determine the DC.S. of EPC hydrogens in water or in the presence of each phenol. Table 1 shows the C.S. and DC.S. for
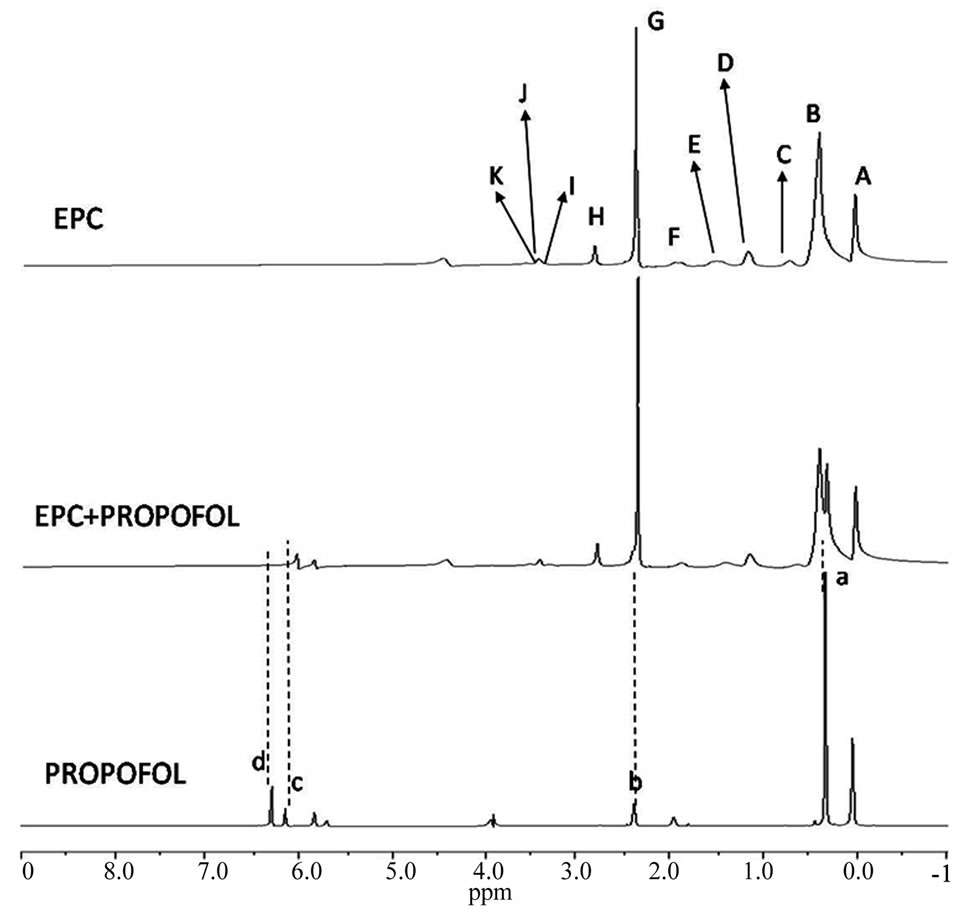
Figure 1. 1H-NMR spectra (600 MHz) of propofol in D2O, EPC small unilamellar vesicles and propofol in EPC vesicles (pD 7.4; 25˚C).
Tab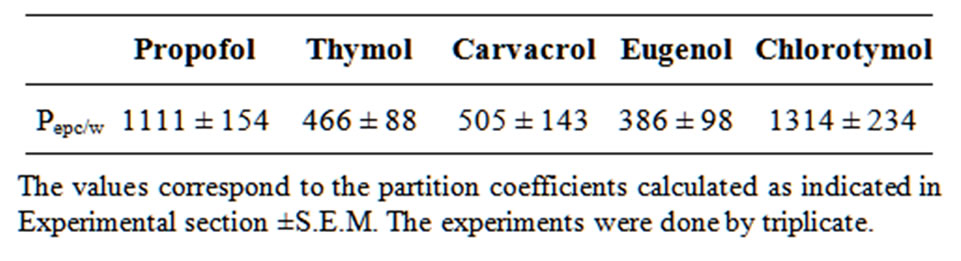 le 1. Partition coefficients (PEPC/w) of phenol compounds between egg-phosphatidylcholine liposomes and phosphate buffer, pH 7.4.
le 1. Partition coefficients (PEPC/w) of phenol compounds between egg-phosphatidylcholine liposomes and phosphate buffer, pH 7.4.

