Food and Nutrition Sciences
Vol.07 No.14(2016), Article ID:72538,13 pages
10.4236/fns.2016.714122
Effect of a 12-Week Dietary Intervention with Folic Acid or Folate-Enhanced Foods on Folate Status in Healthy Egyptian Women
Mohammed E. Hefni1,2*, Mohamed T. Shalaby1, Rasha A. Mohamed3, Ahmad M. Elwa4, Cornelia M. Witthöft2
1Food Industries Department, Faculty of Agriculture, Mansoura University, Mansoura, Egypt
2Department of Chemistry and Biomedical Sciences, Linnaeus University, Kalmar, Sweden
3Faculty of Nursing, Mansoura University, Mansoura University, Mansoura, Egypt
4Clinical Pathology Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 22, 2016; Accepted: December 2, 2016; Published: December 5, 2016
ABSTRACT
The Egyptian government introduced wheat-flour fortification with iron and folic acid to reduce the incidence of neural tube defects, but suspended it for technical reasons. We previously developed novel legume foods with enhanced folate content. In this study, we investigated the efficacy of 12-week intervention with folate-en- hanced foods versus folic acid supplement in improving folate status in Egyptian women. A randomized, parallel intervention trial with two active groups (n = 19, n = 18) and one blinded control group (n = 20) was executed over 12 weeks. Volunteers received either germinated legume foods and orange juice (≈250 μg/d folate) or folic acid supplement (500 μg/d) or apple juice (0 µg/d folate). Folate status was assessed by erythrocyte and plasma folate and total homocysteine (tHcy) at day 0, and after 8 and 12 weeks of intervention. After 12 weeks, mean plasma folate increased by 14 (P < 0.0001) and 12 (P < 0.0001) nmoL in the folic acid and food group, respectively. Erythrocyte folate concentration increased in the folic acid group from 614 to 912 (P < 0.0001) and in the food group from 631 to 914 nmoL (P < 0.0001). After 12 weeks, 90% of subjects in the folic acid group and 70% in the food group had erythrocyte folate concentrations exceeding 906 nmol/L. tHcy concentration was decreased by 20% (P = 0.007) and 18% (P = 0.006) in the folic acid and food group, respectively, but remained unchanged in the control group during intervention. Folate-enhanced foods effectively improve folate status in women of reproductive age. These foods could be used as a complement to folic acid fortification.
Keywords:
Folic Acid, Folate-Enhanced Legume Foods, Human Intervention, Folate Status

1. Introduction
Dietary folate is of interest due to its protective role against fetal abnormalities such as neural tube defects (NTD) [1] . Mandatory folic acid fortification of cereal grain foods has been implemented in several countries, e.g. US [2] , Canada [3] and Chile [4] , to cover the daily recommended folate intake. Folic acid fortification effectively improves folate status [4] [5] [6] and reduces the prevalence of neural tube defects [1] [7] . In intervention studies, folate-rich foods are also reported to improve folate status [8] [9] [10] .
The Egyptian public health authorities previously introduced mandatory folic acid and iron fortification of the subsidized wheat flour used for making baladi bread (type of pita bread) [11] , with the aim of reducing the incidence of NTD (currently estimated at 4.5/1000 pregnancies; [12] ) and the prevalence of anemia (estimated to affect 30% - 50% of women of reproductive age; [13] ). The Egyptian folic acid fortification program was repeatedly interrupted for technical reasons and therefore, additional strategies for improving folate intake in the Egyptian population are required.
Legumes are recognized as important food sources of folate, while germinated legumes are reported to be an even richer folate source [14] . In previous work [15] , we developed new candidate functional foods with increased folate content that are suitable for the contemporary Egyptian diet, such as germinated canned faba beans and cookies baked with germinated chickpea flour. The aim of the present study was to investigate the effects of regular consumption of these novel folate-rich foods, in comparison with a folic acid supplement, on folate status.
2. Study Design and Methods
2.1. Subjects
Sixty-two apparently healthy women of reproductive age (19 - 32 years) were recruited in February 2013, from the student and staff population at Mansoura University, Mansoura, Egypt, and the surrounding community. After recruitment (n = 62), one subject withdrew from the folic acid group before the intervention started and two subjects each from the food and control groups withdrew after 8 weeks of the study. Subjects were deemed eligible for inclusion if the following criteria were met: no history of acute or chronic disease, no use of vitamin or mineral supplements or folic acid-fortified foods (within the past month), body mass index (BMI) >18 and <30 kg/m2, no medication interfering with folate metabolism (e.g., antiepileptic drugs, antibiotics, methotrexate, sulfasalazine, or anticonvulsants), non-smokers, not consuming a special diet (vegetarian), and no pregnancy, planned conception, or lactation. For inclusion in the trial, a normal biochemical range was required for fasting plasma glucose, iron status (hemoglobin, serum ferritin), liver status (aspartate transaminase, alanine transaminase, and γ-glutamyl transferase activity), lipid profile (triglycerides, LDL, HDL), folate status (plasma and erythrocyte folate), plasma total homocysteine (tHcy), and vitamin B-12. To prevent accidental consumption of folic acid-fortified bread during the course of the trial, all participants were given baladi bread from a private bakery (baked using non-subsidized, unfortified flour, extraction rate 82%). The subjects were asked not to donate blood one month prior to and during the intervention. The study was approved by the Institutional Review Board of the Faculty of Nursing, Mansoura University. A signed informed consent form was obtained from all subjects after informing them about the study.
