Food and Nutrition Sciences
Vol.4 No.6(2013), Article ID:32732,4 pages DOI:10.4236/fns.2013.46081
Antiproliferative Activity of “Lycopersicon esculentum” Leaves (Var. Paul Robenson): Preliminary Study
![]()
1Department of Pharmaceutical Sciences, University of Salerno, Via Ponte Don Melillo 84084, Fisciano, Italy; 2Council of National Research (CNR)—Institute of Biomolecular Chemistry, Pozzuoli, Italy.
Email: *saturnino@unisa.it, lisaspa83@hotmail.com, chiara24@hotmail.it, apopolo@unisa.it, gtommonaro@icb.cnr.it, roccodeprisco1@virgilio.it, pintoal@unisa.it
Copyright © 2013 Carmela Saturnino et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 5th, 2012; revised February 23rd, 2013; accepted March 2nd, 2013
Keywords: Lycopersicon esculentum; Antiproliferative Activity; Glioma Cell Line
ABSTRACT
Among plants, the Lycopersicon esculentum (Solanaceae) is the most important for its beneficial effects on health. Several epidemiological studies have shown the benefits of tomato consumption in the cancer and cardiovascular disease prevention. Tomato products constitute the major source of lycopene, the most potent antioxidant among carotenoids in vitro. In tomatoes leaves are also present many secondary metabolites including phenolic compounds, phytoalexins, protease inhibitors and glycoalkaloids who protect against adverse effects of hosts including fungi, bacteria, viruses, and insects and are involved in host-plant resistance. In this work we evaluated the antiproliferative activity of tomato leaves extract (var. Paul Robenson) in vitro.
1. Introduction
The beneficial effects related to consumption of tomato are due to the presence of many antioxidant compounds of carotenoids and vitamins family [1]. Tomato contains carotenoids, ascorbic acid, phenolic compounds and α- tocopherol. Tomato carotenoids include: lycopene, lycopene-5, 6-diol, lutein, ζ-, γ-, β-, carotenes, neurosporene, phytoene and phytofluene. Lycopene protects against lipids, proteins and DNA, oxidative damage and it is involved in several processes that affect the maintenance of cells [2], such as the inhibition of cancer cells proliferation [3], the block of cell transformation by reducing the loss of cancer cells inhibition contact [4,5]. Lycopene also induces phase II enzymes that help to eliminate carcinogens and toxins [6] and enhances gap junctional communication [7]. In tomatoes, lycopene is more abundant in the leaves and in the outer part of the mesocarp, where, following the ripening of the fruit, it replaces the chlorophyll. In fact, in addition to the edible parts of the plant, the leaves are used in traditional medicine for their therapeutic properties. Traditionally, the leaves of chopped tomatoes are applied to the skin as a remedy for insect bites. In Mozambique the fresh leaves are used against diarrhea, dysentery, gonorrhea, anal irritation and eyes infections. In Latin America, the tomato leaves are used as a remedy for buccal candidiasis. In Cuba and Honduras an infusion of leaves is used as a remedy for coughs. In Peru, the leaves are crushed to prepare a mixture for the treatment of bone fractures. In Haiti they are crushed and mixed to obtain a remedy for headaches and dizziness. Even in Mexico the crushed leaves are used against eczema and are prepared by an infusion of fresh leaves and shoots to be used against colds and coughs. These properties are likely to be linked to alpha-tomatine [8], contained only in the green part of the plant, with antibiotic, insecticide, insectifuge, fungicide and anti-bacterial properties. The tomato plants biosynthesize glycoalkaloids [9] de-hydrotomatine and alpha-tomatine probably as a defence mechanism against bacteria, fungi, viruses and insects [10]. This study was aimed to evaluate also the cytotoxic activity of Black Tomato leaves extracts (var. Paul Robenson).
2. Materials and Methods
2.1. Plant Material
The seeds of Black Tomato, Paul Robenson variety, have been grown in the experimental field of the Agro Nocerino Sarnese (Campania region, Italy), which showed the optimal climatic conditions: temperature 25˚C - 30˚C, good sun exposure and a good system of irrigation. The collection of leaves (Black Tomato) was carried out in September when the fruits were fully ripe. The leaves were collected and transported to the laboratory, separated from the stalk, catalogued and stored in refrigerator at −80˚C until analysis.
2.2. The Extraction Process
50 g of Black Tomato fresh leaves were frozen for 3 hours at −80˚C. Frozen leaves were crushed and lyophilized for 3 days. After total dried leaves were macerated in ethanol (96%) at room temperature, until exhaustion. After 5 days the crude extract were filtered and concentrated in vacuo. The crude ethanol extract (5.0 g) was dissolved in water (50 ml) (pH 8 - 9), 10 ml of this solution were extracted (CHCl3/H2O) The extraction was repeated until exhaustion and monitored by thin-layer chromatography (TLC) in BAW (n-buthanol-acetic acid-water, 60:25:15) and CHCl3-MeOH-H2O (80:18:2).
