Journal of Minerals and Materials Characterization and Engineering
Vol.1 No.4(2013), Article ID:34033,7 pages DOI:10.4236/jmmce.2013.14024
Preparation of Nano-TiO2 Thin Film Using Spin Coating Method
1Department of Materials Science and Engineering, Obafemi Awolowo University, Ile Ife, Nigeria
2Engineering Materials Development Institute, Akure, Nigeria
Email: *daniyanayodele@yahoo.com
Copyright © 2013 Ayodele Abeeb Daniyan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 3, 2013; revised May 5, 2013; accepted May 15, 2013
Keywords: Titanium Dioxide; ITO; Spin Coating; Absorption; Band Gap
ABSTRACT
This paper presented the preparation of TiO2 thin film on empty glass and Indium Tin Oxide (ITO) glass by spin coating method. Highly transparent titanium oxide thin films were obtained. The Optical absorption and transmission of the film prepared from both the synthesized and the commercially available TiO2 were measured by the UV-Visible Spectrophotometer. A sharp absorption edge of the TiO2 film was observed. The estimated energy band gap for the film of the commercially available sample was larger than that of the synthesized one.
1. Introduction
Nanotechnology is a part of science and technology that is about the control of matter on the atomic and molecular scale—this means things that are about 100 nanometres or smaller. Nanotechnology includes making products that use these small parts such as electronic devices, catalysts, sensors, etc. Some of the most current research and development activities are in the area of functional nanotechnology. This term also describes applications in which material nanostructures are used to produce optical electronics or magnetic properties [1].
Titanium dioxide (also known as titanium (IV) oxide or Titania) is the naturally occurring oxide of titanium with chemical formula TiO2. It has a wide range of applications, from paint to sunscreen to food colouring. Titanium dioxide (TiO2) has attracted significant attention from researchers because of the many interesting physical and chemical properties that make it suitable for a variety of applications. For instance, TiO2 has high corrosion resistance and chemical stability, excellent optical transparency in the visible and near infrared regions as well as high refractive index that makes it useful for antireflection coatings in optical devices [2]. It has been used mostly as a pigment in paints, sunscreens, ointments toothpaste etc. Therefore, this work seeks to investigate the structural and the optical properties of synthesized nano-TiO2 for opto-electronics applications.
Recently, titanium oxide (TiO2) thin films have been emerged as one of the most promising oxide materials owing to their optical, electrical and photo electrochemical properties.
Many studies on synthesis of TiO2 and its thin films formed by conventional and advanced sol-gel processes have been reported. Previous studies indicate that the properties of TiO2 films appear to strongly depend on the process conditions and starting materials used in the processes. Therefore, many researchers have used sol-gel method to synthesize TiO2. For instance, synthesis of nano rutile TiO2 powder at low temperature by sol-gel method using Ti(OC4H9)4 and HNO3 with mean particle size of about 50 nm after calcination at 600˚C in rutile phase has been reported by [3]. Similarly, the photocatalytic activity of nano-sized TiO2 powders by sol-gel method, using Ti(OC3H7)4 (titanium isopropoxide ) and EtOH/H2O solution has been shown by [4] and the particle size obtained were 7.9 nm and 7.4 nm respectively. Likewise, [5] also studied the preparation and characterization of nano-TiO2 powder by sol-gel method whose result yielded a mean size of about 10 nm after calcination. By similar method, the microstructure control of thermally stable TiO2 obtained by hydrothermal process has been investigated by [6]. They reported 13 and 34 nm in average diameter after calcinations at 600˚C and 800˚C respectively. Furthermore, by sol gel route, the synthesis, processing and characterization of nano-TiO2 was studied by [7]. The results obtained were 5 - 10 nm in diameter after calcination at 400˚C, 22.6 - 29.3 nm after 600˚C and the average particle size of 46.2 nm was obtained after calcinations at 800˚C in pure rutile phase.
2. Experimental Procedure
1) Synthesis of TiO2 (rutile)
5 grams of chitosan powder was poured into a vessel containing 100 ml of deionized water and 5 ml of acetic acid. The mixture was stirred for 6 h at 90˚C, and finally cooled naturally to room temperature. 10 grams of this as-synthesized chitosan solution was added dropwise into a vessel containing 40 ml of acetone and 4 ml of TiCl3 solution (the precursor). Thereafter, the vessel was covered with parafilm and left at room temperature for 2 weeks. The resulting white deposit was immersed in water to dissolve the chitosan, and then the suspension was centrifuged and washed several times with deionized water and ethanol. Finally, the powder was dried at 60˚C [8].