2.2. Study Design
The study was designed as a randomized, controlled, parallel intervention trial with two active groups and one blind control group and ran for 12 weeks (March to June, 2013). For an increase in erythrocyte folate concentrations of 50 nmol/L with 80% power (2-sided P < 0.05) [16] , 14 subjects had to complete each intervention diet, as calculated from the standard deviations (50 nmol/L) of another intervention trial with similar doses [10] . Subjects were randomly assigned into groups by using a block design based on screening concentrations of erythrocyte folate. One active food group (n = 19; called “food folate group”) consumed folate-rich foods: germinated canned faba beans, chick pea cookies and orange juice (providing an additional 250 μg folate/d); the other active folic acid group (n = 18; called folic acid group) consumed a folic acid supplement (500 μg/d); and the blind control group (n = 20; called control group) received placebo apple juice containing no folate or folic acid (0 μg folic acid/d) (Table 1) in addition to their freely chosen diet.
2.3. Intervention Foods and Supplement
Germinated canned faba beans were produced in a single batch at the Harvest Foods Company (6th October City, Giza, Egypt). Dried faba beans were soaked and germinated for 48 h and thereafter canned [15] . The resulting product contained 45 ± 2.3 μg folate/100g fresh weight. Cookies were produced in a single batch at The Egyptian Company for Foods, “BiscoMisr” (Alexandria, Egypt) by replacement of the wheat flour with 40% germinated chickpea flour. A 100 g portion of these bio-fortified biscuits provides 85 ± 5 µg folate, compared with 15 µg/100g in normal cookies (produced from white wheat flour, extraction rate 72%). Orange juice (containing 37 ± 2 µg folate/100mL) and apple juice (0 µg folate) were each produced in a single batch at The Egyptian Canning Company “Best” (Dakahlia, Egypt), packed in individual portions (240 mL) and stored at 4˚C. Baladi bread baked from non-fortified wheat flour (extraction rate
Table 1. Folate content in intervention foods, folic acid content in supplements and intervention doses.
*Folate content according to analyses using an in-house HPLC method [15] . **According to supplier information.
82%) was produced weekly using routine procedures at a private local baladi bread bakery (Mansoura, Egypt). Folic acid tablets (containing 500 µg folic acid/tablet, Arab Co. for Pharmaceuticals & Medicinal Plants MEPACO, Sharkeiya, Egypt) were purchased from a local pharmacy in Mansoura, Egypt.
Subjects in the active food group were asked to replace the ordinary faba bean meal with the new product (germinated canned faba beans). The 200 g/day portions of canned faba beans (tin containing 150 g bean solids and 50 mL canning medium) were warmed, mashed and eaten as bean stew (traditional Egyptian dish “foul”) with bread (Table 1). The cookies (100 g/day, one box) and 245 mL orange juice (one package/day) were to be consumed at any time of the day. Subjects in the active folic acid group were asked to eat one folic acid tablet daily. The supplement containing 500 µg folic acid was the lowest available commercial dose in Egypt. In the control group, subjects were given 240 mL apple juice (Table 1), and were asked to consume it with their habitual diet. All participants were asked to replace any subsidized baladi bread with baladi bread (provided with the other foods) made from non-fortified flour during the 12 weeks of the intervention study. Participants were asked to record deviations from the diet, in particular regarding the amount of intervention foods, in a daily record, together with any medication or sickness during the intervention and their background diet.
2.4. Blood Collection and Sample Preparation
At day 0, 8 and 12 weeks, venous blood samples were collected by qualified nurses from subjects after overnight fasting (9 h) in 3.0-mL EDTA, heparin, and fluoride vacutainers (all GD Vacutainers; Zhejiang Gongdong Medical Technology Co., Ltd.). Blood samples drawn into EDTA vacutainer tubes were used for measurement of folate status (erythrocyte folate, plasma folate, plasma tHcy), hematocrit, and hemoglobin. Heparin blood tubes were used for liver status, plasma cobalamin, and ferritin determination. Fluoride blood tubes were used for blood glucose determination.