Figure 2. Hydrogen peaks assignments for each compound (lower-case letters) and EPC molecule (capital letters) in NMR spectra.
phenols and Figure 3 represent the DC.S. values determined for EPC.
Changes in the chemical shifts of hydrogens (DC.S. ≠ 0) would indicate variations in the chemical environment of the nuclei, being considered more significant those changes higher than 0.05 ppm [15]. All phenolic compounds assayed showed significant DC.S., especially in their aromatic hydrogens, indicating that, in the presence of EPC vesicles, they experience a different chemical environment, and confirming their interaction with the vesicles (Table 2).
Figure 3 shows the effect (DC.S.) of the five phenol compounds on chemical shift of EPC hydrogens. Eugenol, the less hydrophobic analog essentially affected hydrogen I (choline group nearby the phosphate atom of EPC), changing it downfield. All the other phenol compounds induced upfield shifts in EPC hydrogens around the glycerol backbone region. In the presence of thymol, the main DC.S. found in the EPC molecule was observed in hydrogens H, E and C. In the presence of propofol, a significant alteration was observed in hydrogen E and,

Figure 3. Changes in the chemical shifts of EPC hydrogen peaks in NMR spectra induced by the presence of phenolic compounds. Assignments are given in Figure 2. [EPC] = 65 mM, pH 7.4, 25˚C, 600 MHz. Just DC.S. higher than 0.05 ppm is represented.
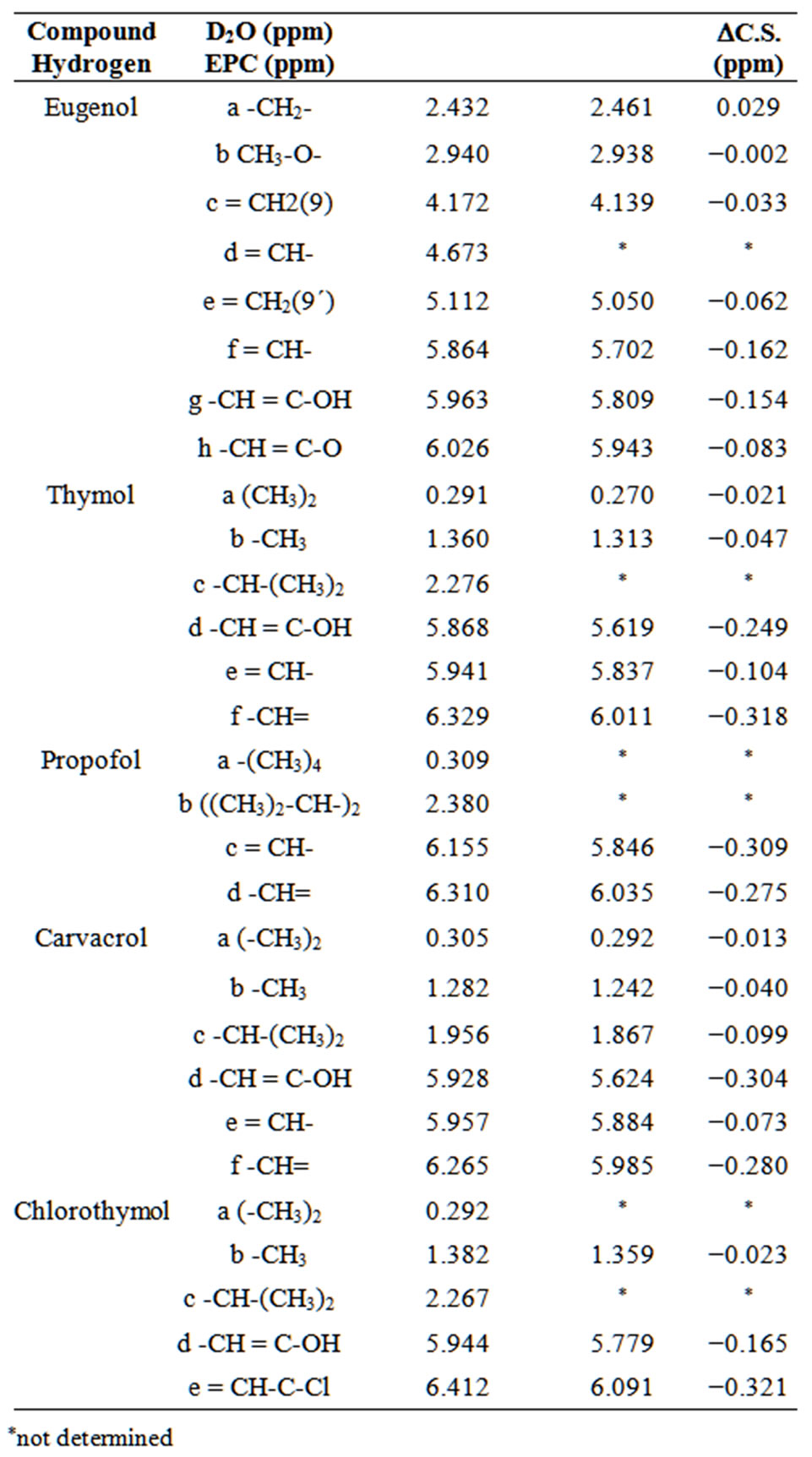
Table 2. Changes in the chemical shifts of 1H-NMR signals of phenolic compounds in the aqueous phase (D2O) and with EPC vesicles, at pH 7.4 and 25˚C.
*not determined with minor intensity, in hydrogens I and K. Carvacrol and chlorothymol induced important alterations in hydrogens E and C.
