Food and Nutrition Sciences
Vol.4 No.5(2013), Article ID:31014,5 pages DOI:10.4236/fns.2013.45065
Calculating the Silicon in Horsetail (Equisetum arvense L.) during the Vegetation Season
![]()
1Department of Ecology, Faculty of Humanities and Natural Sciences, Presov University in Presov, Presov, Slovak Republic; 2Centre of Excellence for Animal and Human Ecology, Presov University in Presov, Presov, Slovak Republic; 3Faculty of Natural Science, Institute of Chemistry, Comenius University in Bratislava, Bratislava, Slovak Republic.
Email: *pavollabun@yahoo.com
Copyright © 2013 Pavol Labun et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 5th, 2013; revised April 5th, 2013; accepted April 12th, 2013
Keywords: Environmental Factors; Equisetum arvense L.; Statistical Variability; Silicon
ABSTRACT
Horsetail (Equisetum arvense L.) is a perennial herb which creates during the life cycle spring and summer stems. The selected species and populations were monitored in the years 2009-2011 in three different natural locations in Laborecká vrchovina (Slovakia). Samples were collected by destructive methods in all three locations. Silicon content was determined in dry biomass by AAS. Silicon content in plants ranged from 21.11 ± 3.24 g·kg−1 to 32.80 ± 8.03 g·kg−1. The highest content of silicon exhibited samples of the September collection. We found that the location and the year in terms of silicon content were not statistically significant. The main sources for statistical variability in the accumulation of silicon were during the collections.
1. Introduction
Horsetail (Equisetum arvense L.) is a herbaceous perennial plant. It has separate sterile non-reproductive and fertile spore-bearing stems, growing from a perennial underground rhizomatous stem system.
The plant contains several substances which can be used medicinally. It is rich at minerals silicon (10%), potassium, and calcium. Silicon is generally classified as an element, which is important for plants at their structure, physiology and for protection [1]. Silicon is taken up by the roots in the form of silicic acid [Si(OH)4], an uncharged monomeric molecule, when the solution pH is below 9 [2]. Silicic acid is a soluble form of silicon and one of the basic form, which is absorbed and used by plants. Polymerized silicates belong to the group of the hardest materials in plant tissues. Silicon helps to raise the plant health by the creating of strongest and more resistant structures. Plants which are attacked by the herbivores tend to accumulate more oxides [3].
It is one of the oldest plants on earth and what remain today from tree-sized fossils are the field horsetails. They were used in historical times for scouring pots and polishing pewter and were commonly called “scouring rushes”. Horsetails have found extensive application in medicine as a source of silica, as it can amount to 25% of the dry weight of the plant [4]. Silicon from horsetail promotes the growth and stability of the skeletal structure. An invention describing a pharmaceutical composition based on Equisetum arvense for the treatment of bone diseases, particularly osteoporosis [5]. A few European clinical studies have determined that fractured bones heal much more quickly when horsetail is taken. The incidence of osteoporosis is, likewise, more greatly reduced when some horsetail is added to the diet. Horsetail extract is also added in a composition used against psoriasis [6].
Researchers [7,8] believe that the medicinal property of horsetail is due to its high silica content. Horsetail has been used as a folklore medicine for treatment of various conditions such as tuberculosis, as a catarrh in the kidney and bladder regions, as a hematostatic for profuse menstruation, nasal, pulmonary and gastric hemorrhages, for brittle fingernails and loss of hair, for rheumatic diseases, gout, poorly healing wounds and ulcers, swelling and fractures and for frostbite [9].
Silicon increases the resistance against mold. Recent researches noted that first reaction after fungi attack is higher at silicic acid presence [10]. Growing of the plants is supported by the silicates, which allows better flexibility and extensibility of cells walls [11]. The content of silicon had negative correlation with lignin and cellulose at wetland macrophytes. It substitutes the mechanical role of these polymers [12].
The aim of this research was to determine the content of the silicic acid, its relation to spatial structure and environmental conditions at the natural habitat of selected plant species.
2. Matrial and Methods
2.1. Plant Material and Description of Collection Locality
Samples of horsetail were collected by the destructive methods in the years 2009, 2010 and 2011, each year from May to September (June—first collection, July— second collection, August—third collection, September— fourth collection). The plant material reduced to its biomass after drying to a constant weight (minimum 24 hours) in 85˚C - 105˚C in a dryer with ventilation.