2.5. Assessment of Dietary Folate Intake before and during the Study
In order to estimate dietary folate and energy intake, all participants were asked to complete a food-frequency questionnaire (FFQ, one month recall) comprising 85 items at screening, at baseline (day 0), and every 4 weeks during the study. Dietary folate intake was estimated using the USDA database (http://ndb.nal.usda.gov) as no national food composition data for folate is available. For foods, for which no data were available in the US database, folate data determined by HPLC [17] were used. Energy was calculated using national food composition data [18] . The analyzed folate content of the intervention foods [15] was used to calculate folate doses during the intervention.
2.6. Analytical Methods
2.6.1. Quantification of Folate Content in Intervention Foods
Folate content in intervention foods was analyzed in duplicate every 4 weeks throughout the trial using HPLC-UV/FLD detection [17] . Food samples were extracted using tri-enzyme treatment for beans and di-enzyme treatment for bread, juice, and cookies. After purification, folates were quantified using RP-HPLC-UV/FLD (Shimadzu LC10, Kyoto, Japan) after separation on an Aquasil C18 column (3 µm, 150 × 4.6 mm, Thermo Scientific) based on an external multilevel (n = 8) calibration curve.
2.6.2. Assessment of Folate Status and Other Clinical Parameters
Erythrocyte folate, plasma folate, and plasma vitamin B-12 concentrations were quantified using the commercial boil, liquid-phase, competitive, ligand-labeled protein binding chemiluminescent procedure on Immulite 1000 (Immulite/Immulite 1000 Folic Acid, document PILKF; Siemens Healthcare Diagnostics, Deerfield, IL) (intraassay CV = 4%, and n = 4 interassay CV = 6%, n = 12). Erythrocyte folate was analyzed in collected whole blood samples (3.0-mL EDTA) and hematocrit was measured. Samples were diluted (1:21) with 1% freshly prepared ascorbic acid solution, allowed to incubate for 90 min at room temperature (20˚C - 28˚C), and then analyzed for erythrocyte folate. Erythrocyte folate concentration was calculated from the measured whole blood folate concentration, adjusted for red blood cell volume and corrected for plasma folate concentration (according to the manufacturer’s guidelines). For the determination of plasma folate, blood samples were immediately placed on ice and centrifuged within 60 min at 3000 g for 10 min at 4˚C. Plasma was separated and stored at −20˚C until analysis within one week. Plasma total homocysteine was quantified using an automatic biochemistry analyzer BT1500 (Biotecnica Instruments SpA, Rome, Italy).
2.7. Statistical Analysis
Data on clinical samples and food folate were expressed as mean ± SD. The absolute values for folate status and tHcy were not normally distributed, while the changes in erythrocyte folate, plasma folate, tHcy from day 0 to week 12 were normally distributed. A normality test was performed using SAS. One-way ANOVA on log-transformed data was used to analyze changes in erythrocyte folate, plasma folate, and tHcy from day 0 to week 8 or week 12. Student’s t-tests with a significance level of P < 0.05 were used to analyze differences in endpoint response between the active intervention groups and the control group. The responses of folate biomarkers (erythrocyte folate, plasma folate, tHcy) to treatments were also analyzed by within-between repeated measures ANOVA (intervention group as between-factor and different time points as within-factor). All statistical analyzes were carried out using SAS software (SAS 9.1, SAS institute Inc. Cary, NC, USA).
3. Results
3.1. Subjects and Characteristics
Screening results were used as a baseline (d 0) (as the time between the recruiting and the beginning of the study was only ≈2 weeks). The baseline characteristics did not differ significantly between groups (mean age 22 y, mean BMI 24 kg/m2; Table 2). Body
Table 2. Characteristics of study participants at screening (day 0).
*Mean energy intake of the habitual diet was estimated using FFQs and Egyptian food composition data [18] . **Folate content analyzed in the laboratory by HPLC. ***According to supplier information of tablets. ****Mean folate intake of the habitual diet was estimated using FFQs and folate data from the USDA database (http://ndb.nal.usda.gov). Screening data were used as baseline (d 0) as the time between the recruiting and the beginning of the study was only ≈2 weeks.
weight and estimated energy intake was maintained throughout the study, with changes within 5% of baseline values in all subjects (data not shown), which indicated that the subjects did not change their habitual consumption pattern and energy intake during the intervention period. Estimated folate intake based on FFQs did not differ significantly over the 12-week intervention period for the control group. In the food group, the average folate intake at baseline was estimated to 195 ± 50 µg/d (calculated by FFQ) which theoretically increased by 250 µg/d, based on analytical data on folate content in the intervention foods (germinated canned faba beans (75 µg/d), cookies (85 µg/d), and orange juice (90 µg/d) (Table 1). In the folic acid group, the level of folate intake from food at baseline was 220 ± 30 µg/d (calculated by FFQ), which was increased during the intervention by 500 µg/d folic acid from the supplement during intervention (Table 1). The baseline concentrations of fasting blood glucose, iron and liver status did not differ significantly between all groups (Table 2).