The NMR literature reports that aromatic or electronegative molecules in the bilayer can shift resonances by the short-range ring current effect [15,20]. Chemical shifts to minor frequencies (upfield) reveal that the group is inducing the shield of the spins of the observed nuclei, indicating the proximity of the aromatic ring and probably the localization of the compound in that spot at the bilayer [15].
The presence of phenolic compounds within the EPC molecules induced also changes in the molecular dynamics, as revealed by relaxation studies. A decrease in T1 values was detected for all several hydrogens belonging to the phenols (data not shown), indicating an important mobility restriction and suggesting a strong phenol-EPC interaction.
Figure 4 shows the T1 profile of EPC hydrogens with and without phenolic compounds. In this work we presented the T1 data as described by Fraceto et al. [15, 16], with an EPC molecule shown in its extended form, from hydrogens of the polar head-group (left) to those of the acyl chain region (right).
The T1 values measured for EPC hydrogens on unilamellar vesicles (Figures 4(a)-(e), full symbol) are in good agreement with those reported before [15]. The T1 are small for the polar headgroup hydrogens (peaks G, H), reflecting the restrictions caused by the electrostatic interaction between the amine group of one phospholipid and the phosphate groups of the next phospholipid [21, 22]. In the glycerol backbone region (peaks I, J, K) hydrogens have intermediate, smoothly higher mobility than hydrogen at carbons α and β of the acyl chain (peaks E, C) due to the high molecular packing of that region. The profile of acyl chain EPC hydrogens (peaks B to A) dynamics is in agreement with that described by other authors and obtained through 13C, 2H and 1H-NMR or EPR [23,24] showing increased mobility towards the terminal methyl group—peak A.