When we chose the locality for collecting the plant samples we encountered regular dispersion of horsetail in Laborecka vrchovina (Slovakia). The three localities are at different altitudes, different distances from rivers and are orientated depending on sun radiation and slope of the terrain. Locality 1 (L1) is on level ground and 30 m from the river, 196.4 m altitude, 49˚03'44.47''N, 21˚57'45.22''E. Locality 2 (L2) has high groundwater and is oriented to the south, 225.5 m altitude, 49˚03'57.71''N, 21˚58'00.89''E. Locality 3 (L3) is shaded and oriented to the north, 205.5 m altitude, 49˚03'43.20''N, 21˚58'40.42''E.
2.2. Analytic Methods and Equipment
The mineralization technique for the sample analysis required nitric acid HNO3 (65%) and hydrofluoric acid HF (46%) which were bought from the Sigma Aldrich Co. The silicon standard (Si) was from company Ultra scientific. We used a 2% solution of HNO3 and deionized water with the conductivity of <0.1 µS for extract stabilization. We used a Speedwave 2 Berghof for mineralization, with a voltage of ~230 V, frequency of 50/60 Hz, power consumption of 1610 W, and a magnetron frequency of 2450 MHz. Pressure vessels DAP-60K, volume 60 ml, maximum pressure 40 bar, maximum temperature 230˚C, maximum weight < 300 mg, minimum volume of acids > 5 ml. Atomic Absorption Spectrophotometers (AAS 7000) from Shimadzu company. Fully automatic dual—beam instrument with 3D-optic system, automatic 6-lamp holder, correction of background by D-lamp with SR-correction of spectral interferences. Software shares preset operating parameters for all elements and standard samples.
2.3. Sample Preparation by Microwave Mineralization
Dried samples of horsetail were weighted at 0.1 g with the precision of 0.0001 g. Thereafter, they were stored in pressure vessels, DAP-60K, and then 8 ml HNO3 (65%) and 2 ml HF (46%) were added. The thermal program was 0˚C - 145˚C/5min, 220˚C/40min, 200˚C/15min. Mineralized samples were cooled until room temperature. After that, we prepared samples for AAS analysis to determine the silicon at wave length 251.61 nm. The aim of this research was to determine the content of the silicic acid, its relation to spatial structure and environmental conditions in the natural habitat of selected plant species.
3. Results
We consider the effect of three factors for the silicon content in the dry matter of horsetail (Table 1), which are from three locations (L1, L2, L3), three years (2009, 2010, 2011) and four collection times (1, 2, 3, 4). Since we do not have values for each combination of factors (we did not collect samples 3 and 4 at L3 locality) and to assess the significance of factors influencing the content of silicon in the dry matter of horsetail, we used an analytical model of variance to calculate the main effects.
Our calculations show the highest average content of silicon was measured at the sample from locality L2 (27.23 ± 7.59 g·kg−1) and the lowest content was from the locality L3 (23.79 ± 2.73 g·kg−1). Relative to the year, the highest average silicon content was from the year 2011 (26.96 ± 3.41 g·kg−1), but the amounts reached
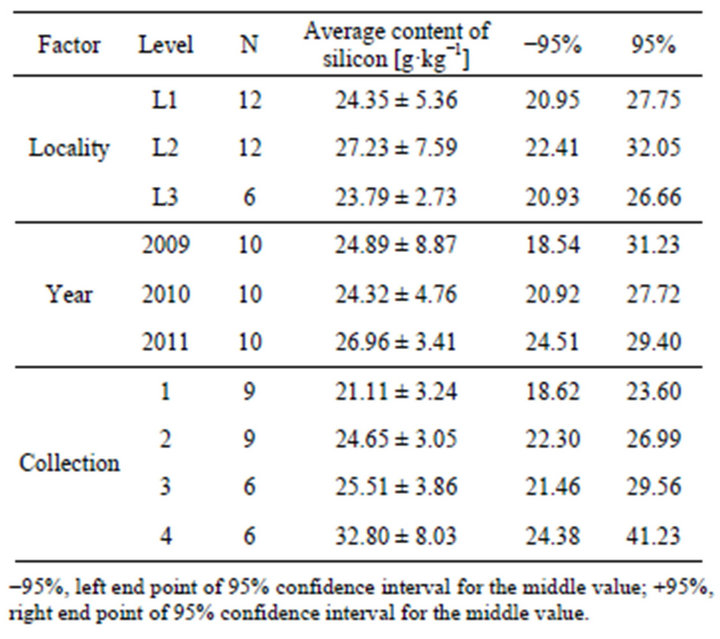
Table 1. Descriptive statistics of silicon content at different factors.
