Journal of Biomaterials and Nanobiotechnology
Vol.3 No.1(2012), Article ID:16698,11 pages DOI:10.4236/jbnb.2012.31004
Influence of Alginates on Tube Nerve Grafts of Different Elasticity—Preliminary in Vivo Study
![]()
1Department of Neurosurgery, Wroclaw University Hospital, Wroclaw, Poland; 2Department of Biomaterials, AGH University of Science and Technology, Krakow, Poland; 3Department of Neurosurgery, Wroclaw Medical University, Wroclaw, Poland; 4Laboratory of Electron Microscopy Studies, Wroclaw University of Environmental and Life Sciences, Wroclaw, Poland; 5Department of Pathomorphology, Wroclaw Medical University, Wroclaw, Poland.
Email: szarekdariusz@gmail.com
Received October 9th, 2011; revised November 19th, 2011; accepted December 20th, 2011
Keywords: Tube nerve grafts; Polyurethane; Polylactide; Terpolimer; Sodium alginate; Sciatic nerve; in vivo
ABSTRACT
This preliminary research project has been conducted to evaluate different elastic polymer materials in terms of their applicability in peripheral nerve regeneration. Poly(tetrafluoroetylene-co-difluorovinylidene-co-propylene), poly(Llactide-co-D,L-lactide), and polyurethane were used for the manufacture of tubular implants. Alginate sodium gel and fibers were used as a scaffold to fill in tube nerve grafts and enhance nerve regeneration. The tubes were implanted to reconstruct a 10 mm gap in the sciatic nerve in rats. After 3, 7, 14, 28 days the tubes were retrieved for histological examination. Among tested tubes polyurethane implants were found to be the most suitable because of their mechanical and surgical properties. Other tested implants were found to be unfavorable due to their inappropriate rigidity, elasticity or surgical convenience. Alginate transformation into dense gel form was observed that hindered inner tube space cellular colonization. In consequence of this transformation nerve regeneration was inhibited inside tube nerve grafts. Histological examination showed massive colonization of the implants with Schwann cells, and growth of new axons was found within Schwann cells growing on tubes external surface. Appropriate time rates for alginate gelation and dissolveing must be determined to allow undisturbed tissue growth and maturation.
1. Introduction
Traumatic injuries of the peripheral nervous system usually lead to long-lasting disability due to loss of motor and/or sensory functions and may cause permanent neurological deficits. The best method to treat peripheral nerve transections is direct surgical coaptation of nerve ends without tension. If such coaptation is impossible, typically due to traumatic nerve tissue loss, the nerve is reconstructed with a graft from the patient’s own nerve. This method is used as a “gold standard” for nerve tissue reconstruction and is highly effective; however, it also includes negative attributes. The most important disadvantages are the necessity of additional surgery for nerve harvesting, new sensory deficits in donor nerves and the risk of neuroma formation [1-3]. For reconstructions of short gaps in sensory nerves in the hand or forearm it is also possible to use veins as autografts [4,5]. However, necessity of additional surgery is also disadvantageous in this case and the nerve regeneration process is not as effective as in nerve grafts.
It is quite obvious that replacement of the autografts with artificial implants, in the surgery of peripheral nerves, is of crucial importance. Rapid developments in tissue engineering give the opportunity for the design and creation of artificial implants for different purposes. For more than 30 years intensive research on various artificial materials for the nerve guide channels has been conducted [6-13]. As a result of this research several polymer implants in the form of tubes have been put into clinical practice. They can be used in the treatment of short gaps of less than 3 cm in length in skin nerves [14-17], but longer nerve gaps require nerve grafting for reconstruction.
Using tubes or veins for nerve reconstruction is limited by the gap length. Implants longer than 10 mm in rats and about 20 - 30 mm in humans tend to collapse. Internal filling of the tubes allows to minimize this effect, and provides an extracellular matrix scaffold for cellular infiltration, eliminates the process of a fibrin bridge creation inside an empty tube and enhances the regenerative properties of the implant [11,12,18-25]. Fibres and hydrogels of natural and synthetic origin have been adapted with various effects [12,21,26]. Synthetic materials are readily accessible, free of infection transfer risk, easily modifiable and processable into scaffolds. They can be infiltrated and colonized by Schwann cells, that produce natural extracellular matrix components, fibres like collagen, laminin or fibronectin, creating basal lamina for regenerating axons [12,13,16,27,28]. In this process the gradually degrading synthetic scaffold can be replaced by the newly formed natural extracellular matrix. For this use alginates could be one of the best solutions. These are highly biocompatible natural polysaccharides, and are open to wide-ranging chemical modifications. In vertebrates they are degraded by hydrolysis, i.e., dissolved and removed from the body via urinary system [26,29]. Alginates are widely used as extracellular matrix artificial scaffolds for tissues, especially for sensitive cell cultures and regenerating tissues [29-33]. The benefits of using alginates as a scafold in the regeneration of neural tissue have been described, amongst others, by Hashimoto [31,32,34-38].
In presented study two aspects of tube nerve grafts construction were analyzed. First we investigated the influence of alginates used to provide tubes internal scaffold on nerve regeneration process. We expected that filling the tubes with alginates will enhance the rate of Schwann cell infiltration into the implant and, due to this process, implant’s neuroregenerative properties. Secondly the suitability of different elastic polymers was analyzed as materials, used in combination with alginates, for implant manufacture in the form of tubes for peripheral nerve regeneration.
2. Materials and Methods
2.1. Preparation of Implants
Poly(tetrafluoroetylene-co-difluorovinylidene-co-propylene), PTFE-PVDF-PP (Terpolymer), was purchased from Aldrich. Poly(L-lactide-co-D,L-lactide), (PLDL), with a molar ratio L-lactide to DL-lactide—80:20, was purchased from Boehringer Ingelheim, Germany. Biodegradable elastomeric polyurethane (PU) was produced by Bayer according to the Gogolewski procedure [24]. Sodium alginates were purchased from BioPolymer, Norway. Alginate fibers were fabricated by the Department of Artificial Fibers at the Technical University of Lodz (Poland). Solvents were purchased from POCh, Poland.
The terpolymer and PLDL were dissolved in acetone at ambient temperature, and 20 wt% solutions were obtained. The polyurethane was dissolved in DMF at different concentrations (from 3 to 50 wt%) at a temperature of 60˚C, upon constant stirring. The time of dissolving was 6-8 hrs when magnetic stirring was used, and 30 mins when the mixture was ultrasonicated.
Polymer tubes with a diameter of 1.2 mm and wall thickness 0.2 mm were prepared by the dip-coating of an appropriate glass or metal capillary in the polymer solution. After withdrawing the capillary tube from the solution it was rotated horizontally for 3 - 4 minutes to obtain a similar wall thickness along the axis of the tube and to evaporate the solvent. The dry coated capillary tubes were immersed in hot water (~70˚C) for 2 hrs and then left in water overnight. Porosity was achieved by processing the blend from the solution in the presence of pentane as a volatile additive or by salt-leaching. Tubes were filled with alginate gels prepared during surgery by dissolving alginate powder in distillated water (the concentration of the obtained solution was 5 wt%), with eight drops of 3% calcium chlorate solution, or filled prior to surgery with sodium alginate fibers. The obtained samples were characterized under a scanning electron microscope (Nova Nano SEM 200, FEI Company).