In the presence of eugenol, hydrogens A, B and D of EPC showed large decrements in T1 values, indicating a decrease in mobility in the hydrocarbon chains region. In the presence of chlorothymol, the hydrogens more affected were I, K and A, indicating the insertion of its aromatic (voluminous) group in that zone of the bilayer (principally hydrogens I and K), also explaining the changes found in chemical shifts (Figure 3) which could be due to the ring current effect inducing changes in the chemical environment of the hydrogens below the glycerol zone (E and C). Similar results have been described for local anesthetics [25]. Propofol induced T1 reduction mainly in the hydrocarbon chains (hydrogens D, F, B and A) but changed C.S. at the glycerol level and the beginning of the acyl chains. Thymol also caused comparable
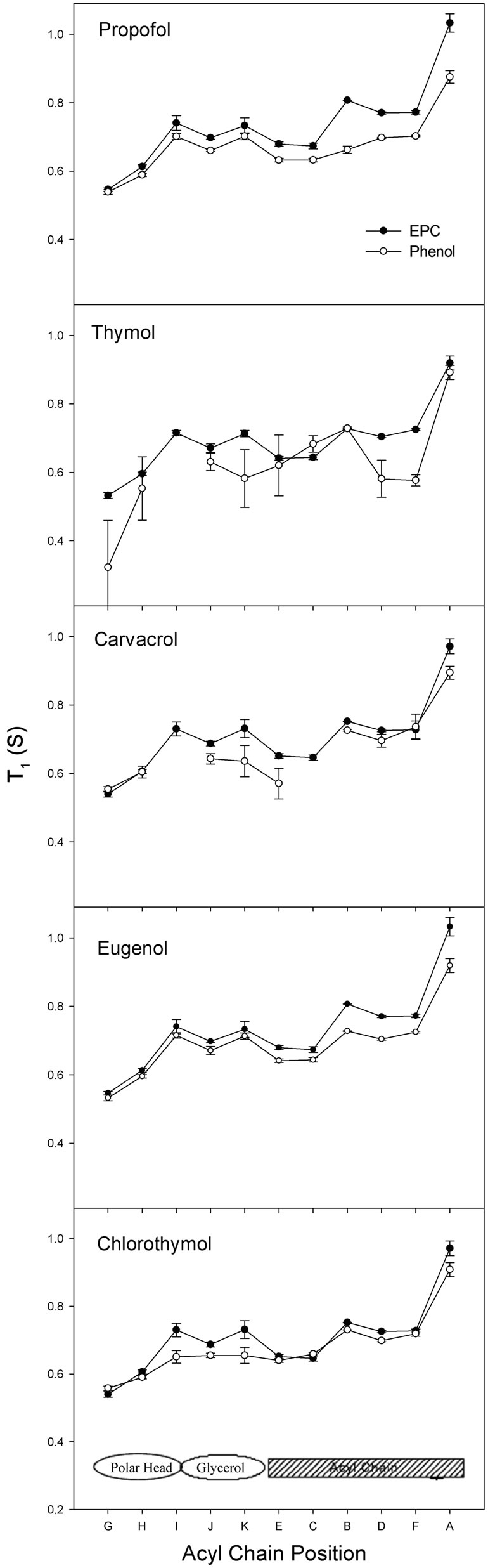
Figure 4. Changes in the longitudinal relaxation times (T1, s) of EPC hydrogens at SUV in the presence of propofol, thymol, carvacrol, eugenol and chlorothymol. [EPC] = 65 mM, pH 7.4, 25˚C, 600 MHz. The letters without values were not determined.
effects on T1 values of hydrogens K (glycerol), D and F (acyl chain). However, alterations in the hydrogens C, E and H were also found, indicating the possible insertion of the compound in this region (between the polar group and the beginning of the acyl chain). In the presence of carvacrol, some T1 values of EPC hydrogens were diminished in the glycerol region (K) and at different depths of the hydrocarbon chains (E and A).