2) Preparation of TiO2 thin film A commercially available TiO2 powder was gotten to comparatively study the synthesized TiO2 powder sample.
The commercially available TiO2 powder and the synthesized TiO2 were used to prepare TiO2 thin film on both empty glass substrate and Indium Tin Oxide (ITO) coated glass substrate using Spin coater. Prior to the preparation of TiO2 thin film, the glass substrate and the Indium Tin Oxide (ITO) coated glass substrate used were cut and ultrasonically cleaned by degreasing with acetone, methanol, rinsed in iso-propanol, kept in staining jar and allow to dry in vacuum oven.
5 mg of commercially available powder of TiO2 and synthesized sample were thoroughly mixed with 5 ml of Polyethylene oxide (PEO) solution. The resulting colloidal solution was spin coated on glass substrate and ITO at 1500 srpm. The thickness of the film were measured using the weight differential method [9].
3. Results and Discussion
The results are shown in Figures 1-11 and Table 1.
X-ray diffraction (XRD) pattern (radiation used: CuKα) of both the synthesized and commercially available powder samples are shown in Figures 1 and 2 (from the diffraction patterns). There are appreciable similarities in their structures, which showed that the two samples are of similar material. The d-values of the synthesized sample obtained in this work are in agreement with the standard d-values of rutile structure with error of about ±1. The average particle sizes of Figures 1 and 2 were calculated using Sherrer’s equation. Sherrer’s equation is as follows:
 (1)
(1)
where λ is the wavelength of X-ray (0.1540 nm), β is the full-width at half-maximum of the peak (in radian), and the θ is the Bragg’s angle of the X-ray diffraction peaks.
The sharp diffraction peaks indicated the polycrystallinity of the TiO2 powder. The broadening of some peaks observed on the synthesized sample, according to [10], the broadening could be as a result of smaller crystals. Other causes of broadening are instruments used and synthesis temperature of the powder [11]. The broadening could be improved by calcination [12]. The little shift observed in the diffraction peaks that correspond to the main peaks of the synthesized samples could be caused by particle size variation [13]. Also, the correlation between the values of diffraction angles of the synthesized sample with the commercially available one with respect to the standard value of diffraction angles of rutile structure obtained by the XRD machine (Table 1) shows that the two powder samples are of the same material. According to [13], this variation in the particle size and the type of instrument (X-ray diffractometer) used could be responsible for the differences observed in the angle between the synthesized TiO2 powder and the commercially available one, compared with the standard TiO2 powder in Table 1. Hence, the structure further confirmed the sample synthesized to be rutile.
The particle size, 15.9 nm obtained from the synthesized sample was smaller than the commercially available one (26.7 nm) and much more smaller than 50 nm, the result obtained by [3] who used sol gel method from Ti(OC4H9)4 precursor. The smaller particle size of the synthesized TiO2 makes its XRD pattern looks rougher than the XRD Pattern of the commercially available TiO2 powder. Reducing the particle size further to about 10 nm will cause the TiO2 to show amorphous pattern [12- 14].
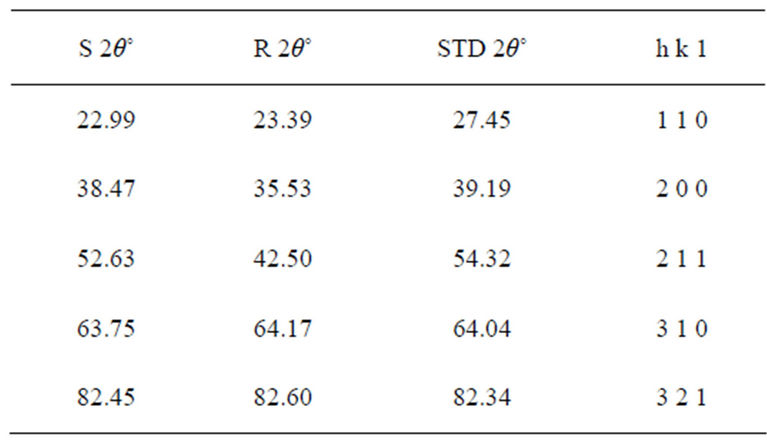
Table 1. Diffraction angles of the synthesized (S 2θ˚) commercially available TiO2 powder (R 2θ˚) and the standard (STD 2θ˚) with the lattice planes.