2.2. Animals and Surgery
All surgical and animal care procedures were carried out according to the guidelines and after acceptation by the Local Ethical Committee for Experiments on Animals. According to the recommendations of the committee the number of experimental animals and tested implants was reduced to a minimum.
Twenty one Wistar rats aged about 3 months (~300 g) were used in the experiments. Animals were housed in group cages one week before the experiment to accustom to the new environment. For the operative procedures rats were anaestetised by a mixted solution of ketamine and xylazine, 10 mg and 1mg per 1ml respectively given intraperitonealy 1 ml per 100 g rat body weight. Left sciatic nerve was exposed and after additional local anaesthesia with several drops of 1% lidocaine, 10 mm gap was created. An implant was sutured to the nerve using two Dafilon 8/0 epineurial stitches per stump. During implantation nerve ends were slightly entubulated. This maneuver slightly shortened the gap to about 9mm. In a control group nerve reconstruction was performed with autotransplantation with excised nerve piece.
Four terpolimer and four polylactide implants, two empty and two filled with alginate gel were implanted for 28 days. Eight poliurethane implants filled with alginate sodium fibres were implanted for 3, 7, 14, 28 days, two for each evaluation point. Five animals in a control group were housed for 28 days. After each observation period, rats were sacrificed with pentobarbital overdose, macroscopic inspection of the implant wound bed was conducted, implants together with the 2 mm pieces of each nerve stump were extracted.
2.3. Sample Staining and Histoological Examination
Samples were fixed in 10% buffered formalin for 24 hours and paraffin-embedded. 5 μm thick paraffin sections for staining with haematoxylin and eosin were obtained on a Zeiss Microm HM 340E microtome, deparaffinized with xylene and ethanol/water solutions of decreasing concentrations of ethanol, finally washed with water. Sections were stained with haematoxylin (Shandon) 3 min., rinsed with running tap water for 10 min. and eosin (Shandon) for 10 min. and dehydrated by subsequent rinsing with ethanol (70% - 100%) and xylene.
2.4. Immunochemistry
Samples were fixed in 10% buffered formalin for 24 hours. Paraffin-embedded 3 μm thick sections were obtained on a Zeiss Microm HM 340E. Slides were deparaffinized and dehydrated with xylene and alcohol treatment series. Heat-induced antigen retrieval was performed: slides were incubated in Tris/EDTA buffer (pH = 9.0) for 20 min. Endogenous peroxidase activity was blocked in 3% hydrogen peroxide for 5 min. Slides were washed with TBS pH 7.6 for 5 min each. Tissue samples were labeled with S100 (N1573, DAKO) and NF (N1591, DAKO) antibody solutions, for Schwann cells and axons, respectively. A primary antibody was incubated for 10 min at room temperature, and monoclonal antibodies for 15 min. Detection was performed with EnVision™ Systems (Dako). Sections were counterstained with Mayer’s haematoxylin, dehydrated with alcohol and xylene.
2.5. Scanning Electron Microscopy
For scanning electron microscopy, 10 μm sections of paraffin-embedded nerve samples were obtained. Samples were placed on histological slides, deparaffinized with graded alcohol content water solutions, and covered with gold powder layer using Scancoat6 (Edwards, London, England). Slides were analyzed with an EVO LS 15 Zeiss scanning electron microscope.
2.6. Histomorfometry
Histomorphometric analysis was done for polylactide and polyurethane tubes, and autotransplants. Slices of proximal and distal part of the grafts were used. Photographs were taken with digital camera system Cannon Powershot using optical microscope Zeiss Axio Imager. A1. Counting of axons was performed with the use of ZEISS software AxioVision Rel. 4.6.3. Terpolimer implants were postponed because only insignificant neuronfillament growth was found in immunocytochemically stained slices.
3. Results
Three polymers showing different mechanical properties were used for reconstruction of injured peripheral nerves, poly(tetrafluoroetylene-co-difluorovinylidene-co-propylene) (PTFEPVDF-PP)-(terpolimer), poly(L-lactide-D,L-lactide) (PLDL), and polyurethane (PU) (Figure 1).

Figure 1. Scanning electron microscopy images external surface and the transverse cut of the polymer tubes made of A: Terpolimer; B: Polylactide; C: Polyurethane; D: Alginate fibers, and the sciatic nerve crossection (small picture).
The terpolimer tubes were highly flexible and elastic, could be deformed effortlessly, showed a good shape memory and restored their shape shortly after the deforming force subsided. They could be bent and twisted during implantation to adapt to the nerve anastomosis alignment, but at the same time, they could easily collapse. These tubes did not induce tensions in the junction with the nerve, but they were very delicate, and could easily be torn with the microsurgical needle.
The polylactide tubes were rigid what made them pressure resistant. They did not collapse after implantation under compression from surrounding tissues thus protecting the tube’s inner space. However, due to this stiffness they did not adapt their shape to the profile of stump anastomosis. That caused some bending of nerve ends at the site of anastomosis. The tubes also cracked upon puncture with a surgical needle. Any cutting to resize the implant was usually associated with the distortion of the tube walls, and its deformation.
The polyurethane employed for implant manufacture was designed to be elastic and biodegradable. The tubes made of that polymer were soft, deformed easily and adjusted to the place of the implantation, did not cause compression or bending of the nerve stumps. The durability of the walls allowed to puncture and suture tubes to nerve stumps. The PU tubes also possessed very good shape memory. However, due to their elasticity and flexibility they tended to collapse readily under compression from surrounding tissues in the wound.
Filling the tubes with alginate fibres or gel allowed to prevent them from collapsing. Tubes filled with the freshly prepared gel were easy to operate, however, rheological properties of the gel were changing very quickly. The gel became very sticky which made, in time, implantation difficult. Preparation of the alginate gel just before surgery prolonged the time of the operation. Applying alginate fibres instead of gel made tubes more surgically friendly and allowed the above mentioned difficulties to be avoided (Figure 2).
3.1. Histological and Scanning Electron Microscopy Observations
Terpolimer tubes after four weeks of observation were covered with a layer of connective and inflammatory tissue, and the wounds were properly healed. Immunohistochemical studies revealed Schwann cells and axon growth on the external surface of tube walls, a slight colonization of inner tube space by Schwann cells was noticed. No toxic or excessive inflammatory reaction was found.
The polyurethane tubes were subjected to in vivo examination in four observational periods, 2 animals each. The early reaction was estimated after 3 and 7 days from the implantation. After 3 days macroscopically implants were correctly attached to the nerve ends, and typical postsurgical blood clots were present. Histological examination showed a large amount of cell infiltration accumulated around the implant and nerve stumps consisting mainly of neutrophiles. The most intensive infiltration was found around the implant, especially close to its central part. On the nerve stumps infiltration was signifycantly lower and occurred only on the surface and the most external layer of the perineurium, did not penetrate or disturb the nerve structure. Alginate fibres underwent hydration and transformed into gel. This alginate gel partially sequestrated and diffused outside the implant were it mixed with the infiltrate. Staining for neurofilaments and S-100 protein showed the activation of Schwann cells, i.e., enlargement in number and size, and the degeneration of axons in the distal stump, both their enlargement in diameter and fragmentation were visible.
