Food and Nutrition Sciences
Vol. 3 No. 5 (2012) , Article ID: 19072 , 7 pages DOI:10.4236/fns.2012.35084
Functional Property of Honey from Echium vulgare
![]()
1Yamagata University, Yamagata, Japan; 2Graduate School, Prince of Songkla University, Songkhla, Thailand; 3National Fisheries University, Yamaguchi, Japan; 4Nagoya Research Institute, Aichi, Japan.
Email: *nandemofreefree@yahoo.co.jp, tnagai@tdsl.tr.yamagata-u.ac.jp
Received October 31st, 2011; revised March 9th, 2012; accepted March 16th, 2012
Keywords: Echium vulgare; Honey; Total phenolic compound; Amylase activity; Antioxidative activity; Antihypertensive activity
ABSTRACT
Chemical property of honey from Echium vulgare was investigated. In comparison with other honey species, the contents of total phenolic compounds and total flavonoids were the highest. α-Amylase activity was also extremely high: about three to nine hundred times as much as those of other honey species. The antioxidative activity of honey was investigated using four different methods. Honey from E. vulgare showed the best performance in inhibiting lipid peroxidation and scavenging superoxide anion radicals, hydroxyl radicals, and DPPH radicals. Moreover, it exhibited stronger inhibition activity of ACE. It is known that higher antioxidative activity and scavenging activity against active oxygen species in honey species related to their colour. In the present study, however, it suggests that the phenolics in honey from E. vulgare with yellow gold colour might be the major active component responsible for the strong antioxidative activity and radical scavenging activity.
1. Introduction
Oxidative stress can be defined as a disproportion between creation of reactive oxygen species and a biological system’s capability to detoxify reactive intermediates. As one of the consequences of oxidative stress, free radical generation may cause severe damage to cells and associated with many diseases such as cancer, cardiovascular diseases, neurological disorders, and metabolic diseases [1]. The use of antioxidant supplementation is beneficial to prevent these diseases. Antioxidant capacity can be generally assessed by two main mechanisms, as quenching of various free radicals and reduction of metal ions such as iron, cupper, and chromium [2]. Antioxidants of natural or synthetic origins are added to foodstuffs to prevent undesirable deterioration. However, synthetic antioxidants as butylated hydroxylanisole, butylated hydroxytoluene, and propyl gallate may be quite unsafe because of their side effects and toxicity to nontarget organs, which are of concern.
It is well known that diets rich in fruits and vegetables have been associated with lower risk of cancer and lower dietary intake of the same doubles the risk of cancer as compared to high intake [3]. This association may be attributed from the antioxidants in foods including vitamin C and E, carotenoids, polyphenolic compounds and flavonoids, which prevent free radical damage [4]. Individual antioxidant compounds do not act alone [5], and their compounds act in combination with other antioxidants, as interactions among them can affect total antioxidant capacity, producing synergistic or antagonistic effects [6]. Knowledge of their total antioxidant capacity, which is the cumulative capacity of food components to scavenge free radicals, would be useful for epidemiologic purposes [7].
Honey, a viscous and aromatic product appreciated since ancient Grecian times, is prepared by bee mainly from nectar of flowers or honeydew [8]. The characteristics of appearance, flavour, sweetness, and texture of honey, as well as its medicinal properties, have attracted thousands of consumers [8]. The typical composition of honey is: moisture, 20.0%; carbohydrate, 79.7%; proteins, 0.2%; and ash, 0.1% [9]. Honeys also contain a number of components to act as preservatives such as vitamin C, flavonoids and other phenolics, and enzymes as glucose oxidase, catalase, and peroxidase [10]. It suggested that any of these substances owe their preservative properties to their antioxidative activity [11]. Honey has been termed value-added products ever since the initial studies confirmed that antioxidant properties of polyphenols lie at the heart of their cosmetic [12], medical [13], and alimentary applications [14].