2.3. Cell Lines and Culture Conditions
Human embryonic kidney (HEK-293), rat glioma (C6) and human breast cancer cells were maintained and grown in adhesion on Petri dishes with DMEM supplemented with FCS (10%), HEPES (25 mM), penicillin (100 U/mL) and streptomycin (100 U/mL).
2.4. Cytotoxic Activity
Cytotoxic activity of Black tomato leaves extracts was tested in vitro by using MTT assay [11] on three cell lines. HEK-293, MCF-7 and C6 cells were plated on 96-well microtiter plates at the concentration of 3.5 × 10−4 cells/well and allowed to adhere at 37˚C in 5% CO2 and 95% air for 2 h. Subsequently, the medium was replaced with 50 L of fresh medium and a rate of 75 L of serial dilution of total extract, hydrophilic extract and chloroform extract were added, and then the cells were incubated for 72 h. In some experiments, serial dilutions of cis-platinum were added for the sole purpose of testing the susceptibility of our cell lines to cytotoxicity.
Cell viability was assessed through 3-(4,5-dimethyltiazol-2yl)-2,5-phenyl-2H-tetrazolium bromide (MTT) assay. Briefly, 25 μL of MTT (5 mg/mL) was added and the cells were incubated for an additional 3 h. Thereafter, cells were lysed and the dark blue crystals solubilised with 100 μL of a solution containing 50% (mL/L) N,Ndimethylformamide, 20% (mL/L) sodium dodecyl sulphate (SDS) with an adjusted pH of 4.5. The optical density (OD) of each well was measured with a microplate spectrophotometer (Titertek Multiskan MCC/340) equipped with a 620 nm filter. The viability of each cell line in response to treatment with tested compounds was calculated as follows:
% dead cells = 100 − (OD treated/OD control) × 100 The cytotoxic activity of each compound was expressed as IC50 (µM) indicating the concentration able to reduce cell viability by 50% (Table 1).
3. Results and Discussion
Cytotoxic activity of hydrophilic and chloroform extracts of Black Tomato leaves was evaluated by MTT-assay on three different cell lines: human embryonic kidney (HEK), rat glioma (C6) and human breast cancer cells (MCF-7). As reported in Table 1, hydrophilic and chloroform extracts exert a cytotoxic activity comparable to cis-platin on C6 cells. On the contrary, no significant activity was exerted by total extract on this cell line. No significant activity was exerted by all three extracts on MCF-7 and HEK-293 cell lines. Regarding MCF-7, our data was in agreement with a previous study of Friedman et al. (1997) who demonstrate that these cells are insensitive to lower concentration of glycoalkaloid contained in tomato and that lower concentration of the extracts cause an initial increase in cell growth. As HEK-293 human cell and not tumor cells, the inactivity of our extract could be advantageous because there wasn’t cytotoxic activity. The cytotoxic effect of tomato leaves could be due to the “sistemin intracellular signalling” [12].
Sistemin is a 18 aminoacid peptide that induces the synthesis of protease inhibitors by interacting with a cell surface receptor, SR160 [13]. The interaction of sistemin with its receptor is the first step of a cascading series of reactions involving the activation of several processes including: mitogen activated protein kinase (MAPK), alkalinization of extracellular medium, phospholipase, release of linoleic acid 12-oxo-phytodienoic acid and jasmonic acid that are converted to strong signals of cellular defense [9]. The precursor Prosistemin, released from cells at the wound site, is probably processed by proteinase and is also released from damaged cells. This would allow the spread to neighbouring cells to settle where interaction with its receptors to induce the synthesis of jasmonate. Furthermore, when the jasmonic acid is produc-
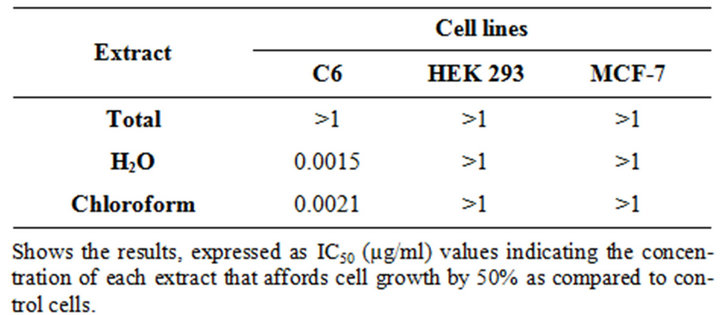
Table 1. Cytotoxic activity of black tomato leaves extracts.
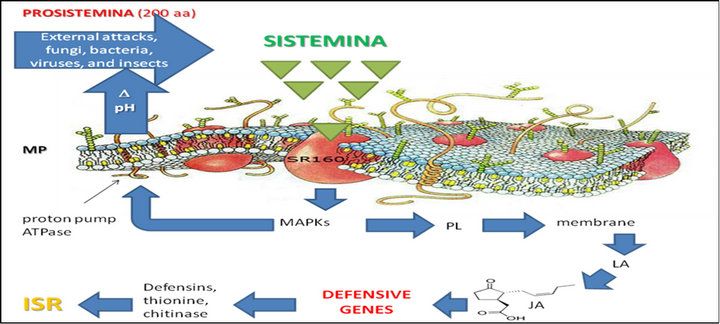
Figure 1. Hypothesized mechanism of induced systemic resistance in tomato leaves. Sistemin interacts with its putative receptor thus activating a series of reactions involving the activetion of MAPK, alkalinisation of extracellular medium, phospholipase, linoleic acid and jasmonic acid.
ed and released into the circulation would cause more prosistemin by proteolytic enzymes that are known to be induced by jasmonic with a defense system and amplification continues. One of the main sources of jasmonate in wound sites is linoleic acid produced from the degradation of lipids in membrane cell. This source of jasmonate probably provides an important “site-start” the signalling of defence alert, following transported to parenchymal cells and trigger a system up-regulation of defence genes, including gene prosistemin (in Solanaceae) (Figure 1).