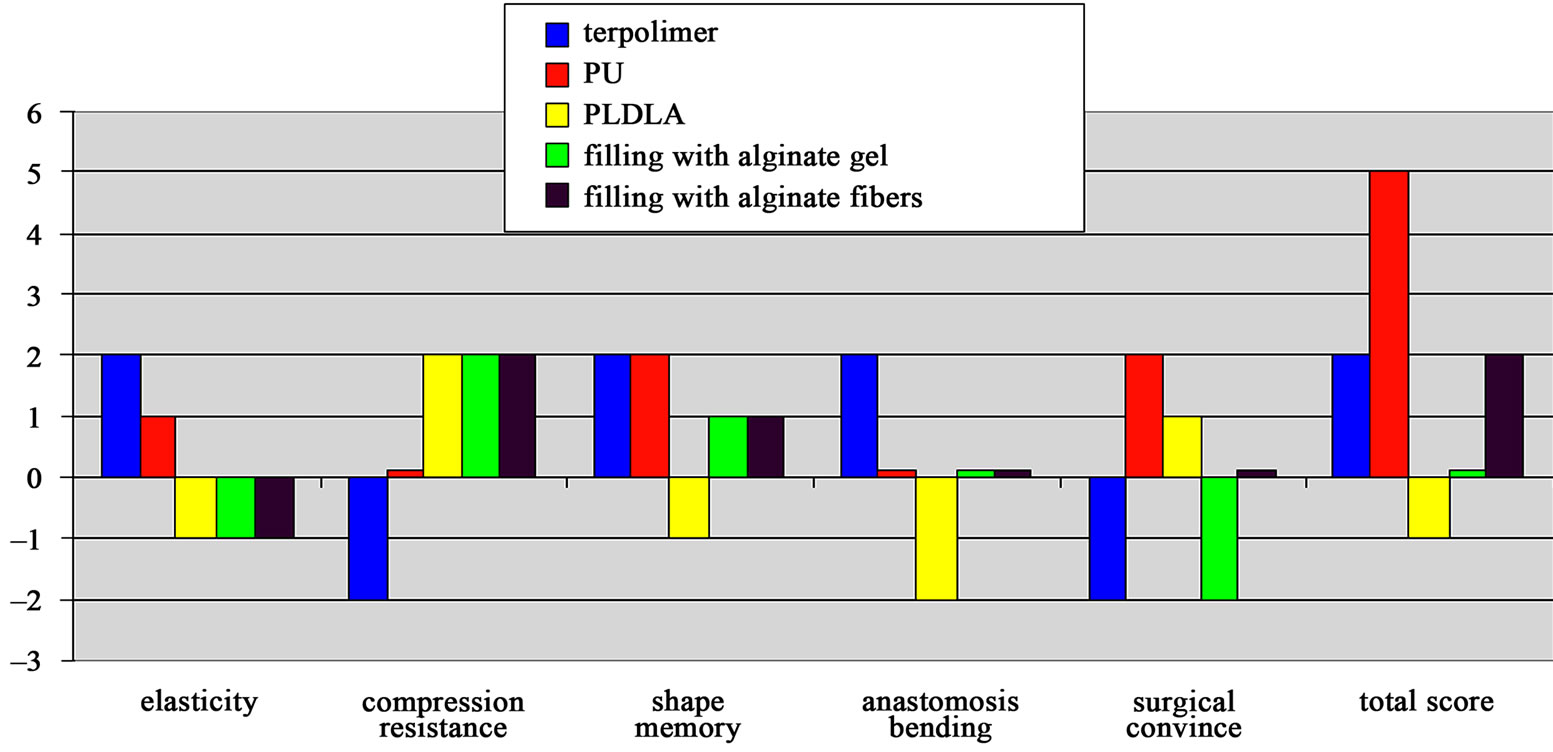
figure 2. macroscopic evaluation and surgical properties of tube nerve grafts. influence of each property scored: very positive +2, positive +1, no influence 0, negative 1, very negative –2; (pu—polyurethane implant; pldla—polylactide implant).
Examination of implants after 7 days showed that the surgical wound was healed. Finely organized infiltration along the implant was present. At this period inflammatory cell response was more expressed near the stumps, more at the distal site. Higher concentrations of plasma neutrophiles with sporadic necrosis, and the activation of stroma in the surroundings could be noticed. The structure of the nerve ends, outside the coaptation sites, was almost unchanged, and covered only with a thin layer of the infiltrate. Alginate fibers inside the implant underwent further hydration and gelation, and some degradation upon the activity of macrophages. However their fibrous structure was partially preserved. Alginate residues were present in the infiltrate surrounding the tube. Staining against S-100 protein showed stronger activation of Schwann cells that were smaller and more numerous, presence of macrophages and histiocytes. Neurofillament staining revealed more pronounced axon degradation with preserved nerve ends structure.
After 14 days the implant was coated with a thicker layer of yellow fibrous tissue. Both, implant and nerve stumps, had significant thickening of inflammation on the side adjoining the neighbouring muscle. Histological analysis showed progression of the infiltrate organization, especially around the distal stump. Inflammation was also visible in the internal structure of the nerve ends, but mainly in external layers with solitary histiocytes and macrophages inside the nerve, while the internal structure of the nerve was unchanged. Residues of alginate fibers were visible in the interior of the tubes, however their degradation and fragmentation caused by infiltrate was vast and only alginate gel was visible. Deposits of alginate gel in inflammation surrounding implant were significantly more evident than in the previous observation period. S-100 staining revealed small amounts of tissue consisting mainly of neutrophil granulocytes, histiocytes, and macrophages entering inside the implant near the stumps and mixed with a degrading alginate gel. Implant surrounding inflammation was build mainly with histiocytes, macrophages and granulocytes and, near distal stump, Schwann cells. Schwann cells seemed to colonize this new tissue surrounding the implant. NF staining showed degeneration of the axons in the distal stump with few remaining positive staining remnants. Activation of proximal nerve stump was evident. Numerous thick axons were present, and between them, new thin NF positive fibres, growth cones were visible. They were not present in the samples examined after 3 and 7 days of implantation.
After 28 days, the surgery site was healed, implants were covered with a layer of yellow/brown tissue. The layer was thinner than in the earlier observation period, and thickened only around the distal stump. Inflammation was more organized and the formation of new tissue was evident. In that tissue beside previously observed cells, also foreign-body giant cells were present, but they were not numerous. As in the previous observation period, tissue surrounding implant was thickened on one side and this thickening was even more evident (Figure 3).

Figure 3. Scanning electron microscopy images, midgraft section A and C: Polyurethane tube after 4 weeks implantation; B and D: Healthy nerve. Inflammatory tissue surrounding implant is visible. It is markedly thicker on the side neighboring muscle. In that area structures of axons surrounded with Schwann cells similar as in the healthy nerve are present.