The annual production of honey in 2002 is about 132 million tons worldwide. In 2003, world production is 134 million tons and in next year has continued to slightly expand to 135 million tons. Japan produces some honeys about 3000 tons per year and imported 4.5 million tons of honey: 90% of China, and Argentine Republic are dependent on passing. Its use of honey species in food has been as a sweetening agent. However, interest has been growing in recent years in the discovery of natural antioxidants from honey species as they contain significant amounts of bioactive compounds. Therefore, the present investigation explores the functional properties, in particular the antioxidative and antihypertensive activities of honey from Echium vulgare that is one of traditional medicinal herb.
2. Materials and Methods
2.1. Materials
Pure honey (n = 3) from Echium vulgare was purchased from Sasaki Yohoen Bee Farm Inc. (Mie, Japan) and used in this study. Neoamylase test was purchased from Daiichi pure chemicals Co. Ltd. (Tokyo, Japan). Angiotensin I-converting enzyme (ACE) from bovine lung (1 U), 2,2’-azobis(2-amidinopropane)dihydrochloride (AAPH), bovine serum albumin (BSA), catechin, 2-deoxy-D-ribose, ethylenediaminetetraacetic acid disodium salt (EDTA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ethyl acetate for spectrochemical analysis grade, hippuryl-L-histidyl-Lleucine as substrate peptide, linoleic acid, nitroblue tetrazolium salt (NBT), α-tocopherol, and xanthine were from Wako Chemicals Co. Ltd. (Osaka, Japan). Xanthine oxidase from butter milk (XOD; 0.33 U/mg powder) was from Oriental Yeast Co. Ltd. (Tokyo, Japan). Other chemicals were of an analytical grade.
2.2. Preparation of Sample Solution
Pure honey was diluted with distilled water and 1.0%, 10%, 25%, and 50% (v/v) honey solutions were used for the following tests.
2.3. Chemical Analysis
The protein content was determined by the method of Lowry et al. [15] using BSA as standard. The total phenolic compounds were measured spectrophotometrically at 760 nm using chlorogenic acid as standard [16]. The total flavonoid content was determined according to the colorimetric assay [17]. Total vitamin C content was measured by the α,α’-dipyridyl method [18]. The α- amylase activity was assayed by the method using blue starch as a substrate [19].
2.4. Auto-Oxidation Test
Antioxidative activity was assayed by using a linoleic acid model system. A 0.083 ml of sample solution and 0.208 ml of 0.2 M sodium phosphate buffer (pH 7.0) were mixed with 0.208 ml of 2.5% (w/v) linoleic acid in ethanol. The preoxidation was initiated by the addition of 20.8 μl of 0.1 M AAPH and carried out at 37˚C for 200 min in the dark. The degree of oxidation was measured according to the thiocyanate method for measuring peroxides by reading the absorbance at 500 nm using a PerkinElmer model Lambda 11 (PerkinElmer, Tokyo, Japan) UV/VIS spectrophotometer after colouring with FeCl2 and ammonium thiocyanate [20]. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control.
2.5. Effect of Superoxide Anion Radical
Superoxide anion radical scavenging activity was evaluated by the method of Nagai et al. [20]. The system contained 0.48 ml of 0.05 M sodium carbonate buffer (pH 10.5), 0.02 ml of 0.15% of BSA, 0.02 ml of 3 mM EDTA, 0.02 ml of 0.75 mM NBT, 0.02 ml of 3 mM xanthine, and 0.02 ml of sample solution. After pre-incubation at 25˚C for 10 min, the reaction was started by adding 6 mU XOD and carried out at 25˚C for 20 min. The reaction was stopped by adding 0.02 ml of 6 mM CuCl. The absorbance of the reaction mixture was measured at 560 nm and the inhibition rate was calculated by measuring the amount of formazan that was reduced from NBT by the superoxide. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control. The IC50 value was defined as the concentration of honey required to inhibit 50% of superoxide anion radical activity.