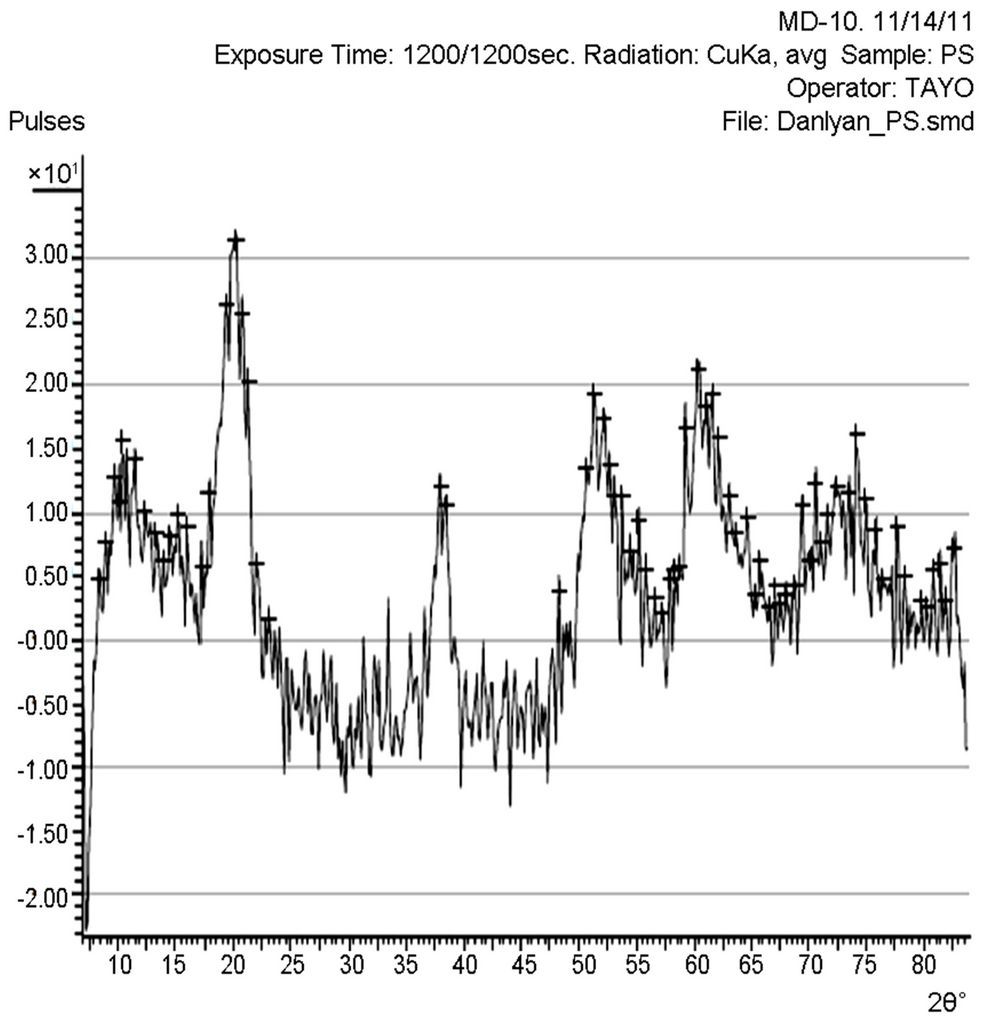
Figure 1. XRD pattern of the synthesized TiO2 powder.