Some alginate inclusions were still present in the surrounding tissue, with the highest concentration around the distal stump. In the interior of the tube the quantity of alginate was significantly lower in the comparison with the previous observation period. Schwann cells were present in the infiltrate surrounding the implant, a particularly high number of Schwann cells were found in the area of its thickening. Schwann cells created a platform along the whole implant from proximal to the distal nerve stump. In that regions with Schwann cells, almost to the end of implant, NF positive cells were present. It can be assumed, then, that the Schwann cells created pathways for the regenerating axons along the outer wall of the implant. The growing axons were thin, and arranged in rosette-like structures around the cells identified as Schwann cells. Figure 4 Inside the tubes deposits of gel together with the advancing infiltrating tissue and Schwann cells were visible. The quantity of the infiltrateing tissue, and the number of Schwann cells were greatly in-creased compared with the previous examination period. It consisted of mixed neutrophiles and Schwann cells growing in the central part of the tube, they were surrounded by alginate deposits. Growth was more intensive on the side of distal stump and reached about 2 mm into the implant.
Observations of polylactide implants were similar to those of polyurethane tubes. Regenerating neural tissue was found to form within inflammatory infiltration around the implant, but the inflammation was less distinct than in the polyurethane group. More Schwann cells were found within this tissue compared to the polyurethane implants, with a greater number on the distal side. Neurofilament positive nerve fibers were found to accompany Schwann cells from the proximal side over half of the implant’s length. This implies axon regeneration along the external wall of the implant similar to polyurethane implants. Alginate gel deposits were found in tissue surrounding the implant, but their amount was larger than in polyurethane implants. Inflammatory infiltration around alginate deposits was less distinct and its greater organization was evident.
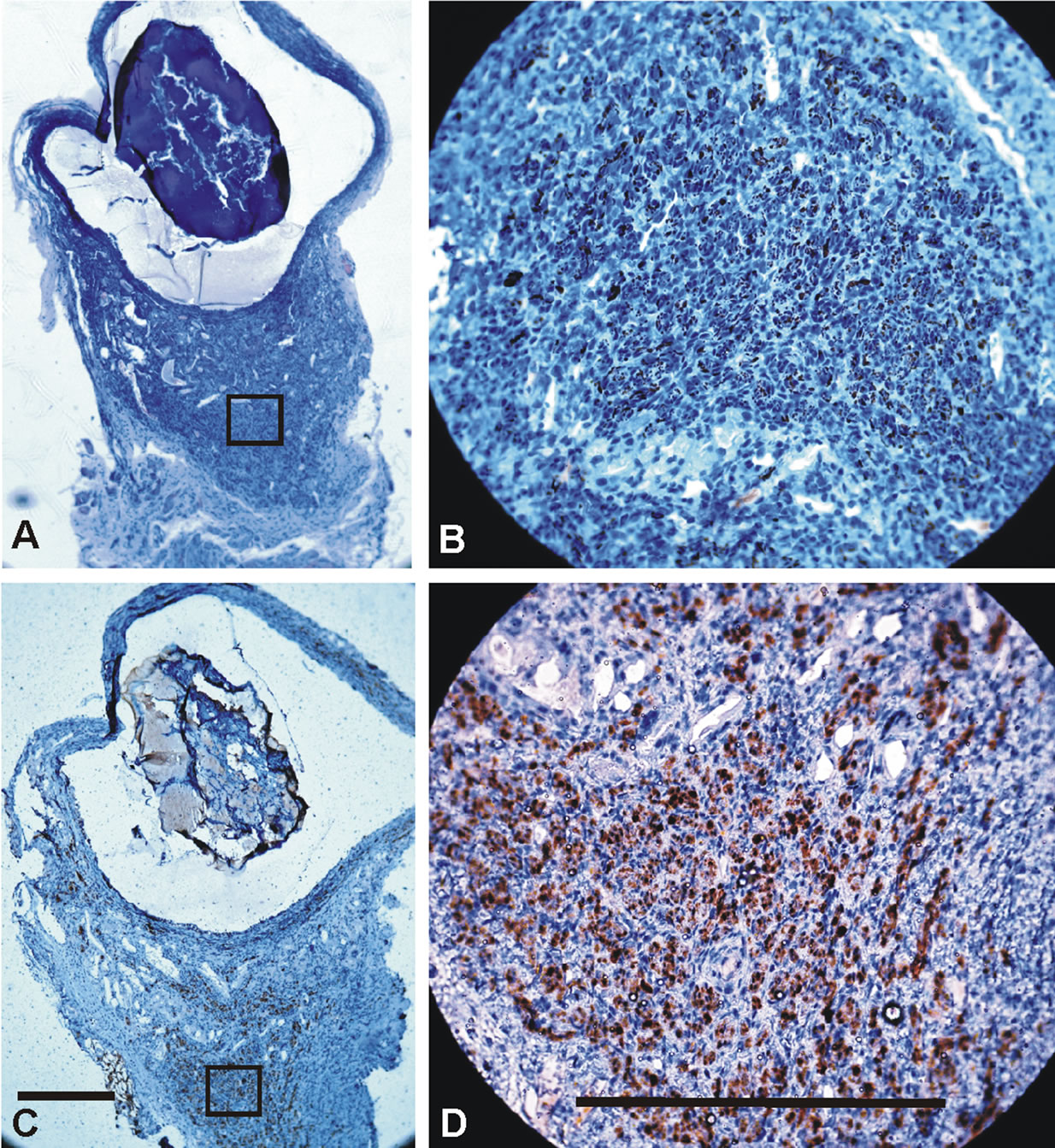
Figure 4. Polyurethane tube crossections after implantation in rat. The same nerve as on figure 3. Slides A and B: Neurofilament staining; C and D: S-100 staining. Left 10×, right 40×. Inflammatory tissue surrounding implant is visible. It is thickened on side neighboring muscle. Alginate deposits are present in that tissue visible as colorless areas in tissue around the tube. Infiltrating tissue growing around those deposits is markedly more mature with greater number of nerve specific elements. Multiple Schwann cells with accompanying axons are noted in implant surrounding inflammation, mostly between alginate deposits and in the thickening of infiltration. Scale bars 200 μm.
In all cases when the tubes were filled with alginate fibers or alginate gels, the alginate deposits were found inside the tube and in inflammatory infiltration surrounding the tubes. The volume of outside-the-tube alginates increased over longer observation periods with a corresponding decrease inside the tubes. Most of the alginates were observed around the tube ends, more around the distal end. Enhanced distal evacuation was probably caused by gravitation, the lower position of the rear limb in rats and the direction of muscle contraction. In every observation period inflammatory infiltration around alginate deposits was less profound and its organization was markedly faster than in tissues with no alginates. This infiltrate organization was increasing with longer observation periods providing new tissue formation around alginate deposits. Evident colonization of this new tissue with neural elements, Schwann cells and axons, was markedly greater around alginates. Axons were found typically in tissues with alginates, and only small number was visible in other areas of infiltrate. Infiltrate distal from alginate deposits remained it’s inflammatory character and it’s transformation in new tissue was significantly lower (Figure 5).