2.6. Effect of Hydroxyl Radical
The effect of hydroxyl radical in honey from E. vulgare was investigated using the deoxyribose method [20]. The reaction mixture contained 0.45 ml of 0.2 M sodium phosphate buffer (pH 7.0), 0.15 ml of 10 mM 2-deoxy-D-ribose, 0.15 ml of 10 mM FeSO4-EDTA, 0.525 ml of distilled water, and 0.075 ml of sample solution in an Eppendorf tube. The reaction was started by the addition of 0.15 ml of 10 mM H2O2. After incubation at 37˚C for 4 h, the reaction was stopped by adding 0.75 ml of 1.0% (w/v) of 2-thiobarbituric acid in 50 mM NaOH and 0.75 ml of 2.8% (w/v) trichloroacetic acid. Then the solution was rapidly boiled for 10 min, and was cooled in water. The solution was centrifuged at 12,000 rpm for 5 min, and the absorbance of the supernatants was measured at 520 nm. Hydroxyl radical scavenging activity was evaluated as the inhibition rate of 2-deoxy-D-ribose oxidation by hydroxyl radicals. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control. The IC50 value was defined as the concentration of honey required to inhibit 50% of hydroxyl radical activity.
2.7. Effect of DPPH Radical
DPPH radical scavenging activity was measured by the method of Nagai et al. [20]. The reaction mixture contained 0.03 ml of 1.0 mM DPPH solution in ethanol, 0.24 ml of 99% of ethanol, and 0.03 ml of sample solution. The mixture was rapidly mixed and scavenging ability was measured by monitoring the decrease in absorbance at 517 nm. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control. The IC50 value was defined as the concentration of honey required to inhibit 50% of DPPH radical activity.
2.8. Antihypertensive Activity
The ACE inhibitory activity assay was performed by the method of Nagai et al. [20]. Twenty five microliters of sample solution and 75 μl of 0.1 M sodium borate buffer (pH 8.3) containing 5.83 mM hippuryl-L-histidyl-L-leucine and 1.0 M NaCl in an Eppendorf tube were preincubated at 37℃ for 5 min. Then the mixture was incubated with 25 μl of 0.1 M sodium borate buffer (pH 8.3) containing 1 mU ACE and 1.0 M NaCl at 37˚C for 60 min. The reaction was stopped by the addition of 125 μl of 1.0 M HCl. The resulting hippuric acid was extracted with 750 μl of ethyl acetate by mixing for 15 s. After centrifugation at 6000 rpm for 3 min, 500 μl of the upper layer was transported into the other tube and evaporated at 40˚C for 2 h. The hippuric acid was dissolved in 500 μl of distilled water, and then the absorbance was measured at 228 nm. The IC50 value was defined as the concentration of honey required to inhibit 50% of the ACE activity.
2.9. Statistical Analysis
Each assay was repeated 3 times independently and the results were reported as means ± standard deviation (SD). The significance of differences means as determined by a one-way analysis of variance with a significant level at p < 0.05.
3. Results and Discussion
Chemical properties of honey from E. vulgare were investigated. The protein, total phenolic components, and total vitamin C were about 3.2 mg/ml, 289.0 μg/ml, and 2.1 mg/100ml, respectively (Table 1). α-Amylase activity of honey was measured and it showed the highest activity about 710.8 IU/L. Nagai et al. [21] investigated the properties of honey species from different floral sources. As a result, the protein contents of honey from
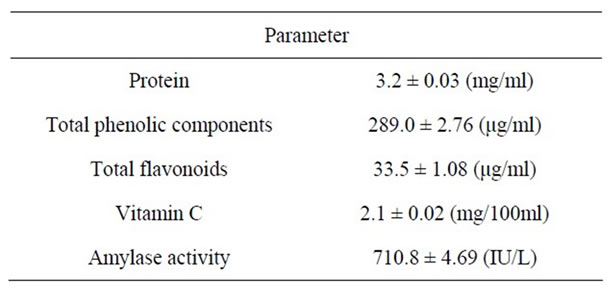
Table 1. The contents of protein, total phenolic components, total flavonoids, and vitamin C, and amylase activity of honey from Echium vulgare.