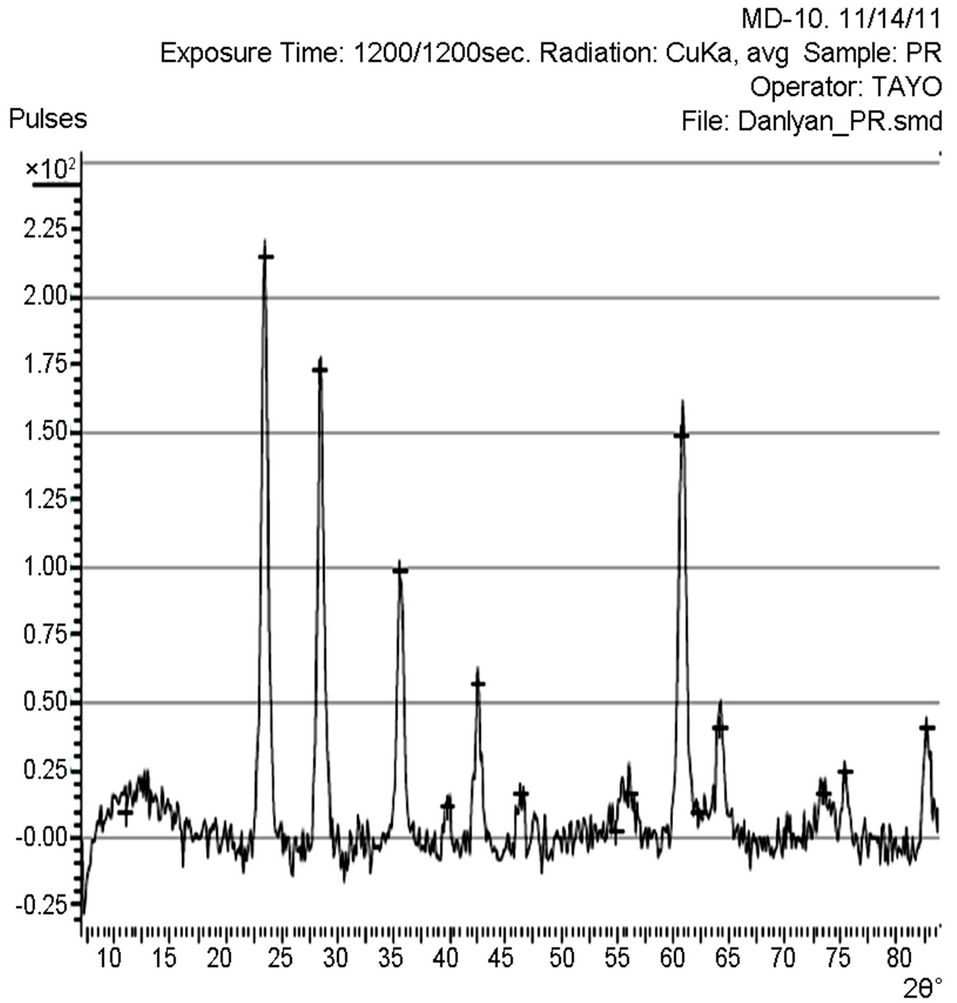
Figure 2. XRD pattern of the commercially available TiO2 powder.

Figure 3. The XRD pattern of the TiO2 film prepared from the synthesized TiO2 powder.
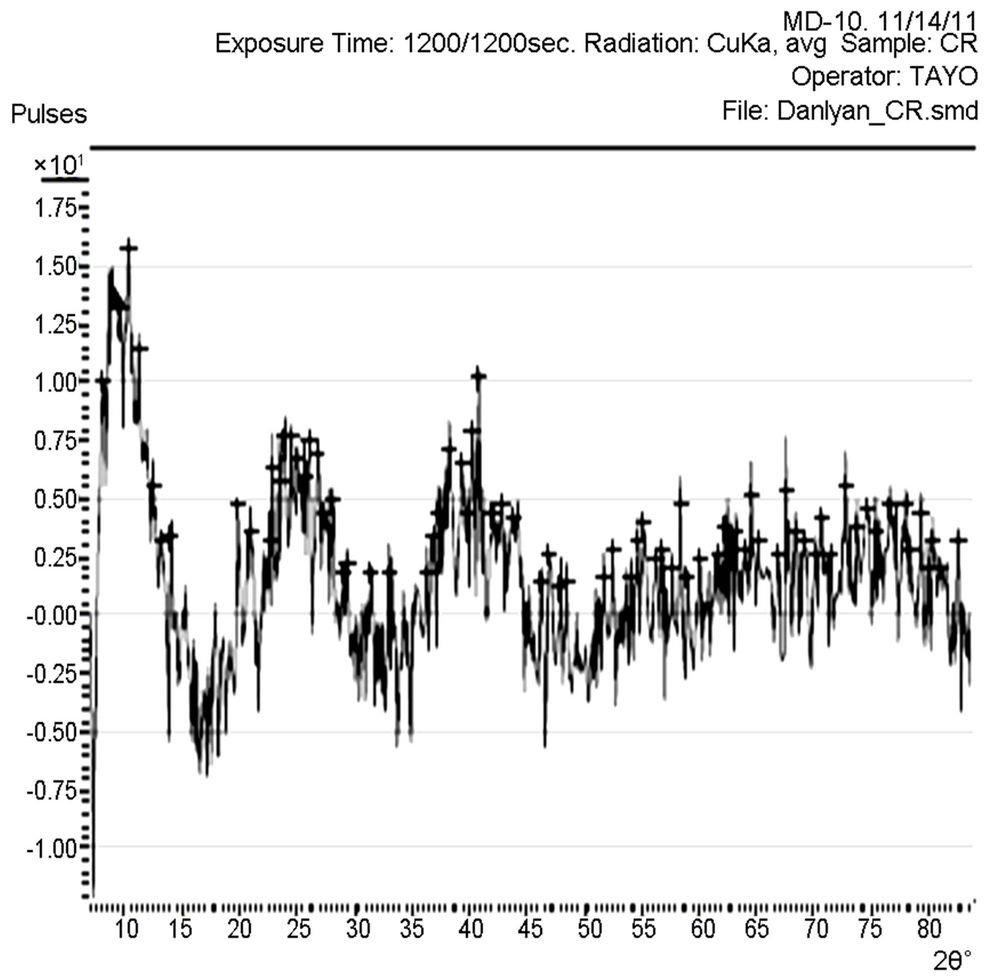
Figure 4. XRD pattern of TiO2 film prepared from the commercially available TiO2 powder.
Figure 5 shows the comparison trend among the optical transmittance plots of the polyethylene oxide (PEO), synthesized and commercially available TiO2 solution. Figures 6 and 7 show the transmittance of their thin films on both the empty glass and the ITO glass. TA represents the transmittance of the solvent used (PEO), TS represents the transmittance of the synthesized sample and TR represents the transmittance of the commercially available sample.
The optical transmission spectra of the films, for both the synthesized and the commercially available samples in Figure 5, indicate that the synthesized TiO2 possessed higher transmission in the visible region of the spectrum than the commercially available one. This can be traced to the particle size difference between the two samples. That is, the synthesized sample is of smaller size.