3.2. Folate Status
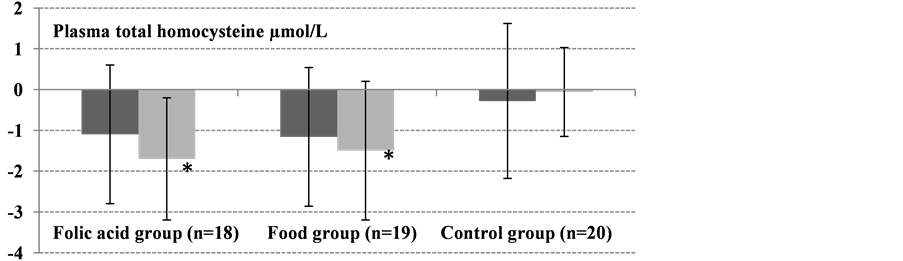
The baseline (day 0) concentrations of plasma and erythrocyte folate did not differ significantly between all groups (Table 2). However, plasma tHcy differed significantly between groups (P = 0.005). After 12 weeks of intervention, folate status (erythrocyte folate, plasma folate, and plasma tHcy) improved significantly in both active groups (Table 3, Figure 1). In the control group, there were no significant changes in concentrations of erythrocyte folate (P = 0.0534), plasma folate (P = 0.297), or plasma tHcy (P = 0.412) between day 0, 8 weeks and 12 weeks.
Mean erythrocyte folate concentration in the food group increased by 20% (P = 0.018) and 45% (P < 0.0001) after 8 and 12 weeks, respectively (Table 3, Figure 1). At day 0, the erythrocyte folate concentration in the folic acid group was 614 nmol/L and reached 788 and 912 (P < 0.0001) after 8 and 12 weeks, respectively (Table 3). No significant difference in erythrocyte folate concentrations between the two active groups (food and folic acid) was observed at week 8 (P = 0.363) or week 12 (P = 0.868).
In the food group, plasma folate concentration increased from 22 nmol/L at day 0 to 27 and 34 nmol/L after 8 (P = 0.011) and 12 weeks (P < 0.0001), respectively (Table 3). In the folic acid group, the increase in plasma folate was 8 and 14 nmol/L (P < 0.0001) after 8 and 12 weeks, respectively (Table 3). Plasma folate concentrations in both active groups did not differ significantly between week 8 (P = 0.279) and week 12 (P = 0.821) (Table 3).
The plasma tHcy concentration decreased in the folic acid group from 8.4 to 7.4 µmol/L (−12%; P = 0.226) after 8 weeks and reached 6.8 µmol/L (−20%; P = 0.007) after 12 weeks. In the food group, the tHcy concentration decreased by 1.16 µmol/L (−12%; P = 0.087) and 1.47 µmol/L (−18%; P = 0.006) after 8 and 12 weeks, respectively (Table 3). The decrease in plasma tHcy did not differ significantly between the folic acid group and the food group at weeks 8 and 12 (Table 3).
Table 3. Measured folate status (erythrocyte, plasma folate and total homocysteine) in active groups and control group during the intervention.
Values are means ± SD. The absolute values were not normally distributed, but the changes from day 0 to week 12 were. Significant difference in changes from day 0 to week 12 in the intervention groups versus changes in the control group, Student’s t-test (*P < 0.01, **P < 0.001).