E. vulgare was slightly low as compared with other honey species [21]. The contents of total phenolic compounds of honey from E. vulgare were the highest among these honey species tested. Total flavonoid content of honey from E. vulgare was lower than total phenolic content as reported by Khalil et al. [22]. The α-amylase activity of honey from E. vulgare was extremely high and was about three to nine hundred times as much as those of other honey species [21].
Antioxidative activity on the peroxidation of linoleic acid was investigated to evaluate in vitro effect of honey from E. vulgare at the initiation stage of lipid peroxidation. As shown in Table 2, each fraction showed the antioxidative effect and the activity decreased with passage of time to 200 min. However, the activity increased with increasing of the concentration of honey. The activity for 1% honey was very low. The activity for 50% honey was fairly high and was similar to that of 5 mM ascorbic acid, although this did not amount to that of 1 mM α-tocopherol. Nagai et al. tested the antioxidant abilities of 6 species of honey using the same technique [14]. As a result, the activity of honey from E. vulgare was higher than those of commercially available honey and pure honeys from acacia and Chinese milk vetch, but lower than those of honeys from buckwheat, Japanese bee, and mixed-breed.
Superoxide anion is an initial free radical formed from mitochondrial electron transport systems. Mitochondria generate energy using a 4-electron chain reaction, reducing oxygen to water. Some of the electrons escaping from the mitochondrial chain reaction directly react with oxygen and form superoxide anion, that plays an important role in the formation of other reactive oxygen species in living systems, such as hydrogen peroxide, hydroxyl radical or singlet oxygen [23]. Superoxide radical has been implicated in playing crucial roles in ischaemia-reperfusion injury [24]. Superoxide anion radical scavenging activity of honey from E. vulgare was measured using xanthine-xanthine oxidase system. Each fraction exhibited the activity and the activity increased with increasing the concentration of honey (Table 3). The
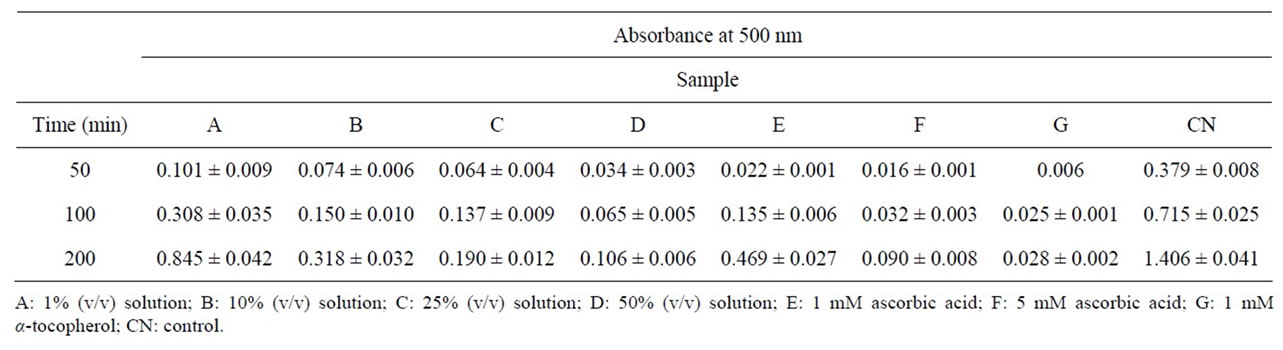
Table 2. Antioxidant activity of honey from Echium vulgare.