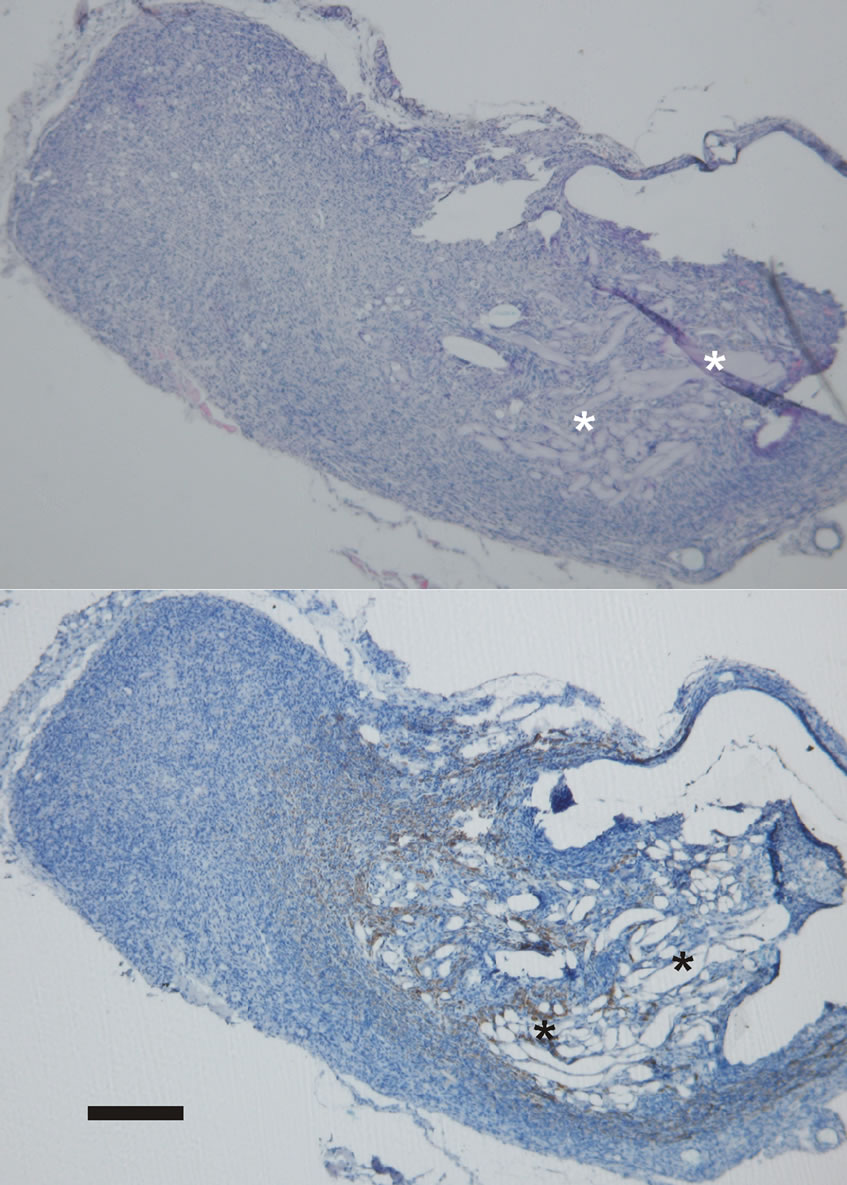
Figure 5. Alginate residues in tissue growing around tube graft visible as amorphous substance. Inflammatory infiltration around those deposits is more organized, new tissue creation is visible with less inflammation typical cells. NF positive cells tend to colonize areas close to alginate deposits. Upper photograph HE staining, lower photograph NF immunostaining, asterisk—alginates, scale bar 400 μm.
3.2. Histomorphometric Analysis
Neurofillament-positive nerve fibers were found in tissue growing on the external walls of the proximal sections of PLDL and PU tubes. The number of the fibers was greater in PLDL group, but markedly lower comparing to autograft group. This difference was more prominent in distal parts of the graft, where autograft group revealed advanced nerve regeneration process and greater number of nerve fibers (Table 1).
4. Discussion
The process of peripheral nerve regeneration in tube nerve grafts is significantly affected by the gap length. Depending on the different animal model and biomaterial used, the length of this gap can be different, but typically it is very short. Secondly, longer empty implants tend to collapse due to surrounding tissue pressure. Internal tube filling is needed to provide a scaffold for the regenerating nerve and to prevent tube collapse. Alginate fibers and gels were introduced into the implants to investigate how the inner tube filling with this biomaterial influences the regeneration processes. To estimate the usefulness of the chosen polymers for tube graft construction we have focused mainly on the observation of the regenerating tissue and the processes occurring in the implantation site. As this was a short-time preliminary research we have decided not to introduce electrophysiologic, functional or behavioral examinations. In these tests changes start to be clearly noticeable after more than 4 weeks observations [39-42]. Polymer tubes were made of three different polymers, namely: terpolimer, polilactide and polyurethane. Polymers were chosen according to the differences in their mechanical properties and biodegradability. Terpolymer and polyurethane are soft and elastic, while polylactide is very rigid. On the other hand, terpolymer is not biodegradable or resorbable, while PLDL and PU were biodegradable with different times of biodegradetion. According to the data from the producers, biodegradation of PLDL occurs in 4 to 6 months, and PU in ~3 years. All three polymers are anticipated to be biologically inert.
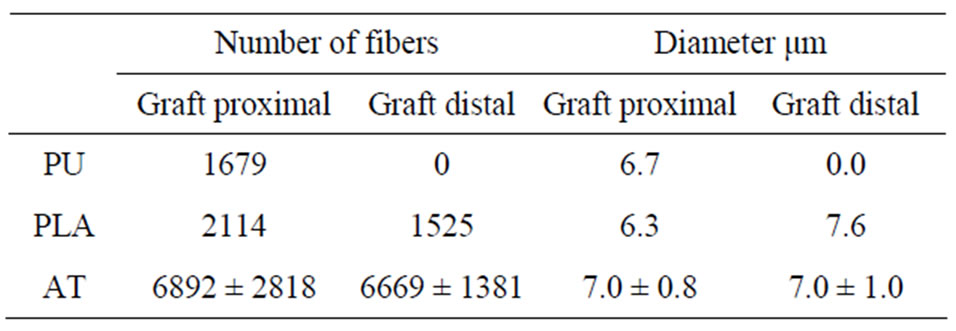
Table 1. Mean number and diameter of axons in proximal and distal part of implants and nerve autograft. 4 weeks observation period. (PU—polyurethane implant group, PLDL— polylactide implant group, AT—nerve autotransplantation group).
Terpolimer tubes were highly elastic and showed good shape memory. Their highly elastic character guaranteed no tension effect on the stumps. Polylactide tubes were very rigid and resistant to mechanical load, but at higher loads they could crack, and lacked the ability to restore their shape. High stiffness of tube walls made atraumatic microsurgical implantation almost impossible due to the great force needed for stitching and suturing. Mechanical properties of polyurethane tubes, in terms of softness and elasticity, were between those of terpolimer and polylactide. These tubes were more resistant to compression than terpolymer, but soft and elastic enough to allow comfort in operating with them during surgery, they also showed very good shape memory.