Figures 6 and 7 indicated that the coated thin films showed excellent transparency, about 100% over 285 and 1085 nm on the empty glass and 85% (high transparency) on the ITO glass substrate, in the visible-light region of the spectrum. More so, it was shown that, the difference observed on the film deposited on the ITO glass is slight, compared to the one on the empty glass, which is very sharp. That is, the transmittance spectrum of nano TiO2 coating on ITO substrate was quite similar to that of bare substrates. To measure the energy band gap from the absorption spectra, a graph of α2 versus E (eV) is plotted as shown in Figures 8-11.
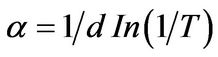 (2)
(2)
where d is film’s thickness, T is transmittance and α is the absorption coefficient.
The linear extrapolation of the plot to energy axis gives the value of the energy band gap of the film. The value obtained for the synthesized sample was 3.95 eV which agrees to 3.75 eV obtained by [13] and comparable to 3.98 eV obtained from the commercially available sample. The estimated value of the band gap for the film is larger than for the TiO2 bulk solid (3.3 eV). It was assumed, according to [15] that the increase in the band gap is due to the quantum size effect (QSE), which occurs for semiconductor particles below 100 nm. Also, this could also be inferred from the increment, 4.28 and 4.30 eV observed respectively on the ITO films. These results are also in agreement with the values reported by [13,14,16-18].