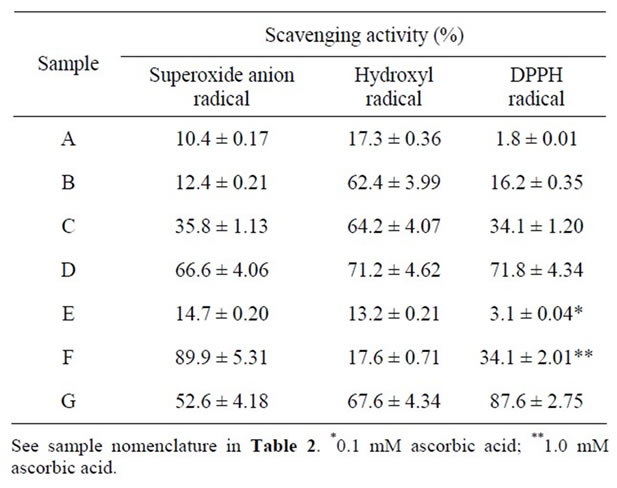
Table 3. Superoxide anion radical, hydroxyl radical, and DPPH radical scavenging activities of honey from Echium vulgare.
activity of 50% honey was higher than that of 1 mM α-tocopherol, but was lower than that of 5 mM ascorbic acid. Only the activities about 10% - 12% were detected for 1% and 10% honeys. In addition, the IC50 value against superoxide anion radical was calculated to 37.0%. As compared with other honey species, 50% honey from E. vulgare showed markedly higher activity than commercially available honey and pure honeys from acacia and Chinese milk vetch, but lower than honey from buckwheat [14]. Honey from E. vulgare exhibited the same activity as those from Japanese bee and mixed-breed [14].
Hydroxyl radical is the most reactive free radical and is formed from superoxide anion and hydrogen peroxide in the presence of metal ions such as copper or iron. Hydroxyl radical have the highest 1-electron reduction potential and can react with everything in living organisms at second-order rate constants of 109-10 mol/s. Hydroxyl radicals react with lipids, polypeptides, proteins, and DNA, especially thiamine compounds, it can add across a double bond, resulting in the hydroxycyclohexadienyl radical. The resulting radical can undergo further reactions, such as with oxygen, to give peroxyl radical or can decompose to phenoxyl-type radicals by water elimination [23]. Hydroxyl radical scavenging activity was investigated on honey from E. vulgare using the Fenton reaction mechanism. Each fraction showed hydroxyl radical scavenging activity, and its activity tended to increase with increasing degree of the concentration of honey (Table 3). The activity for 1% honey was low: this was similar to that of 5 mM ascorbic acid. Honey for 10% scavenged hydroxyl radical more than 62%, and it showed a similar activity to 1 mM α-tocopherol. Honey for 50% exhibited much higher scavenging activity than 1 mM α-tocopherol. The IC50 value against hydroxyl radical was calculated to 23.4%. The activity of honey from E. vulgare was lower than those from other honey species (about 90%) [14].
DPPH is a stable nitrogen-centered free radical, and its colour changes from violet to yellow when reduced by either the process of hydrogen or electrondonation. Substances to perform it above reaction can be considered as antioxidants and therefore radical scavengers [25]. DPPH radical scavenging activity was known to correlate well with the inhibitory capacity of lipid peroxidation of test compound [26]. DPPH has been widely used to investigate the free radical scavenging ability of various food samples. To evaluate the scavenging effect of DPPH on honey from E. vulgare, DPPH inhibition was tested and the results are shown in Table 3. Each fraction exhibited DPPH radical scavenging activity, although the activity was hardly detected in 1% honey. The activity for 25% honey was the same as that of 1.0 mM ascorbic acid and was about 34% (Table 3). Moreover, 50% honey scavenged this radical about 72%, although the activity did not amount to that of 1 mM α-tocopherol. High correlation was demonstrated between the concentration of honey and DPPH radical scavenging activity, with R2 = 0.998. That is, it suggested that honey from E. vulgare captured this radical in a concentration-dependent manner.