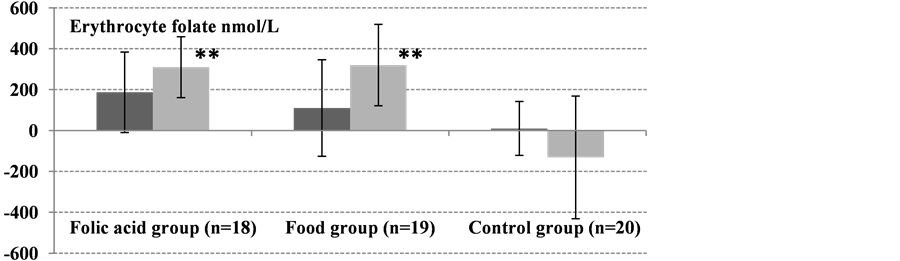
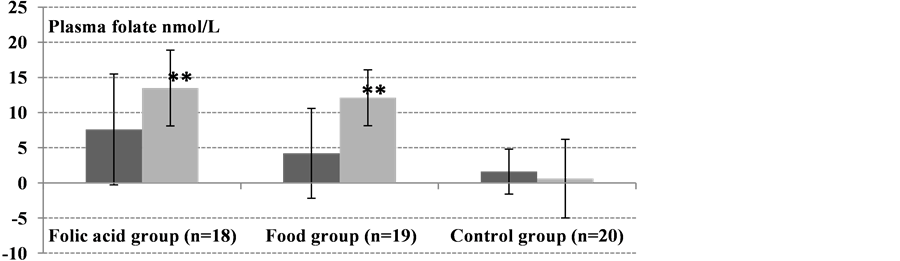
Figure 1. Absolute changes from baseline (day 0) to 8 weeks (dark gray bars) and 12-weeks (light gray bars) in erythrocyte folate, plasma folate, and plasma tHcy (all were normally distributed). Values are means ± SD. Significant differences in absolute changes between each active group, the food group (n = 19) and folic acid group (n = 18), and the control group (n = 20) were determined using Student’s t-test. No significant changes in erythrocyte folate, plasma folate, and plasma tHcy concentrations were observed from day 0 to 8 and 12 weeks in the control group (*P < 0.01, **P < 0.001).
Two-way ANOVA was applied to explore the effect of treatment and intervention time and their interaction (treatment X time) on measured folate biomarkers. There was a significant interaction between the effects of treatment and time on folate status (erythrocyte folate, plasma folate and plasma tHcy) (P ≤ 0.001).
4. Discussion
The results from this trial with healthy, non-pregnant women showed similar high bioefficacy of folate-rich foods as of synthetic folic acid in improving folate status. The findings from this trial and others [8] [9] [19] reporting improved folate status through non-fortified foods could be useful for health professionals in countries that have not implemented mandatory folic acid fortification because of the ambiguous role of synthetic folic acid in prevention and promotion of e.g. colorectal cancer or delayed diagnosis of vitamin B-12 deficiency. However, some previous intervention studies with non-fortified foods have reported no effects on different folate status markers [20] [21] . This discrepancy can be explained by differences in study design and the response parameters (e.g., different intervention times, folate doses and different folate status at the beginning of the study) used. In the present study, we measured three markers of folate status, namely changes in erythrocyte folate, plasma folate, and tHcy.
Erythrocyte folate concentration is considered a long-term indicator reflecting the risk of NTD pregnancies [22] [23] . In the present 12-week intervention study, a significant increase in erythrocyte folate was observed in both active groups. These results are in agreement with those of other trials [8] [9] [10] [24] [25] . At the end of the study, 90% of the subjects that had received 500 µg synthetic folic acid/d and 70% of the group that had received 250 µg food folate/d had erythrocyte folate concentrations above 906 nmol/L, the threshold associated with a reduced risk of NTDs [22] . Similarly, Öhrvik et al. [9] reported that 60% of subjects had median erythrocyte folate concentration >900 nmol/L after 12 weeks of intervention with a small dose of 125 µg folate from foods, which was half the amount of food folate ingested in the present study. Interventions using 400 or 200 µg folic acid supplements for 24 weeks have been reported to increase median erythrocyte folate to >906 nmol/L [22] . Another study [26] reported an increase in average erythrocyte folate concentration to above 906 nmol/L already after 8 weeks of intervention with a dose of 400 µg folic acid/d or 416 µg 5-CH3-H4 folate/day.
The present study confirmed that folate-rich foods (providing an additional dose of 250 µg food folate/d) are as effective as synthetic folic acid supplement in increasing erythrocyte folate to a safe range above 906 nmol/L.
The results from the present 12-weeks intervention with 500 µg folic acid/d and 250 µg food folate/d showed significant mean increases in plasma folate concentration of 14 and 12 nmol/L respectively, which is much higher than the increases of 5.8 and 6.5 nmol/L reported previously in subjects receiving similar doses of 250 µg folic acid/d and 350 µg food folate/d, respectively, after only four weeks of intervention [10] . Johansson et al. [5] also reported a more modest increase in plasma folate of >4 nmol/L after 4 weeks in subjects receiving 166 and 355 µg folic acid/d from fortified breakfast rolls. However, a subsequent increase in plasma folate concentration after 8 and 12 weeks of the intervention could not be quantified due to the limited quantification range of the method, but further increased erythrocyte folate concentrations showed improved folate status of subjects [5] .