similar percentages in the years 2009 and 2010. The collection factor showed the highest disproportion in the measured values. The highest accumulation of silicon was from the fourth collection (32.80 ± 8.03 g·kg−1) and the lowest content was measured in the first collection (21.11 ± 3.24 g·kg−1).
3.1. Analysis of Variance of the Silicon Content at Dry Mater
The results from the analysis of the variance of the silicon accumulation in the dry mater of horsetail (Eqisetum arvense L.) are shown in Table 2. It describes the insignificance of the locality factor (F = 1.37, p = 0.275). Analysis of the year factor showed the same—the content of silicon in three different years (2009-2011) in horsetail was not statistically significant (F = 0.92, p = 0.413).
The only statistically significant factor was the collection (F = 7.79, p = 0.001) (Figure 1). It means that the content of silicon in the dry matter of horse tail was different in each collection.
Figure 1 shows the average silicon concentration in the dry matter from each collection. The highest average values were recorded in the fourth collection from 26.44 ± 1.32 g·kg−1 to 32.80 ± 8.03 g·kg−1. The lowest contents were measured in the first collection in each locality and
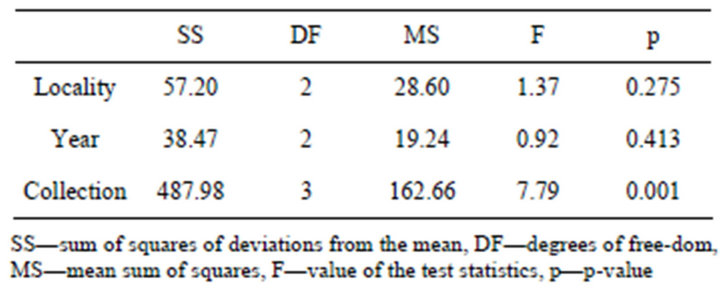
Table 2. Analysis of variance of the main factor for silicon accumulation at dry matter.
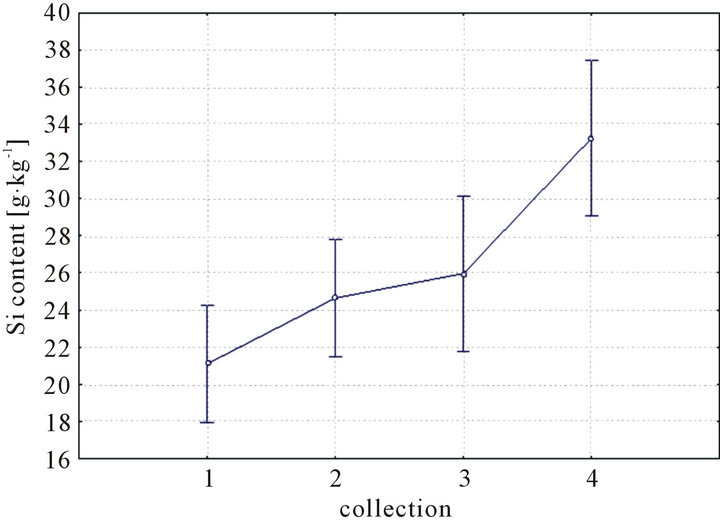
Figure 1. The content of silicon at dry matter of horsetail in each collection.
each year. The range was from 21.11 ± 3.24 g·kg−1 to 25.16 ± 1.25 g·kg−1.
The analysis of the results of variants from each collection shows that the collection factor was statistically significant (F = 7.79, p = 0.001), that means that the content of silicon in the dry matter of horsetail is not the same in each collection. The highest content of silicon accumulated in plants that were measured in the fourth collection, which can be the result of stress from climatic factors at the end of the vegetation season.