NMR results indicate that hydrogens from all the five compounds presented significant variations in C.S. in the presence of EPC vesicles, providing evidences of their interaction with the lipid phase of such model membrane. T1 values allowed us to monitor molecular dynamics at different depths of the bilayer when the phenols interact with the membrane components (and vice versa), revealing the preferential location of each phenol compound inside the bilayer.
Taking into account the short-range ring effects (∆C.S. values) in EPC hydrogens after the partition of each phenol at the same (1:3 phenol:lipid) molar ratio in the membrane, we suggest that the phenols are placed between choline molecule and the glycerol backbone, reaching the first acyl chain carbons for carvacrol and chlorothymol. Similarly, the changes found in the molecular dynamics (increased T1 values) of EPC hydrogens in the presence of all phenol compounds are coherent with these amphiphiles acting as spacers between the lipid molecules (16), mainly in the hydrocarbon chains and glycerol regions (see graphical hypothesis in Figure 5" target="_self"> Figure 5).
Finally, we can conclude that all compounds are able to insert in EPC phospholipid vesicles and to locate in the region between the polar group (choline molecule), the glycerol and the first atoms of the acyl chains, being the more lipophilic compounds (propofol and chlorothymol) (see logP values determined in similar systems [13]) those that would occupy deeper preferential positions in the regions above described. This location of the phenol molecules would induce the reduction of the repulsive forces among phospholipids head groups allowing closer molecular packing and finally diminishing the mobility degree of the hydrocarbon chains. This description is supported by recently reported epifluorescence imaging analysis at phospholipidic Langmuir films [14].
4. Final Remarks