Plasma tHcy can be considered a functional indicator of folate status and has been repeatedly used in studies assessing the bioefficacy of food folate and folic acid [27] [28] . It is well demonstrated that folic acid supplementation efficiently reduces tHcy concentration [5] [10] . In this study, a significant decrease in tHcy concentration of 1.7 µmol/L in the folic acid group and 1.5 µmol/L in the food folate group was observed. Similar decreases in plasma tHcy (1.5 and 1.8 µmol/L, respectively) were reported by Brouwer et al. [19] , who compared the effects of comparable doses of food folate (350 µg/d) and folic acid supplement (250 µg/d) over 4 weeks. Öhrvik et al. [9] also reported a 2.3 µmol/L decrease in tHcy after a 12-week intervention with additional 125 µg/d folate from a breakfast. However, Vahteristo et al. [6] only observed a non-significant reduction in tHcy after a 4-weeks intervention with 184 µg natural food folate and 188 µg folic acid per day. The short intervention period and the small intervention dose could explain that low response.
The potential to lower tHcy levels through dietary interventions longer than 4 weeks may be important, because elevated plasma homocysteine concentrations have been identified as an independent risk factor for cardiovascular disease [29] [30] [31] .
The quantified folate content in the intervention foods might be underestimated mainly because the sum of individual folate forms quantified by the HPLC method is generally lower than the total folate content quantified by microbiological assay [14] [32] . The selection of the intervention foods in the current trial was based on the Egyptian dietary habits [33] . Faba beans are very popular and widely consumed, commonly eaten together with baladi bread. Most (>80%) of our subjects were students and living on the campus and they received similar foods every week. They already consume faba bean stew (foul) for their habitual dinner and subjects of the food group were simply asked to replace their ordinary faba bean stew with the new product (germinated canned faba beans) and to consume the cookies and orange juice during the day. Therefore, the intervention foods did not change the subjects’ habitual diet. This was confirmed by the stability of subjects’ BMIs, which suggested only minor changes (<5%) in energy intake. The dietary folate intake was also constant during the intervention (10% variation). Compliance with supplementation was monitored in the folic acid group by use of a tablet calendar where subjects documented whether or not they took the tablet. Only three subjects had forgotten to take the supplements (on 3 - 5 single days) throughout the intervention.
5. Conclusion
The high folate content of legume foods indicates that they are a good source of folate and could be candidate functional products. In this study, we have shown that the folate-rich legume foods had similar high bioefficacy in improving folate status (based on erythrocyte folate, plasma folate, and tHcy) in healthy, non-pregnant women as a folic acid supplement and might be an adequate complement to folic acid fortification.
Acknowledgements
We are most grateful to all subjects for their participation in the study. Prof. Nazem Shalaby (Faculty of Agriculture, Mansoura University) is gratefully acknowledged for statistical advice. The folate standards used were a kind gift from Merck & Cie, Schaffhausen, Switzerland. We would like to thank the Egyptian Canning Company (Best) for producing the apple and orange juice, Harvest Foods Company (Egypt) for producing the canned germinated faba beans and the Egyptian Company for Foods BiscoMisr for producing the cookies.
Funding
This study was supported by Formas-SIDA (FormasSida Dn 222-2009-1975).
Declaration of Interest
The authors declare that they have no conflict of interest.
Cite this paper
Hefni, M.E., Shalaby, M.T., Mohamed, R.A., Elwa, A.M. and, Witthöft, C.M. (2016) Effect of a 12-Week Dietary Intervention with Folic Acid or Folate-Enhanced Foods on Folate Status in Healthy Egyptian Women. Food and Nutrition Sciences, 7, 1339-1351. http://dx.doi.org/10.4236/fns.2016.714122
References
- 1. De Wals, P., Tairou, F., Van Allen, M.I., Uh, S.-H., Lowry, R.B., Sibbald, B., Evans, J.A., Van den Hof, M.C., Zimmer, P., Crowley, M., Fernandez, B., Lee, N.S. and Niyonsenga, T. (2007) Reduction in Neural-Tube Defects after Folic Acid Fortification in Canada. The New England Journal of Medicine, 357, 135-142.
http://dx.doi.org/10.1056/NEJMoa067103 - 2. Institute of Medicine Food and Nutrition Board (1998) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B-6, Folate, Vitamin B-12, Pantothenic Acid, Biotin, and Choline. National Academy Press, Washington DC.
- 3. Health Canada (1997) Food and Drug Regulations, Amendment Schedule No. 1066. Health Canada, Ottawa.
- 4. Hertrampf, E., Cortés, F., Erickson, J.D., Cayazzo, M., Freire, W., Bailey, L.B., Howson, C., Kauwell, G.P. and Pfeiffer, C. (2003) Consumption of Folic Acid-Fortified Bread Improves Folate Status in Women of Reproductive Age in Chile. Journal of Nutrition, 133, 3166- 3169.