To secure the mechanical stability of the tubes and provide a scaffold for regenerating tissue, alginates were placed inside the implants. Tubes filled with alginate gel blocked tissue growth inside the tube most probably due to its thickness. To change this process sodium alginate in the form of solid fibers were applied but their fast transformation into gel was observed. In spite of the literature data showing the usefulness of alginate in the processes of nerve regeneration [31-38], our study showed that alginate gel blocked cellular colonization and most importantly Schwann cell migration inside the tubes. Due to this process neural tissue growth and organization was observed mainly on the external surface of the tubes within inflammatory infiltration. Within this tissue migration of Schwann cells and axons with a fast organization of infiltration was observed. It has been described by Matsuura [32], Nawarro [19,30] or Hashimoto [31] that regenerating tissue colonizes alginate gel, not through its porosity but through its partially in vivo degraded form. Those authors described hydrolytic degradation to progresses from the perimeter to the inside of the implant enabling scaffold replacement by tissue. In our study alginate gel, and fibres transformed into gel, appeared to inhibit penetration of the tubes with ingrowing tissue. It proved the negative effect of a dense scaffold of nerve growth as described previously by Meek [43]. After evacuation from the tube into external tissues alginate gel was able to dissolve freely. Due to that process thickness and structure of alginates in these deposits changed and became appropriate for nerve regeneration [31,32,44,45]. Marked neural tissue formation was found in tissues surrounding alginate deposits. This neuroregeneration process was similar to neuroregeneration around free alginate implants described by Matsuura and others [19,30-32], Figures 3 and 4. It indicates importance of alginate degradation to support nerve regeneration. We have found a diminishing effect on inflammatory reaction in tissues with alginate deposits: inflammatory cells were less numerous and faster organization of inflammation was observed. Schwann cells and axons were infiltrating this tissue more quickly and in greater numbers. This influence expressed in greater number of axons growing on the external surface of PLA tubes than PU tubes, Table 1. Slightly greater number of axons in PLA group was probably connected with greater alginate evacuation from the tube’s inner space and greater amounts of alginate deposits in tube’s surrounding tissue. This alginates migration process from the tube’s inner space into surroundings was enhanced by PLDL tubes porosity. The lack of porosity in polyurethane tubes blocked fast alginate diffusion from the inner area of tubes and it hampered tissue ingrowth [17,30]. Neural tissue growth inside the tube was also found, but during the longest observation period and after a great part of the alginate gel had been evacuated from the tube into its surroundings. This tissue was also mixed with alginates, Figure 4.
Schwann cell migration into tissue growing around the tubes was more prominent from the distal nerve stump. Process of neural tissue formation within inflammatory infiltration was similar to one observed by Terada, Zao and other authors [13,23,31,46]. The coexistence of inflammatory cells, like granulocytes, plasmatic cells and macrophages with Schwann cells and regenerating axons was clearly noticeable. It seems that inflammatory infiltration has a stimulating effect on neuroregeneration, however the mechanism of this interaction is unclear. Such coexistence has also been found also previously described [13,28,31,47-50]. On the basis of these observations it can be assumed that inflammation positively influences the neuroregeneration process. The most intensive regeneration of neural tissue was found in areas adjacent to muscles neighboring the implantation, Figures 3 and 4. This well vascularized tissue was a great source of nutritional factors that facilitated tissue creation and enhanced nerve regeneration. Positive neurofilament and S-100 staining in the same areas of adjacent slices indicated the simultaneous growth of axons and Schwann cells. It may suggest that nerve tissue growth occurred simultaneously with its maturation. The absence of axons in the distal nerve stump corresponds to nerve regeneration time described as long as even 6 weeks for 10 mm gaps [13].
Findings of this preliminary study have shoved a very interesting influence of alginates on neuroregeneration process. This influence appears to be greatly dependent of physical form of this biomaterial but also local tissue environment. Free hydratation and dissolution conditions may be most important in order achieve and maintain alginate stimulating influence on neuroregeneration.
5. Conclusion
All investigated implants were biocompatible and did not show any toxic character or caused any reaction to foreign bodies in the organism in any observational period. Filling the tubes with alginate gel impeded the regeneration process, and the new tissue appeared mainly on the outer surface of the implant. Remodeling of this extracellular matrix scaffold and prolonging its time of hydration and gelation is necessary. Longer degradation times and a fibrous form could create a scaffold similar to the fascicular anatomy of the nerve with a great surface area for ingrowing tissue. Larger series study is necessary to investigate disolving conditions on alginate influence on neuroregeneration process. In the case of all investigated polymers, connection of the injured nerve with the tubular bridge was found to be a guiding construct for Schwann cells and following them axons. No considerable differences in regenerating process was noticed, but taking the number of newly created axons polylactide tubes were the most efficient. The implants, however, significantly differed with mechanical properties responsible for surgical handiness. Polyurethane tubes were found to have optimal properties for their elasticity, softness, surgical handiness, slow biodegradeability, and biocompatibility.
6. Acknowledgements
This work was supported by the Polish Ministry of Science and Higher Education, Grant No. N N507342733.
REFERENCES
- L. B. Dahlin and J. Brandt, “Basic Science of Peripheral Nerve Repair: Wallerian Degeneration/Growth Cones,” Operative Techniques in Orthopaedics, Vol. 14, No. 3, 2004, pp. 138-145. doi:10.1053/j.oto.2004.06.004
- W. Z. Ray and S. E. Mackinnon, “Management of nerve gaps: Autografts, allogratfs, nerve transfers, and endto-end neuroraphy,” Experimental Neurology, Vol. 223, No. 1, 2010, pp. 203-206. doi:10.1016/j.expneurol.2009.03.031
- T. M. Myckatyn and S. E. Mackinnon, “Surgical Techniques of nerve grafting (Standard/Vascularized/Allograft),” Operative Techniques in Orthopaedics, Vol. 14, No. 3, 2004, pp. 171-178. doi:10.1053/j.oto.2004.06.007
- S. Stahl and J. A. Goldenberg, “The use of vein grafts in upper extremity nerve surgery,” European Journal of Plastic Surgery, Vol. 22, No. , 1999, pp. 255-259. doi:10.1007/s002380050199
- G. Risitano, G. Cavallaro, T. Merrino, S. Coppolino and F. Ruggeri, “Clinical results and thoughts on sensory nerve repair by autologous vein Graft in emergency hand reconstruction,” Chirurgie de la Main, Vol. 21, No. 3, 2002, pp. 194-197. doi:10.1016/S1297-3203(02)00109-9
- D. Szarek, W. Jarmundowicz, A. Frączek and S. Błażewicz, “Biomaterials in the treatment of peripheral nerve injuries—an overview of methods and materials,” Engineering of Biomaterials, Vol. 56-57, 2006, pp. 40-53.