These results indicate that the phenols studied are clearly able to interact with membranes. Considering that many drugs have intracellular targets, which require their transport across phospholipid bilayers, and drug-lipid interactions are unavoidable. Furthermore, although specific pharmacological regulation of membrane protein functions, such as GABAA receptor, can be analyzed using well-described theories of ligand-receptor interactions, it
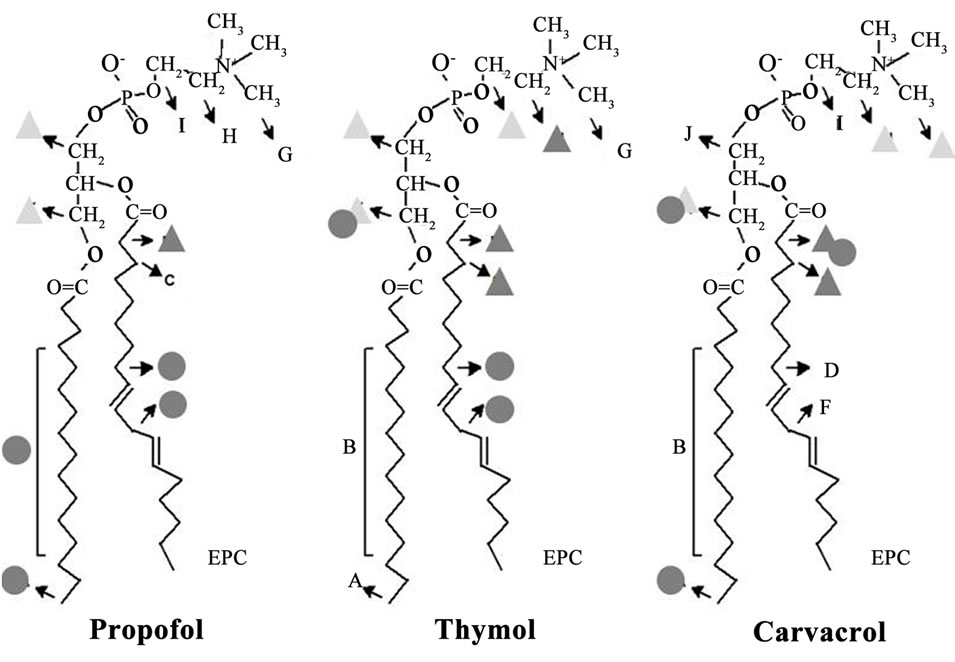
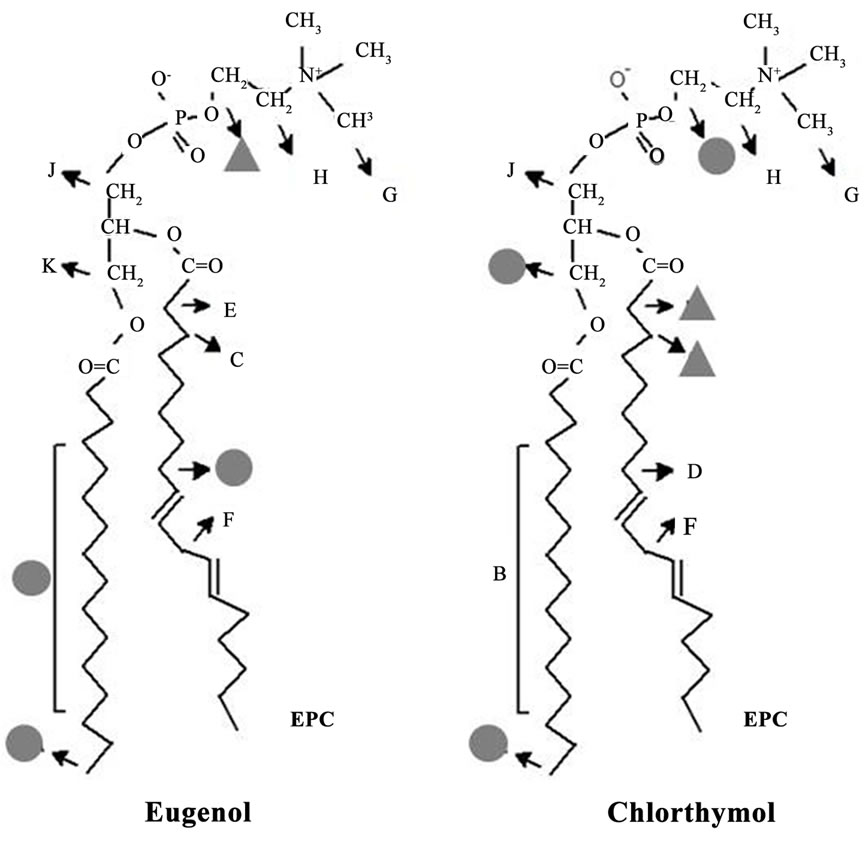
Figure 5. Schematic representation of the proposed insertion of the five phenol groups in EPC liposomes, as revealed by different NMR approaches (see text). More significant changes in C.S. (DC.S.) and T1 values are represented by triangles (dark gray: >0.05 ppm; light gray: 0.03 - 0.05 ppm) or circles (>0.06 s), respectively.
should be considered that many compounds that regulate the receptor protein function are noticeably lipophilic, which may change the physical properties of the lipid bilayer. Thus, it is possible that any anesthetic activity of lipophilic phenols could be the combined result of the interaction of the phenol molecules with specific receptor proteins (GABAA receptor) and with the surrounding lipid molecules modulating the supramolecular organization of the receptor environment.
5. Acknowledgements
This work was partially financed with grants from a bilateral CAPES (Brasil)/MINCyT (Argentina) project, SECyT—Universidad Nacional de Córdoba, MINCyT (Córdoba) and CONICET (Argentina). DAG and MAP are career investigators from CONICET.
REFERENCES
- V. Campagna-Slater and D. F. Weaver, “Anaesthetic Binding Sites for Etomidate and Propofol on a GABAA Receptor Model,” Neuroscience Letters, Vol. 418, No. 1, 2007, pp. 28-33. doi:10.1016/j.neulet.2007.02.091
- R. L. Macdonald and R. W. Olsen, “GABAA Receptor Channels,” Annual Review of Neuroscience, Vol. 17, No. 1994, pp. 569-602.