- 5. Johansson, M., Witthoft, C.M., Bruce, A. and Jagerstad, M. (2002) Study of Wheat Breakfast Rolls Fortified with Folic Acid. The Effect on Folate Status in Women during a 3-Month Intervention. European Journal of Nutrition, 41, 271-278.
http://dx.doi.org/10.1007/s00394-002-0388-9 - 6. Vahteristo, L., Kariluoto, S., Barlund, S., Karkkainen, M., Lamberg-Allardt, C., Salovaara, H. and Piironen, V. (2002) Functionality of Endogenous Folates from Rye and Orange Juice Using Human in Vivo Model. European Journal of Nutrition, 41, 271-278.
http://dx.doi.org/10.1007/s00394-002-0385-z - 7. Carmichael, S.L., Yang, W. and Shaw, G.M. (2010) Periconceptional Nutrient Intakes and Risks of Neural Tube Defects in California. Birth Defects Research Part A: Clinical and Molecular Teratology, 88, 670-678.
http://dx.doi.org/10.1002/bdra.20675 - 8. Fenech, M., Noakes, M., Clifton, P. and Topping, D. (2005) Aleurone Flour Increases Red-Cell Folate and Lowers Plasma Homocyst(e)ine Substantially in Man. British Journal of Nutrition, 93, 353-360.
http://dx.doi.org/10.1079/BJN20051377 - 9. Ohrvik, V.E., Olsson, J.C., Sundberg, B.E. and Witthoft, C.M. (2009) Effect of 2 Pieces of Nutritional Advice on Folate Status in Swedish Women: A Randomized Controlled Trial. American Journal of Clinical Nutrition, 89, 1053.
http://dx.doi.org/10.3945/ajcn.2008.27192 - 10. Brouwer, I.A., van Dusseldorp, M., West, C.E., Meyboom, S., Thomas, C.M.G., Duran, M., van het Hof, K.H., Eskes, T.K.A.B., Hautvast, J.G.A.J. and Steegers-Theunissen, R.P.M. (1999a) Dietary Folate from Vegetables and Citrus Fruit Decreases Plasma Homocysteine Concentrations in Humans in a Dietary Controlled Trial. Journal of Nutrition, 129, 1135-1139.
- 11. Global Alliance for Improved Nutrition (2009) WFP, MOSS and GAIN Celebrate Start of Flour Fortification in Egypt to Reduce Widespread Anemia by 28%.
http://www.ffinetwork.org/country_profiles/country.php?record=59 - 12. Temtamy, S.A., Abdel Maguid, N., Mazen, I., Ismail, S.R., Kassem, N.S. and Bassiouni, R.A. (1998) A Genetic Epidemiological Study of Malformations At Birth in Egypt. Eastern Mediterranean Health Journal, 4, 252-259.
http://apps.who.int/iris/bitstream/10665/118073/1/emhj_1998_4_2_252_259.pdf - 13. EDHS (2005) Egypt Demographic and Health Survey. National Population Council, Cairo.
- 14. Hefni, M. and Witthoft, C.M. (2014) Folate Content in Processed Legume Foods Commonly Consumed in Egypt. LWT—Food Science and Technology, 57, 337-343.
http://dx.doi.org/10.1016/j.lwt.2013.12.026 - 15. Hefni, M., Shalaby, M.T. and Witthoft, C.M. (2015) Folate Content in Faba Beans (Vicia faba L.)—Effects of Cultivar, Maturity Stage, Industrial Processing, and Bioprocessing. Food Science & Nutrition, 3, 65-73.
http://dx.doi.org/10.1002/fsn3.192 - 16. Altman, D.G. (1991) Sample Size. In: Altman, D.G., Ed., Practical Statistics for Medical Research, Chapman & Hall, London, 455-460.
- 17. Hefni, M., Ohrvik, V., Tabekha, M. and Witthoft, C. (2010) Folate Content in Foods Commonly Consumed in Egypt. Food Chemistry, 121, 540-545.
http://dx.doi.org/10.1016/j.foodchem.2009.12.044 - 18. National Nutrition Institute (NNI) (2006) Food Composition Tables for Egypt. 2nd Edition, Cairo.