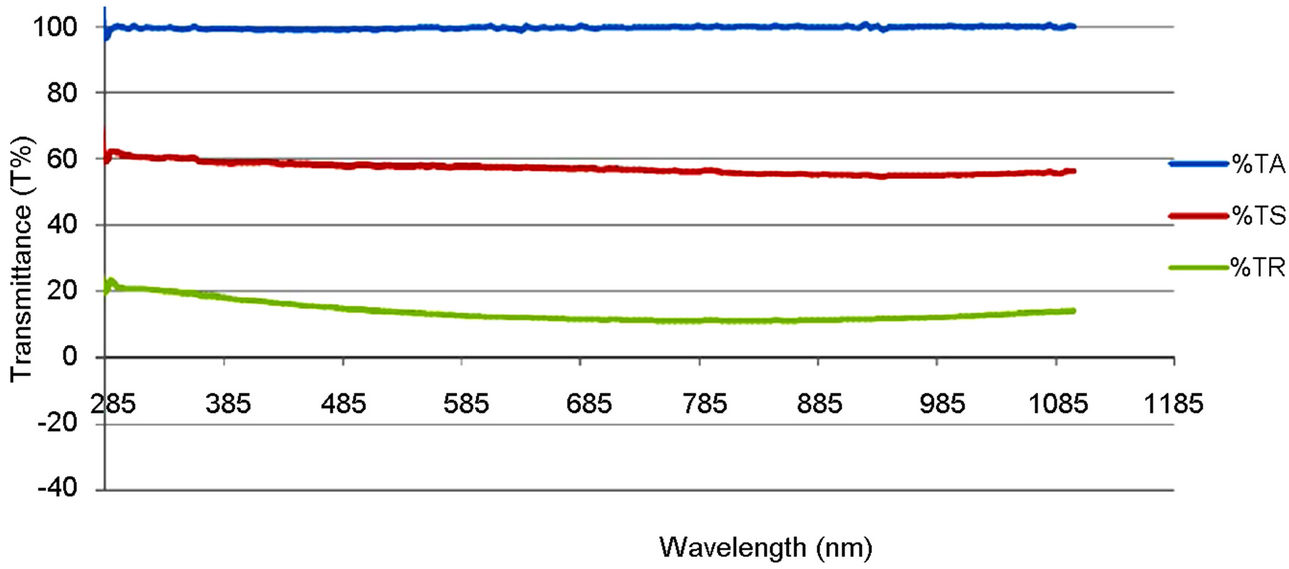
Figure 5. Optical transmittance plot of the PEO solution, synthesized and commercially available TiO2.

Figure 6. Optical transmittance plot of the PEO, the synthesized and commercially available TiO2 film.

Figure 7. Optical transmittance plot of the PEO, the synthesized and the commercially available TiO2 film on ITO glass.
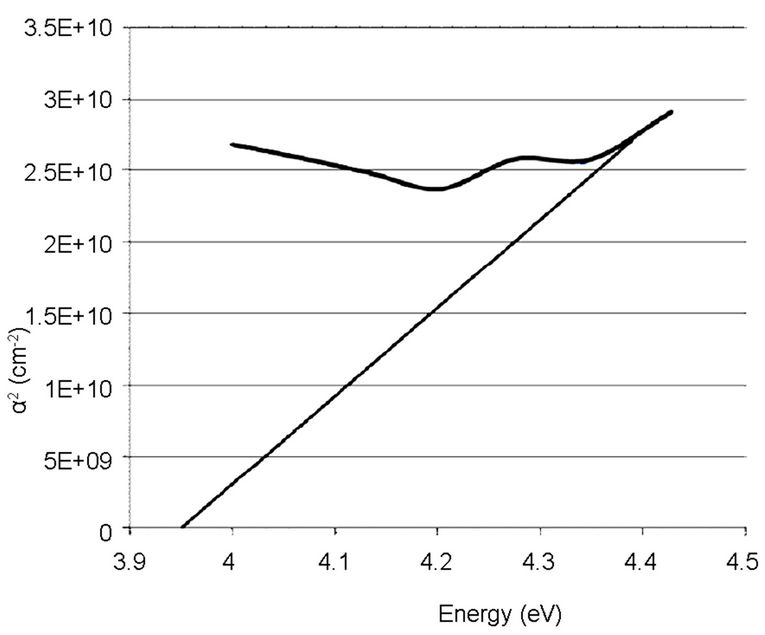
Figure 8. Absorption coefficient square (α2) plots of the synthesized TiO2 on empty glass.

Figure 9. Absorption coefficient square (α2) plots of the commercially available TiO2 on empty glass.