- U. Rudolph and H. Mohler, “Analysis of GABAA Receptor Function and Dissection of the Pharmacology of Benzodiazepines and General Anesthetics through Mouse Genetics,” Annual Review of Pharmacology and Toxicology, Vol. 44, No. 2004, pp. 475-498.
- R. Sogaard, T. M. Werge, C. Bertelsen, C. Lundbye, K. L. Madsen, C. H. Nielsen and J. A. Lundbaek, “GABA(A) Receptor Function Is Regulated by Lipid Bilayer Elasticity,” Biochemistry, Vol. 45, No. 43, 2006, pp. 13118- 131129. doi:10.1021/bi060734+
- F. Sancar and C. Czajkowski, “Allosteric Modulators Induce Distinct Movements at the GABA-Binding Site Interface of the GABA-A Receptor,” Neuropharmacology, Vol. 60, No. 2-3, 2011, pp. 520-528. doi:10.1016/j.neuropharm.2010.11.009
- B. Mohammadi, G. Haeseler, M. Leuwer, R. Dengler, K. Krampfl and J. Bufler, “Structural Requirements of Phenol Derivatives for Direct Activation of Chloride Currents via GABA(A) Receptors,” European Journal of Pharmacology, Vol. 421, No. 2, 2001, pp. 85-91. doi:10.1016/S0014-2999(01)01033-0
- D. A. Garcia, J. Bujons, C. Vale and C. Sunol, “Allosteric Positive Interaction of Thymol with the GABAA Receptor in Primary Cultures of Mouse Cortical Neurons,” Neuropharmacology, Vol. 50, No. 1, 2006, pp. 25-35. doi:10.1016/j.neuropharm.2005.07.009
- G. N. Reiner, L. Delgado-Marin, N. Olguin, S. SanchezRedondo, M. Sanchez-Borzone, E. Rodriguez-Farre, C. Sunol and D. A. Garcia, “Gabaergic Pharmacological Activity of Propofol Related Compounds as Possible Enhancers of General Anesthetics and Interaction with Membranes,” Cell Biochemistry and Biophysics, 2013, pp. 1-11.
- M. R. Witt and M. Nielsen, “Characterization of the Influence of Unsaturated Free Fatty Acids on Brain GABA/ Benzodiazepine Receptor Binding in Vitro,” Journal of Neurochemistry, Vol. 62, No. 4, 1994, pp. 1432-1439. doi:10.1046/j.1471-4159.1994.62041432.x
- D. A. Garcia, M. A. Perillo, J. A. Zygadlo and I. D. Martijena, “The Essential Oil from Tagetes Minuta L. Modulates the Binding of [3H]flunitrazepam to Crude Membranes from Chick Brain,” Lipids, Vol. 30, No. 12, 1995, pp. 1105-1110. doi:10.1007/BF02536610
- M. A. Perillo, D. A. Garcia, R. H. Marin and J. A. Zygadlo, “Tagetone Modulates the Coupling of Flunitrazepam and GABA Binding Sites at GABAA Receptor from Chick Brain Membranes,” Molecular Membrane Biology, Vol. 16, No. 2, 1999, pp. 189-194. doi:10.1080/096876899294652
- M. Pytel, K. Mercik and J. W. Mozrzymas, “Interaction between Cyclodextrin and Neuronal Membrane Results in Modulation of GABA(A) Receptor Conformational Transitions,” British Journal of Pharmacology, Vol. 148, No. 4, 2006, pp. 413-422. doi:10.1038/sj.bjp.0706747
- G. N. Reiner, D. O. Labuckas and D. A. Garcia, “Lipophilicity of Some GABAergic Phenols and Related Compounds Determined by HPLC and Partition Coefficients in Different Systems,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 49, No. 3, 2009, pp. 686-691. doi:10.1016/j.jpba.2008.12.040
- G. N. Reiner, M. A. Perillo and D. A. Garcia, “Effects of Propofol and Other GABAergic Phenols on Membrane Molecular Organization,” Colloids and Surfaces B: Biointerfaces, Vol. 101, No. 2013, pp. 61-67.