- 19. Brouwer, I.A., van Dusseldorp, M., Thomas, C.M.G., Duran, M., Hautvast, J.G.A.J., Eskes, T.K.A.B. and Steegers-Theunissen, R.P.M. (1999b) Low-Dose Folic Acid Supplementation Decreases Plasma Homocysteine Concentrations: A Randomized Trial. American Journal of Clinical Nutrition, 69, 99-104
- 20. Hannon-Fletcher, M.P., Armstrong, N.C., Scott, J.M., Pentieva, K., Bradbury, I., Ward, M., Strain, J.J., Dunn, A.A., Molloy, A.M., Kerr, M.A. and McNulty, H. (2004) Determining Bioavailability of Food Folates in a Controlled Intervention Study. American Journal of Clinical Nutrition, 80, 911-918
- 21. Bogers, R.P., Dagnelie, P.C., Bast, A., van Leeuwen, M., van Klaveren, J.D. and van den Brandt, P.A. (2007) Effect of Increased Vegetable and Fruit Consumption on Plasma Folate and Homocysteine Concentrations. Journal of Nutrition, 23, 97-102.
http://dx.doi.org/10.1016/j.nut.2006.11.002 - 22. Daly, L.E., Kirke, P.N., Molloy, A., Weir, D.G. and Scott, J.M. (1995) Folate Levels and Neural Tube Defects. Implications for Prevention. The Journal of the American Medical Association, 274, 1698-1702.
http://dx.doi.org/10.1001/jama.1995.03530210052030 - 23. Brown, J.E., Jacobs Jr., D.R., Hartman, T.J., Barosso, G.M., Stang, J.S., Gross, M.D. and Zeuske, M.A. (1997) Predictors of Red Cell Folate Level in Women Attempting Pregnancy. The Journal of the American Medical Association, 277, 548-552.
http://dx.doi.org/10.1001/jama.1997.03540310046033 - 24. Venn, B.J., Mann, J.I., Williams, S.M., Riddell, L.J., Chisholm, A., Harper, M.J. and Aitken, W. (2002) Dietary Counseling to Increase Natural Folate Intake: A Randomized, Placebo-Controlled Trial in Free-Living Subjects to Assess Effects on Serum Folate and Plasma Total Homocysteine. American Journal of Clinical Nutrition, 76, 758-765
- 25. Stea, T.H., Mansoor, M.A., Wandel, M., Uglem, S. and Frolich, W. (2008) Changes in Predictors and Status of Homocysteine in Young Male Adults after a Dietary Intervention with Vegetables, Fruits and Bread. European Journal of Nutrition, 47, 201-209.
http://dx.doi.org/10.1007/s00394-008-0714-y - 26. Lamers, Y., Prinz-Langenohl, R., Bramswig, S. and Pietrzik, K. (2006) Red Blood Cell Folate Concentrations Increase More after Supplementation with [6S]-5-Methyltetrahydrofolate than with Folic Acid in Women of Childbearing Age. American Journal of Clinical Nutrition, 84, 156-161
- 27. Jacob, R.A., Pianalto, F.S., Henning, S.M., Zhang, J.Z. and Swendseid, M.E. (1995) In Vivo Methylation Capacity Is Not Impaired in Healthy Men during Short-Term Dietary Folate and Methyl Group Restriction. Journal of Nutrition, 125, 1495-1502
- 28. Brouwer, I.A., van Dusseldorp, M., West, C.E. and Steegers-Theunissen, R.P. (2001) Bioavailability and Bioefficacy of Folate and Folic Acid in Man. Nutrition Research Reviews, 14, 267-294.
http://dx.doi.org/10.1079/NRR200126 - 29. El-Khairy, L., Ueland, P.M., Nygard, O., Refsum, H. and Vollset, S.E. (1999) Lifestyle and Cardiovascular Disease Risk Factors as Determinants of Total Cysteine in Plasma: The Hordaland Homocysteine Study. American Journal of Clinical Nutrition, 70, 1016-1024
- 30. Wald, N.J., Watt, H.C., Law, M.R., Weir, D.G., McPartlin, J. and Scott, J.M. (1998) Homocysteine and Ischemic Heart Disease: Results of a Prospective Study with Implications Regarding Prevention. Archives of Internal Medicine, 158, 862-867.
http://dx.doi.org/10.1001/archinte.158.8.862 - 31. Boushey, C.J., Beresford, S.A., Omenn, G.S. and Motulsky, A.G. (1995) A Quantitative Assessment of Plasma Homocysteine as a Risk Factor for Vascular Disease. Probable Benefits of Increasing Folic Acid Intakes. The Journal of the American Medical Association, 274, 1049-1057.
http://dx.doi.org/10.1001/jama.1995.03530130055028 - 32. Ruggeri, S., Vahteristo, L.T., Aguzzi, A., Finglas, P. and Carnovale, E. (1999) Determination of Folate Vitamers in Food and in Italian Reference Diet by High-Performance Liquid Chromatography. Journal of Chromatography A, 855, 237-245.
http://dx.doi.org/10.1016/S0021-9673(99)00674-3 - 33. Hassan-Wassef, H. (2004) Food Habits of the Egyptians: Newly Emerging Trends. Eastern Mediterranean Health Journal, 10, 898-915.