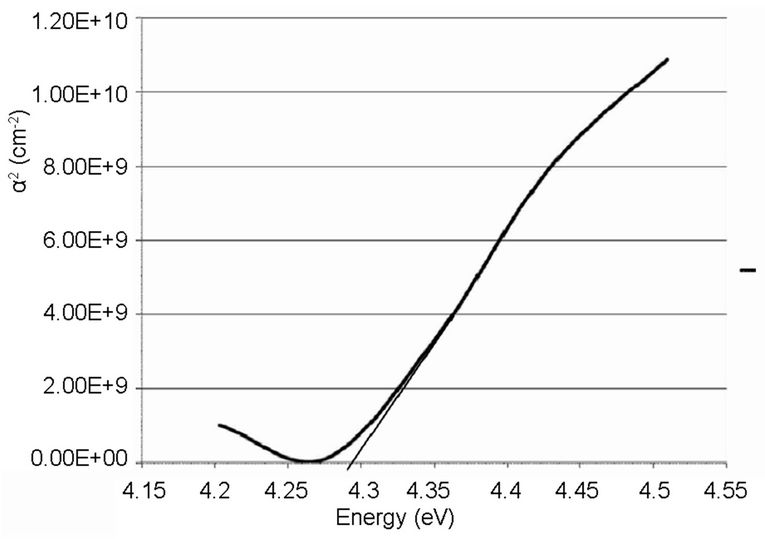
Figure 10. Absorption coefficient square (α2) plots of the synthesized TiO2 on ITO glass.
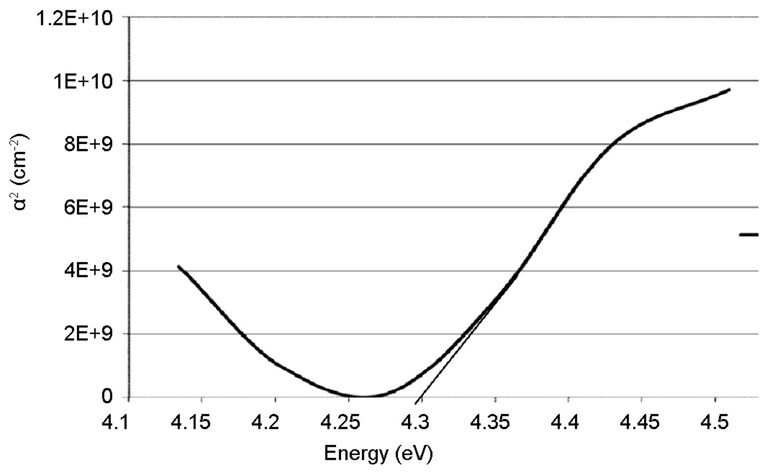
Figure 11. Absorption coefficient square (α2) plots of the commercially available TiO2 on ITO glass.
4. Conclusion
The optical behaviour of the films was investigated at room temperature. It has been found that all the films were highly transparent; they showed high transmission in the visible region of the spectrum, which reveals relatively high surface smoothness without abnormal grain growth. The analysis of the spectra also showed that the film has an estimated energy band gap of 3.95 eV on the empty glass and 4.28 on ITO substrate.
5. Acknowledgements
The authors appreciate the support provided by the management of Engineering Materials Development Institute, Akure, Nigeria where the entire bench work was carried out.
REFERENCES
- O. O. Adewoye, “Advances in Engineering Materials Development,” Proceedings of Nigeria Materials Congress, Ile-Ife, 14-17 November 2007, pp. 118-122.
- C. X. Shan, X. Hou and K. Choy, “Corrosion Resistance of TiO2 Films Grown on Stainless Steel by Atomic Layer Deposition,” Surface and Coatings Technology, Vol. 202, No. 11, 2008, pp. 2399-2402. doi:10.1016/j.surfcoat.2007.08.066
- Z. L. Tang, J. Y. Zhang, Z. Cheng and Z. T. Zhang, “Synthesis of Rutile TiO2 at Low Temperature,” Materials Chemistry and Physics, Vol. 77, No. 2, 2001, pp. 314-317. doi:10.1016/S0254-0584(02)00003-2
- J. C. Yu, J. G. Yu, L. Z. Zhang and W. K. Ho, “Photocatalytic Activity of Nano-Sized TiO2 Powders by SolGel Method, Using Titanium Tetraisopropoxide and EtOH/ H2O Solution,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 148, No. 1-3, 2002, pp. 263-271. doi:10.1016/S1010-6030(02)00052-7
- Y. Z. Li, N. H. Lee, E. G. Lee, J. S. Song and S. Kim, “Preparation and Characterization of Nano-TiO2 Powder by Sol-Gel Method,” Chemical Physics Letters, Vol. 389, No. 1-3, 2004, pp. 124-128. doi:10.1016/j.cplett.2004.03.081
- A. M. Ruiz, G. Sakai, A. Cornet, K. Shimanoe, J. R. Morante and N. Yamazoe, “Microstructure Control of Thermally Stable TiO2 Obtained by Hydrothermal Process,” Sensors and Actuators B: Chemical, Vol. 103, No. 1-2, 2004, pp. 312-317. doi:10.1016/j.snb.2004.04.061
- S. Qiu and S. Kalita, “Synthesis, Processing and Characterization of Nanocrystalline Titanium Dioxide,” Materials Science and Engineering: A, Vol. 435-436, 2006, pp. 327-332.