3.2. Fisher LSD Test of Factor Significance
The main source of statistical variability in the silicon accumulation in horsetail was the collection indicator. Table 3 shows the results of the comparison of average values. The results of the Fisher test show that levels 1, 2, and 3 of the collection factor were not statistically significant. The amount of silicon was approximately the same and the p-value was higher than 0.05. The fourth collection, in which plants contained the highest amount of silicon and the p-value was lower than 0.05, was statistically significant.
4. Discussion and Conclusion
The silicon in plants tissues can be quantified by a few techniques. We used a method using AAS (Atomic Absorption Spectroscopy). It is very important to understand, that the process of mineralization is critical for silicon quantification. One of the doctrines says that plants need 16 basic elements for their growth. In fact, the plant needs many more elements. One of the main inorganic elements is silicon. Silicon is widespread on the Earth. In soil it occurs as silicic acid, which is easily absorbed by plants.
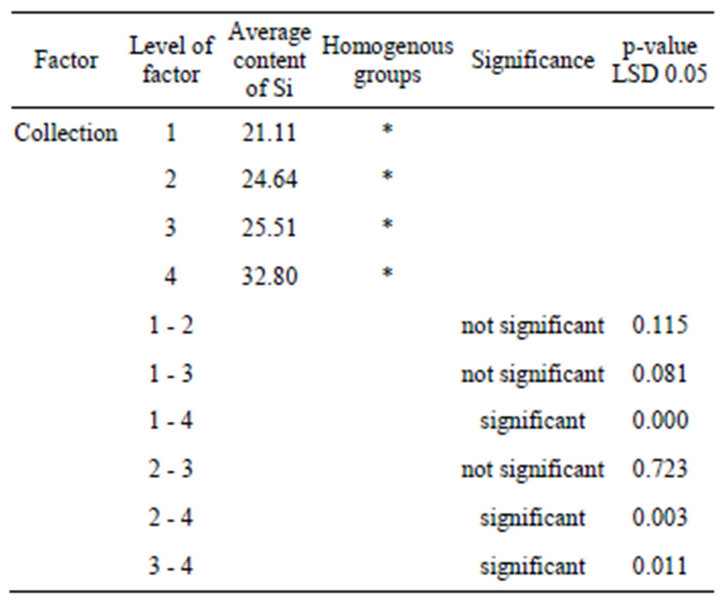
Table 3. Fisher LSD test of comparison of a variable for different factors.
Rafi [13] performed an experiment with wheat (Triticum aestivum). They checked the silicon absorbance. They found that younger plants absorbed lower amount of silicon while the requirement for silicon in growing plants increased. Silicon absorbance varies in different plant species. It is presumed that this element is important for plants, but its amount in the dry matter is at a minimum level. Barker [14] included also potassium, sodium and chlorine.
The different analyses showed the amount of silicon between 2% and 10% in the dry matter of some plant species. It is known that some solutions containing silicon were used for rice growing in China and Japan.
Horsetail is characterized by the high amount of silicic acid. Bey [15] made some experiments for the silicon content at horsetails teas. The silicon was extracted by the using some solutions as hexane, dichloromethane, ethanol, methanol, water and hydroxide. The average amount of silicon was about 5 which is 50 g·kg−1 of dry matter.
Our results for the silicon concentration in horsetail reached from 2.64% to 4.80% of the dry matter. The lowest amount of silicon was in the range between 1.52% and 2.51%. The plant growth was not influenced by the highest levels of silicon. These results suggested that high silicon concentration in the soil did not cause higher concentrations in the plants tissues.
The differences in plants’ growth stages are the differentiating factor for the rate of silicon uptake into the plant populations. Older plants have higher concentrations of silicon in their tissues. If the silicon is localized in the plant organ it is not distributed throughout the different parts of the plants [16].
Our results are comparable to the results from the literature. The collection factor is for our results statistically significant (F = 7.79, p = 0.001). The main source of analysis of variance was the indicator from the collection with the highest impact on silicon accumulation. This result arises from the Fisher test, the multivariate test of comparisons with average values. p-values are lower than 0.05. The amount of silicon in the fourth collection ranged from 26.44 ± 1.32 g·kg−1 to 32.80 ± 8.03 g·kg−1. The lowest amounts were measured at first collection in different localities and years. The rates were from 21.11 ± 3.24 g·kg−11 to 25.16 ± 1.25 g·kg−1.