Hypertension is a significant public problem worldwide. One of the factors affecting blood pressure in mammals is ACE. ACE catalyzes the conversion of angiotensin I to the potent vasoconstrictor angiotensin II and also deactivation of the vasodilator nonapeptide bradykinin [27]. ACE inhibitors reduce blood pressure by decreasing peripheral vascular resistance and stabilizing renal function, making them useful in reducing the progress of diabetic nephropathy [28]. Therefore, finding new sources of ACE inhibitors, especially in food resources, is of great interest. ACE inhibitory activity of honey from E. vulgare was determined and are shown in Table 4. Each fraction inhibited the activity to different degrees, with increasing activities at higher concentrations. For 25% honey possessed fairly high activity about 85%, and 50% honey inhibited ACE activity about 94%. On the other hand, the IC50 value was 21.0% (0.67 mg protein/ml).
E. vulgare is a wild plant that enjoys dry meadows and fields, waste places, and roadsides. It grows tall and its beautiful blue wildflowers, rarely white or pink, flower from late spring to mid-summer. A member of the Borage family, it is native to southern Europe but it found in most countries from United States to New Zealand. It is often one of the many blossoms contributing to multifloral honeys from around the world, but in single flower honeys where it accounts for at least 45% of the content. It is the number one bee plant. New Zealand is the primary source, mainly from the Southern island where it grows wild in the dry mountain valleys and mountain sides during the summer months. This pure environment is ideal because of the low risk of pesticide and chemical contamination. E. vulgare is also a traditional medicinal herb, and is used to treat kidney and respiratory diseases, to soothe irritated tissues, and to aid in the healing of wounds.
E. vulgare honey is a yellow gold colour with a light clean taste, a floral bouquet, and lemon characteristics. It is high in fructose which makes it slow to crystallize. It is delicious in tea or coffee and compliments a strong cheese such as blue or Roquefort. Its honey is also known as Borage honey or Blue Borage honey. This should not be confused with honey made from Borage (Borago officinalis), a commercially grown plant used for seed oil,
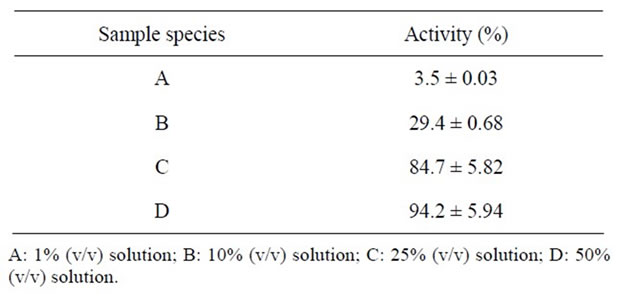
Table 4. ACE inhibitory activity of honey from Echium vulgare.
nor with honey from Purple Vipers Bugloss (E. plantagineu), popularly known in Australia as Patterson’s Curse.
Oxidative stress is involved in the pathology of oxidation-linked diseases such as cancer, heart disease, atherosclerosis, and rheumatoid arthritis and could play a role in neurodegenerative diseases and aging process [29]. Dietary phenolic compounds have generally been considered as nonnutrients, and their possible benefit to human health through their phenolic-linked antioxidant effects has only recently been considered. It is expected that a useful functionality is possessed in honey made from E. vulgare. So, we tried to investigate the functional properties of honey from E. vulgare with various assays. In the present investigation, this honey showed the best performance in inhibiting lipid peroxidation and scavenging superoxide anion radicals, hydroxyl radicals, and DPPH radicals in comparison to the reference compounds ascorbic acid and α-tocopherol, which may be related to the contents of total phenolic compounds. Moreover, it exhibited stronger inhibition activity of ACE. Our previous report [14] reached a conclusion that higher antioxidative activity and scavenging activities against active oxygen species in honey species related to their colour: higher activity was observed in honeys with dark colour such as buckwheat and mixed-breed honeys in comparison with light coloured honeys as acasia and Chinese milk vetch. However, honey from E. vulgare with a yellow gold colour showed high antioxidative activity. It suggests that it owe to markedly high content of phenolic compounds in honey from E. vulgare. The phenolics in honey from E. vulgare might be the major active component responsible for the strong antioxidative activity and scavenging activity. More detailed investigation between the individual phenolic compounds present in honey from E. vulgare and these functional properties needs to be carried out in the future.