- S. F. Chen, J. P. Li, K. Qian, W. P. Xu, Y. Lu, W. X. Huang and S. H. Yu, “Large Scale Photochemical Synthesis of M@TiO2 Nano Composites (M = Ag, Pd, Au, Pt) and Their Optical Properties, CO Oxidation Performance, and Antibacterial Effect,” 2010. www.springerlink.com
- J. Y. Choi, K. Kim, J. Yoo and D. Kim, “Properties of Cadmium Sulphide Films Deposited by Chemical Bath Deposition with Ultrasonication,” Solar Energy, Vol. 64, No. 1-3, 1998, pp. 41-47. doi:10.1016/S0038-092X(98)00047-4
- D. S. Reddy, D. R. Reddy, B. K. Reddy, A. M. Reddy, K. R. Gunasekhar and P. S. Reddy, “Annealing Effect on Physical Properties of Thermally Evaporated MnS Nanocrystalline Films,” Journal of Optoelectronics and Advanced Materials, Vol. 9, No. 7, 2007, pp. 2019-2022.
- R. K. Wahi, Y. Liu, J. C. Falkner and V. L. Colvin, “Solvothermal Synthesis and Characterization of Anatase TiO2 Nanocrystals with Ultrahigh Surface Area,” Journal of Colloid and Interface Science, Vol. 302, No. 2, 2006, pp. 530-536. doi:10.1016/j.jcis.2006.07.003
- M. Addamo, V. Augugliaro, A. Paola, E. García-López, V. Loddo, G. Marcì and L. Palmisano, “Photocatalytic Thin Films of TiO2 Formed by a Sol-Gel Process Using Titanium Tetraisopropoxide as the Precursor,” Thin Solid Films, Vol. 516, No. 12, 2007, pp. 3802-3807. doi:10.1016/j.tsf.2007.06.139
- B. H. Kim, J. H. Ahn, J. H. Jeong, Y. S. Jeon, K. O. Jeon and K. S. Hwang, “Preparation of TiO2 Thin Film on SiO2 Glass by a Spin Coating-Pyrolysis Process,” Ceramics International, Vol. 32, No. 2, 2006, pp. 223-225. doi:10.1016/j.ceramint.2005.01.016
- C. Randorn, J. Irvine and P. Robertson, “Synthesis of Visible-Light-Activated Yellow Amorphous TiO2 Photocatalyst,” International Journal of Photoenergy, Vol. 2008, 2008, Article ID: 426872. doi:10.1155/2008/426872
- S. Ito, P. Chen, P. Comte, M. Khaja, K. Nazeeruddin, P. Liska, P. Péchy and M. Grätzel, “Fabrication of ScreenPrinting Pastes From TiO2 Powders for Dye-Sensitized Solar Cells,” Progress in Photovoltaics: Research and Applications, Published online in Wiley InterScience, 2007. www.interscience.wiley.com
- M. Addamo, V. Augugliaro, A. Paola, E. García-López, V. Loddo, G. Marcì and L. Palmisano, “Photocatalytic Thin Films of TiO2 Formed by a Sol-Gel Process Using Titanium Tetraisopropoxide as the Precursor,” Thin Solid Films, Vol. 516, No. 12, 2008, pp. 3802-3807. doi:10.1016/j.tsf.2007.06.139
- S. Ito, T. Takeuchi, T. Katayama, M. Sugiyama, M. Matsuda, T. Kitamura, Y. Wada and S. Yanagida, “Conductive and Transparent Multilayer Films for Low-Temperature-Sintered Mesoporous TiO2 Electrode of Dye-Sensitized Solar Cells,” Chemistry of Materials, Vol. 15, No. 14, 2003, pp. 2824-2828. doi:10.1021/cm021051t
- P. S. Raghupathi, J. George and C. S. Menon, “The Effect of Deposition Rate on Electrical, Optical and Structural Properties of ITO Thin Films,” E-Journal of Chemistry, Vol. 2, No. 3, 2005, pp. 171-177. doi:10.1155/2005/879524
NOTES
*Corresponding author.