4. Conclusion
Further studies needed to explore these preliminary observations that indicate the involvement of the extracts of tomato leaves in the reduction of tumour cell proliferation.
REFERENCES
- M. L. Balestrieri, R. De Prisco, B. Nicolaus, P. Pari, V. Schiano Moriello, G. Strazzullo, E. L. Iorio, L. Servillo and C. Balestrieri, “Lycopene in Association with Alpha-Tocopherol or Tomato Lipophilic Extracts Enhances AcylPlatelet-Activating Factor Biosynthesis in Endothelial Cells during Oxidative Stress,” Free Radical Biology & Medicine, Vol. 36, No. 8, 2004, pp. 1058-1067. doi:10.1016/j.freeradbiomed.2004.01.014
- A. V. Rao, “Lycopene, Tomatoes, and the Prevention of Coronary Heart Disease,” Experimental Biology and Medicine, Vol. 227, No. 10, 2002, pp. 908-913.
- A. Nahum, K. Hirsch, M. Danilenko, C. K. Watts, O. W. Prall, J. Levy and Y. Sharoni, “Lycopene Inhibition of Cell Cycle Progression in Breast and Endometrial Cancer Cells Is Associated with Reduction in Cyclin D Levels and Retention of p27 (Kip1) in the Cyclin E-cdk2 Complex,” Oncogene, Vol. 20, No. 26, 2001, pp. 3428-3436. doi:10.1038/sj.onc.1204452
- J. Karppi, S. Kurl, T. Nurmi, T. H. Rissanen, E. Pukkala and K. Nyyssönen, “Serum Lycopene and the Risk of Cancer: The Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study,” Annals of Epidemiology, Vol. 19, No. 7, 2009, pp. 512-518. doi:10.1016/j.annepidem.2009.03.017
- K. Wertz, U. Siler and R. Goralczyk, “Lycopene: Modes of Action to Promote Prostate Health,” Archives of Biochemistry and Biophysics, Vol. 430, No. 1, 2004, pp. 127- 134. doi:10.1016/j.abb.2004.04.023
- O. Aust, N. Ale-Agha, L. Zhang, H. Wollersen, H. Sies and W. Stahl, “Lycopene Oxidation Product Enhances Gap Junctional Communication,” Food and Chemical Toxicology, Vol. 41, No. 10, 2003, pp. 1399-1407. doi:10.1016/S0278-6915(03)00148-0
- C. M. Rick, J. W. Uhlig and A. D. Jones, “High Alphatomatine Content in Ripe Fruit of Andean Lycopersicon Esculentum var. Cerasiforme: Developmental and Genetic Aspects,” Proceeding of the National Academy of Sciences USA, Vol. 91, No. 26, 1994, pp. 12877-12881. doi:10.1073/pnas.91.26.12877
- M. Friedman and G. M. McDonald, “Potato Glycoalkaloids: Chemistry, Analysis, Safety and Plant Physiology,” Critical Review in Plant Sciences, Vol. 16, No. 1, 1997, pp. 55-132.
- C. A. Ryan and G. Pearce, “Systemins: A Functionally Defined Family of Peptide Signals That Regulate Defensive Genes in Solanaceae Species,” Proceeding of the National Academy of Sciences USA, Vol. 100, No. 2, 2003, pp. 14577-14580.
- J. Stratmann, J. Scheer and C. A. Ryan, “Suramin Inhibits Initiation of Defense Signaling by Systemin, Chitosan, and a Beta-Glucan Elicitor in Suspension-Cultured Lycopersicon peruvianum Cells,” Proceedings of the National Academy of Sciences USA, Vol. 97, No. 16, 2000, pp. 8862-8867. doi:10.1073/pnas.97.16.8862
- T. Mosmann, “Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxic Assays,” Journal of Immunological Methods, Vol. 65, No. 1-2, 1983, pp. 55-63. doi:10.1016/0022-1759(83)90303-4
- C. A. Ryan, “The Systemin Signaling Pathway: Differential Activation of Plant Defensive Genes,” Biochimica et Biophysica Acta, Vol. 1477, No. 1-2, 2000, pp. 112-121. doi:10.1016/S0167-4838(99)00269-1
- B. McGurl and C. A. Ryan, “The Organization of the Prosystemin Gene,” Plant Molecular Biology, Vol. 20, No. 3, 1992, pp. 405-409. doi:10.1007/BF00040600
NOTES
*Corresponding author.