4. Conclusion
This is the first report concerning to the honey from E. vulgare belongs to the order Boraginaceae for antioxidant and antihypertensive capacities. Honey from E. vulgare showed the best performance in inhibiting lipid peroxidation and scavenging superoxide anion radicals, hydroxyl radicals, and DPPH radicals. Moreover, it exhibited stronger inhibition activity of ACE. It is known that higher antioxidative activity and scavenging activity against active oxygen species in honey species related to their colour. In the present study, however, it suggests that the phenolics and flavonoids in honey from E. vulgare with yellow gold colour might be the major active component responsible for the strong antioxidative activity and radical scavenging activity.
REFERENCES
- D. Gems and L. Partridge, “Stress-Response Hormesis and Aging: That Which Does Not Kill Us Makes Us Stronger,” Cell Metabolism, Vol. 7, No. 3, 2008, pp. 200- 203. doi:10.1016/j.cmet.2008.01.001
- J. A. Imlay, “Pathways of Oxidative Damage,” The Annual Review of Microbiology, Vol. 57, 2003, pp. 395-418. doi:10.1146/annurev.micro.57.030502.090938
- B. N. Ames, M. K. Shigenaga and T. M. Hagen, “Oxidants, Antioxidants and Degenerative Diseases of Aging,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 90, 1993, pp. 7915- 7922. doi:10.1073/pnas.90.17.7915
- R. Chang, “Functional Properties of Edible Mushrooms,” Nutrition Reviews, Vol. 54, No. 11, 1996, pp. S91-S93. doi:10.1111/j.1753-4887.1996.tb03825.x
- G. Strazzulo, A. De Giulio, G. Tommonaro, C. La Pastina, A. Poli, B. Nicolaus, R. De Prisco and C. Satumimo, “Antioxidative Activity and Lycopene and β-Carotene Contents in Different Cultivars of Tomato (Lycopersicon esculentum),” International Journal of Food Properties, Vol. 10, 2007, pp. 321-329. doi:10.1080/10942910601052681
- E. Niki and N. Noguchi, “Evaluation of Antioxidant Capacity. What Capacity Is Being Measured by Which Method?” Life, Vol. 50, No. 4-5, 2000, pp. 323-329.
- N. Pellegrini, M. Serafini, B. Colombi, D. Del Rio, S. Salvatore, M. Bianchi and F. Brighenti, “Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in Vitro Assays,” Journal of Nutrition, Vol. 33, 2003, pp. 2812- 2819.
- J. H. Dustmann, “Honey, Quality and Its Control,” American Bee Journal, Vol. 133, No. 9, 1993, pp. 648-651.
- “New Standard Tables of Food Composition in Japan,” In: New Standard Tables of Food Composition in Japan Editorial Committee, Ed., Tokyo Horei Publishing Co Ltd, Tokyo, 2011.