- J. Laska, A. Frączek, H. Yolsal, S. Błażewicz, D. Szarek, A. Sobczak and J. Chłopek, “Manufacturing and Characterization of polimer tubes designer for nerve regeneration,” Engineering of Biomaterials, Vol. 58-60, 2006, pp. 164-165.
- L. J. Chamberlain, I. V. Yannnas, A. Arrizabalaga, H.-P. Hsu, T. V. Norregaard and M. Spector, “Early nerve healing in collagen and silicone tube implants: myofibroblasts and the cellular response,” Biomaterials, Vol. 19, No. 15, 1998, pp. 1393-1403. doi:10.1016/S0142-9612(98)00018-0
- K. Jansen, J. F. A. van der Werff, P. B. van Wachem, J. P. A. Nicolai, L. F. M. H. de Leij and M. J. A. Luyn, “A hyaluronian-based nerve guide: In vitro cytotoxicity, subcutaneous tissue reactions, and degradation in the rat,” Biomaterials, Vol. 25, No. 3, 2004, pp. 483-489. doi:10.1016/S0142-9612(03)00544-1
- T. Nakamura, Y. Inada, S. Fukuda, M. Yoshitani, A. Nakada, S. Stoi, S. Kanemaru, K. Endo and Y. Shimizu, “Expermental study on the regeneration of peripheral nerve gaps through a poluglycolic acid-collagen (PGAcollagen) tube,” Brain Research, Vol. 1027, No. 1-2, 2004, pp. 18-29. doi:10.1016/j.brainres.2004.08.040
- K. Matsumoto, K. Ohnishi, T. Kiyotani, T. Sekine, H. Ueda, T. Nakamura, K. Endo and Y. Shimizu, “Peripheral nerve regeneration across an 80-mm gap bridged by a polyglicolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves,” Brain Research, Vol. 868, No. 2, 2000, pp. 315-328. doi:10.1016/S0006-8993(00)02207-1
- D. J. Terris, E. T. Cheng, D. S. Utley, D. M. Tarn, P.-R. Ho and A. N. Verity, “Functional recovery following nerve injury and repair by silicon tubulization: comparison of laminin-fibronectin, dialyzed plasma, collagen gel, and phosphate buffered solution,” Auris Nasus Larynx, Vol. 26, No. 2, 1999, pp. 117-121. doi:10.1016/S0385-8146(98)00067-4
- Q. Zhao, G. Lundborg, N. Danielsen, L. M. Bjursten and L. B. Dahlin, “Nerve regeneration in a ‘pseudo-nerve’ graft created in a silicone tube,” Brain Research, Vol. 769, No. 1, 1997, pp. 125-134. doi:10.1016/S0006-8993(97)00620-3
- A. M. Moore, R. Kasukurthi, C. K. Magill, F. Farhadi, G. H. Borschel and S. E. Mackinnon, “Limitations of Conduits in Peripheral Nerve Repairs,” Hand, Vol. 4, No. 2, 2009, pp. 180-186. doi:10.1007/s11552-008-9158-3
- B. Schlosshauer, L. Dreesmann, H.-E. Schaller and N. Sinis, “Synthetic nerve guide implants in humans: a comprehensive survey,” Neurosurgery, Vol. 59, No. 4, 2006, pp. 740-748. doi:10.1227/01.NEU.0000235197.36789.42
- H. Wang and W. C. Lineaweaver, “Nerve conduits for nerve reconstruction,” Operative Techniques in Plastic and Reconstructive Surgery, Vol. 9, No. 2, 2003, pp. 59- 66. doi:10.1016/S1071-0949(03)90011-4
- F. J. Rodriguez, N. Gomez, G. Perego and X. Navarro, “Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps,” Biomaterials, Vol. 20, No. 16, 1999, pp. 1489-1500. doi:10.1016/S0142-9612(99)00055-1
- J. Cai and X. Peng, “Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation,” Journal of Biomedical Meterials Research Part A, Vol. 75, No. 2, 2005, pp. 374-386. doi:10.1002/jbm.a.30432
- X. Navarro, E. Verdu, F. J. Rodriguez and D. Ceballos, “Artificial nerve graft for the repair of peripheral nerve Injuries,” Neurological Sciences, Vol. 22, 2001, pp. 7-13. doi:10.1007/s100720170003
- S. A. Busch and J. Silver, “The role of extracellular matrix in CNS regeneration,” Current Opinion in Neurobiology, Vol. 17, No. 1, 2007, pp. 120-127. doi:10.1016/j.conb.2006.09.004
- N. Terada, L. M. Bjurstem, M. Papaloїzos and G. Lundborg, “Resorbable filament structures as a scaffold for matrix formation and axonal growth in artificial nerve grafts: long term observations,” Restorative Neurology and Neuroscience, Vol. 11, No. 1-2, 1997, pp. 65-69.
- G. Lundborg, L. Dahlin, D. Dohi, M. Kanje and N. Terada, “A new type of ‘bioartificial’ nerve graft for bridging extended defects in nerves,” The Journal of Hand Surgery, Vol. 22, No. 3, 1997, pp. 299-303. doi:10.1016/S0266-7681(97)80390-7
- N. Terada, L. M. Bjursten and G. Lundborg, “The role of macrophages in bioartificial nerve grafts based on resorbable guiding filament structures,” Journal of Materials Science: Materials in Medicine, Vol. 8, No. 6, 1997, pp. 391-394. doi:10.1023/A:1018593219113
- W. Hu, J. Gu, A. Deng and X. Gu, “Polyglycolic Acid Filaments Guide Schwann Cell Migration in Vitro and in Vivo,” Biotechnology Letters, Vol. 30, No. 11, 2008, pp. 1937-1942. doi:10.1007/s10529-008-9795-1
- T. Arai, G. Lundborg and L. B. Dahlin, “Bioartificial nerve graft for bridging extended nerve defects in rat sciatic nerve based on resorbable guiding filaments,” Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery, Vol. 34, No. 2, 2000, pp. 101- 108. doi:10.1080/02844310050159936
- J. L. Drury and D. J. Mooney, “Hydrogels for tissue engineering: scaffold design variables and applications,” Biomaterials, Vol. 24, No. 24, 2003, pp. 4337-4351. doi:10.1016/S0142-9612(03)00340-5
- M. B. Bunge, K. Williams, P. M. Wood, J. Uitto and J. J. Jeffrey, “Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation,” The Journal of Cell Biology, Vol. 84, No. 1, 1980, pp. 184-202. doi:10.1083/jcb.84.1.184
- H. Fansa, W. Schneider and G. Keilhoff, “Revascularization of Tissue-Engineered Nerve Grafts and Invasion of Macrofages,” Tissue Engineering, Vol. 7, No. 5, 2001, pp. 519-524. doi:10.1089/107632701753213147
- J. A. Rowley, G. Madlambayan and D. J. Mooney, “Alginate hydrogels as synthetic extracellular matrix materials,” Biomaterials, Vol. 20, No. 1, 1999, pp. 45-53. doi:10.1016/S0142-9612(98)00107-0