We found that the highest amount of silicon was accumulated in the fourth collection. September is the optimal time for collecting horsetail biomass. The collection in this month is the best time for using this plant as a food supplement.
5. Acknowledgements
The work was supported by the Agency of Ministry of Education, Science, Research and Sport of the Slovak Republic, the project: 00162-0001 (MS SR-3634/2010- 11).
REFERENCES
- H. Marschner, “Mineral Nutrition of Higher Plants,” Academic Press, London, 1995, p. 899.
- J. F. Ma and E. Takahashi, “Soil, Fertilizer, and Plant Silicon Research in Japan,” Elsevier Science, 2002, p. 281.
- J. F. Ma and N. Yamaji, “Silicon Uptake and Accumulation in Higher Plants,” Trends in Plant Science, Vol. 11. No. 8, 2002, pp. 392-397. doi:10.1016/j.tplants.2006.06.007
- F. P. Massey, A. R. Ennos and S. E. Hartley, “Herbivore Specific Induction of Silicabased Plant Defences,” Oecology, Vol. 152, No. 4, 2007, pp. 677-683. doi:10.1007/s00442-007-0703-5
- A. Zampieri and S. Casolaro, “Composition for the Treatment of Bone Disease,” Ita. Appl., 2001.
- C. Andrei, “Antioxidant Composition Based on Natural Extracts for Treating Psoriasis,” Rome, 2006.
- V. E. Tyler, “The Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies,” 3rd Edition, Pharmaceutical Products Press, New York, 1993, pp. 119-120.
- D. Cloutier and A. Watson, “Growth and Regeneration of Field Horsetail (Equisetum arvense),” Weed Science, Vol. 33, No. 3, 1983, pp. 358-365.
- N. S. Sandhu, S. Kaur and D. Chopra, “Equisetum Aervens: Pharmacology and Phytochemistry—A Review,” Asian Journal of Pharmaceutical and Clinical Research, Vol. 3, No. 3, 2010, pp. 146-150.
- F. Fauteux, F. Chain, F. Belzile, J. G. Menzies and R. R. Belanger, “The Protective Role of Silicon in the Arabidopsis—Powdery Mildew Pathosystem,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 103, No. 46, 2006, pp. 17554-17559. doi:10.1073/pnas.0606330103
- M. T. Hossain, R. Mori, K. Soga, K. Wakabayashi, S. Kamisaka and S. Fujii, “Growth Promotion and an Increase in Cell Wall Extensibility by Silicon in Rice and Some other Poaceae Seedlings,” Journal Plant Research, Vol. 115, No. 1, 2002, pp. 23-27. doi:10.1007/s102650200004
- J. Schoelynck, K. Bal, H. Backx, T. Okruszko, P. Meire and E. Struyf, “Silica Uptake in Aquatic and Wetland Macrophytes: A Strategic Choice between Silica, Lignin and Cellulose,” New Phytologist, Vol. 186, No. 2, 2010, pp. 385-391. doi:10.1111/j.1469-8137.2009.03176.x
- M. M. Rafi and E. Epstein. “Silicon Absorption by Wheat (Triticum aestivum L.),” Plant and Soil, Vol. 211, No. 2, 1999, pp. 223-230. doi:10.1023/A:1004600611582
- A. V. Barker and D. J. Pilbeam, “Handbook of Plant Nutrition,” Taylor & Francis Group, Boca Raton, 2007.
- R. Bey, S. F. Thingstad and B. S. Paulsen, “Horsetail (Equisetum spp) as a Source of Silicon Supplement in Human Nutritiona Myth?” Journal of Herbs, Spices and Medicinal Plants, 2010, Vol. 16, No. 2, 2010, pp. 119-125.
- P. B. Kaufman, P. Dayanandan, Y. Takeoka, J. D. Bigelow, J. D. Jones and R. Iler, “Silica in Shoots of Higher Plants,” In: T. L. Simpson and B. E. Volcani, Eds., Silicon and Siliceous Structures in Biological Systems, Springer, 1981, pp. 409-449.
NOTES
*Corresponding author.