- L. F. Fraceto, A. Spisnic, S. Scherier and E. de Paula, “Differential Effects of Uncharged Aminoamide Local Anesthetics on Phospholipid Bilayers, as Monitored by 1H-NMR Measurements,” Biophysical Chemistry, Vol. 115, No. 2005, pp. 11-18.
- L. F. Fraceto, M. Pinto Lde, L. Franzoni, A. A. Braga, A. Spisni, S. Schreier and E. de Paula, “Spectroscopic Evidence for a Preferential Location of Lidocaine inside Phospholipid Bilayers,” Biophysical Chemistry, Vol. 99, No. 3, 2002, pp. 229-243. doi:10.1016/S0301-4622(02)00202-8
- Y. Kuroda and Y. Fujiwara, “Locations and Dynamical Perturbations for Lipids of Cationic Forms of Procaine, Tetracaine, and Dibucaine in Small Unilamellar Phosphatidylcholine Vesicles as Studied by Nuclear Overhauser Effects in 1H Nuclear Magnetic Resonance Spectroscopy,” Biochimica et Biophysica Acta, Vol. 903, No. 3, 1987, pp. 395-410. doi:10.1016/0005-2736(87)90046-0
- S. Fujisawa, Y. Kadoma and Y. Komoda, “1H and 13C NMR Studies of the Interaction of Eugenol, Phenol, and Triethyleneglycol Dimethacrylate with Phospholipid Liposomes as a Model System for Odontoblast Membranes,” Journal of Dental Research, Vol. 67, No. 11, 1988, pp. 1438-1441. doi:10.1177/00220345880670111501
- E. Locci, S. Lai, A. Piras, B. Marongiu and A. Lai, “13CCPMAS and 1H-NMR Study of the Inclusion Complexes of Beta-Cyclodextrin with Carvacrol, Thymol, and Eugenol Prepared in Supercritical Carbon Dioxide,” Chemistry & Biodiversity, Vol. 1, No. 9, 2004, pp. 1354-1366. doi:10.1002/cbdv.200490098
- W. M. Yau, W. C. Wimley, K. Gawrisch and S. H. White, “The Preference of Tryptophan for Membrane Interfaces,” Biochemistry, Vol. 37, No. 42, 1998, pp. 14713-14718. doi:10.1021/bi980809c
- J. Seelig, “31P Nuclear Magnetic Resonance and the Head Group Structure of Phospholipids in Membranes,” Biochimica et Biophysica Acta, Vol. 515, No. 2, 1978, pp. 105-140. doi:10.1016/0304-4157(78)90001-1
- G. Buldt and R. Wohlgemuth, “The Headgroup Conformation of Phospholipids in Membranes,” The Journal of Membrane Biology, Vol. 58, No. 2, 1981, pp. 81-100. doi:10.1007/BF01870972
- J. F. Ellena, S. J. Archer, R. N. Dominey, B. D. Hill and D. S. Cafiso, “Localizing the Nitroxide Group of Fatty Acid and Voltage-Sensitive Spin-Labels in Phospholipid Bilayers,” Biochimica et Biophysica Acta, Vol. 940, No. 1, 1988, pp. 63-70. doi:10.1016/0005-2736(88)90008-9
- B. J. Gaffney and H. M. McConnell, “ParamagneticResonance Spectra of Spin Labels in Phospholipids Membranes,” Journal of Magnetic Resonance, Vol. 16, 1974, pp. 1-30.
- L. F. Fraceto, M. Pinto Lde, L. Franzoni, A. A. Braga, A. Spisni, S. Schreier and E. de Paula, “Spectroscopic Evidence for a Preferential Location of Lidocaine Inside Phospholipid Bilayers,” Biophysical Chemistry, Vol. 99, No. 3, 2002, pp. 229-243. doi:10.1016/S0301-4622(02)00202-8
NOTES
*Corresponding author.