- R. O. Labrador, M. Buti and X. Nawarro, “Peripheral nerve repair: role of agarose matrix density on functional recovery,” Neuroreport, Vol. 6, No. 15, 1995, pp. 2022-2026. doi:10.1097/00001756-199510010-00017
- T. Hashimoto, Y. Suzuki, M. Kitada, K. Kataoka, S. Wu, K. Suzuki, K. Endo, Y. Nishimura and C. Ide, “Peripheral nerve regeneration through alginate gel: analysis of early outgrowth and late increase in diameter of regenerating axons,” Experimental Brain Research, Vol. 146, No. 3, 2002, pp. 356-368. doi:10.1007/s00221-002-1173-y
- S. Matsuura, T. Obara, N. Tsuchiya, Y. Suzuki and T. Habuchi, “Cavernous Nerve Regeneration by Biodegradable Alginate Gel Sponge Sheet Placement without Sutures,” Urology, Vol. 68, No. 6, 2006, pp. 1366-1371. doi:10.1016/j.urology.2006.09.051
- K. Kataoka, Y. Suzuki, M Kitada, K. Ohnishi, K. Suzuki, M. Tanihara, C. Ide, K. Endo and Y. Nishimura, “Alginate, a bioresorbable material derived from brown seaweed, enhances elongation of amputated axons of spinal cord in infant Rats,” Journal of Biomedical Materials Research, Vol. 54, No. 3, 2001, pp. 373-384. doi:10.1002/1097-4636(20010305)54:3<373::AID-JBM90>3.0.CO;2-Q
- Y. Suzuki, M. Tanihara, K. Ohnishi, K. Suzuki, K. Endo and Y. Nishimura, “Cat peripheral nerve regeneration across 50 mm gap repaired with a novel guide composed of freeze-dried alginate gel,” Neuroscience Letters, Vol. 259, No. 2, 1999, pp. 75-78. doi:10.1016/S0304-3940(98)00924-0
- Y. Suzuki, M. Kitaura, S. Wu, K. Kataoka, K. Suzuki, K. Endo, Y. Nishimura and C. Ide, “Electophysiological and horseradish peroxidase-tracing studies of regeneration through alginate-filled gap in adult rat spinal cord,” Neuroscience Letters, Vol. 318, No. 3, 2002, pp. 121-124. doi:10.1016/S0304-3940(01)02359-X
- K. Suzuki, Y. Suzuki, K. Ohnishi, K. Endo, M. Tanihara and Y. Nishimura, “Regeneration of transected spinal cord in young adult rats using freeze-dried alginate gel,” Neuroreport, Vol. 10, No. 14, 1999, pp. 2891-2894. doi:10.1097/00001756-199909290-00003
- K. Kataoka, Y. Suzuki, M. Kitada, T. Hashimoto, H. Chou, H. Bai, M. Ohta, S. Wu, K. Suzuki and C. Ide, “Alginate enhances elongation of early regenerating axons in spinal cord of young rats,” Tissue Engineering, Vol. 10, No. 3-4, 2004, pp. 493-504. doi:10.1089/107632704323061852
- P. Pranga, R. Mullerb, A. Eljaouharib, K. Heckmannb, W. Kunzb, T. Weberc, C. Faberc, M. Vroemena, U. Bogdahna and N. Weidnera, “The promotion of oriented axonal regrowth in the injured spinal cord by alginatebased anisotropic capillary hydrogels,” Biomaterials, Vol. 27, No. 19, 2006, pp. 3560-3569.
- J. R. Dijkstra, M. F. Meek, P. H. Robinson and A. Gramsbergen, “Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex,” Journal of Neuroscience Methods, Vol. 96, No. 2, 2000, pp. 89-96. doi:10.1016/S0165-0270(99)00174-0
- R. Koka and T. A. Hadlock, “Brief communication quantification of functional recovery following rat sciatic nerve transaction,” Experimental Neurology, Vol. 168, No. 1, 2001, pp. 192-195. doi:10.1006/exnr.2000.7600
- A. E. Beris, K. K. Naka, A. Skopelitou, I. Kosta, V. Vragalas, S. Konitsiotis, E. Bontioti and P. N. Soucacos, “Functional assessment of the rat sciatic nerve following intraoperative expansion: the effect of recovery duration on behavioural, neurophysiological, and morphological measures,” Microsurgery, Vol. 17, No. 10, 1996, pp. 568577. doi:10.1002/(SICI)1098-2752(1996)17:10<568::AID-MICR7>3.0.CO;2-M
- C. L. A. M. Vleggeert-Lankamp, “The role of evaluation methods in the assessment of peripheral nerve regeneration through synthetic conduits: a systematic review,” Journal of Neurosurgery, Vol. 107, No. 6, 2007, pp. 1168-1189. doi:10.3171/JNS-07/12/1168
- M. F. Meek, P. H. Robinson, I. Stokroos, E. H. Blaauw, G. Kors and W. F. A. den Dunne, “Electronmicroscopical evaluation of short-term nerve regeneration through a thin-walled biodegradable poly(DLLA-ε-CL) nerve guide filled with modified denatured muscle tissue,” Biomaterials, Vol. 22, No. 10, 2001, pp. 1177-1185. doi:10.1016/S0142-9612(00)00340-9
- K. H. Bouhadir, K. Y. Lee, E. Alsberg, K. L. Damm, K. W. Anderson and D. J. Mooney, “Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering,” Biotechnology Progress, Vol.17 , No. 5, 2001, pp. 945-950. doi:10.1021/bp010070p
- M. S. Shoichet, R. H. Li, M. L. White and S. R. Winn, “Stability of Hydrogels Used in Cell Encapsulation: An in Vitro Comparison of Alginate and Agarose,” Biotechnology and Bioengineering, Vol. 50, No. 4, 1996, pp. 374-381. doi:10.1002/(SICI)1097-0290(19960520)50:4<374::AID-BIT4>3.0.CO;2-I
- T. Hausner, R. Schmidhammer, S. Zandieh, R. Hopf, A. Schultz, S Gogolewski, H. Hertz and H. Redl, “Nerve regeneration using tubular scaffolds from biodegradable polyurethane,” Acta Neurochirurgica Supplementum (How to Improve the Results of Peripheral Nerve Surgery), Vol. 100, 2007, pp. 69-72.
- G. Stoll, J. W. Griffin, C. Y. Li and B. D. Trapp, “Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation,” Journal of Neurocytology, Vol. 18, No. 5, 1989, pp. 671-683. doi:10.1007/BF01187086
- W. Brück, “The Role of Macrophages in Wallerian Degeneration,” Brain Pathology, Vol. 7, No. 2, 1997, pp. 741-752. doi:10.1111/j.1750-3639.1997.tb01060.x
- L. R. Williams, F. M. Longo, H. C. Powell, G. Lundborg and S. Varon, “Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for Bioassay,” The Journal of Comparative Neurology, Vol. 218, No. 4, 1983, pp. 460-470. doi:10.1002/cne.902180409
- J. M. Le Beau, M. H. Ellisman and H. C. Powell, “Ultrastructural amd morphometric analysis of long-term peripheral nerve regeneration through silicone tubes,” Journal of Neurocytology, Vol. 17, No. 2, 1988, pp. 161- 172. doi:10.1007/BF01674203