- F. Ferreres, C. Garciaviguera, F. Tomaslorente and F. A. Tomasbarberan, “Hesperetin C a Marker of the Floral Origin of Citrus Honey,” Journal of the Science of Food and Agriculture, Vol. 61, 1993, pp. 121-123. doi:10.1002/jsfa.2740610119
- P. Cerutti, “Oxy-Radicals and Cancer,” Lancet, Vol. 344, No. 8926, 1994, pp. 862-863. doi:10.1016/S0140-6736(94)92832-0
- M. M. Jimenez, M. J. Fresno and E. Selles, “The Galenic Behaviour of a Dermopharmaceutical Excipient Containing Honey,” International Journal of Cosmetic Science, Vol. 16, No. 5, 1994, pp. 211-226. doi:10.1111/j.1467-2494.1994.tb00098.x
- R. A. Cooper, P. C. Molan and K. G. Harding, “The Sensitivity to Honey of Gram-Positive Cocci of Clinical Significance Isolated from Wounds,” Journal of Applied Microbiology, Vol. 93, No. 5, 2002, pp. 857-863. doi:10.1046/j.1365-2672.2002.01761.x
- T. Nagai, R. Inoue, N. Kanamori, N. Suzuki and T. Nagashima, “Characterization of Honey from Different Floral Sources. Its Functional Properties and Effects of Honey Species on Storage of Meat,” Food Chemistry, Vol. 97, No. 2, 2006, pp. 256-262. doi:10.1016/j.foodchem.2005.03.045
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, 1951, pp. 265-275.
- K. Slinkard and V. L. Singleton, “Total Phenol Analysis,” American Journal of Enology and Viticulture, Vol. 28, No. 1, 1977, pp. 49-55.
- J. Zhishen, T. Mengcheng and W. Jianming, “The Determination of Flavonoid Contents in Mulderry and Their Scavenging Effects on Superoxide Radicals,” Food Chemistry, Vol. 64, 1999, pp. 555-559. doi:10.1016/S0308-8146(98)00102-2
- “Vitamin Handbook,” In: The Vitamin Society of Japan, Ed., Kagakudojin, Kyoto, 1990.
- T. Nagai, R. Inoue, N. Suzuki and T. Nagashima, “AlphaAmylase from Persimmon Honey: Purification and Characterization,” International Journal of Food Properties, Vol. 12, No. 3, 2006, pp. 512-521. doi:10.1080/10942910701867764
- T. Nagai and T. Nagashima, “Functional Properties of Dioscorin, a Soluble Viscous Protein from Japanese Yam (Dioscorea opposita Thunb.) Tuber Mucilage Tororo,” Zeitschrift für Naturforschung, Vol. 61c, No. 11-12, 2006, pp. 792-798.
- T. Nagai, R. Inoue, Y. Takami, N. Suzuki and T. Nagashima, “α-Amylase in Commercially Available Honey Species from Different Floral Sources,” ITE Letters on Batteries, New Technologies & Medicine, Vol. 7, No. 2, 2006, pp. 194-197.
- Md. I. Khalil, M. Mahaneem, S. M. S. Jamalullail, N. Alam and S. A. Sulaiman, “Evaluation of Radical Scavenging Activity and Colour Intensity of Nine Malaysian Honeys of Different Origin,” Journal of ApiProduct and ApiMedical Science, Vol. 3, No. 1, 2011, pp. 4-11. doi:10.3896/IBRA.4.03.1.02
- J. Lee, N. Koo and D. B. Min, “Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals,” Comprehensive Reviews in Food Science and Food Safety, Vol. 3, No. 1, 2004, pp. 21-33. doi:10.1111/j.1541-4337.2004.tb00058.x
- R. Radi, J. S. Beckman, K. M. and B. A. Freeman, “Peroxynitrite-Induced Membrane Lipid Peroxidation: Cytotoxic Potential of Superoxide and Nitric Oxide,” Archives of Biochemistry and Biophysics, Vol. 288, No. 2, 1991, pp. 481-487. doi:10.1016/0003-9861(91)90224-7
- W. Brand-Williams, M. Cuvelier and C. Berset, “Use of a Free Radical Method to Evaluate Antioxidant Activity,” LWT-Food Science and Technology, Vol. 28, No. 1, 1995, pp. 25-30. doi:10.1016/S0023-6438(95)80008-5
- E. Rekka and P. N. Kourounakis, “Effect of Hydroxyethyl Rutosides and Related Compounds on Lipid Peroxidation and Free Radical Scavenging Activity. Some Structural Aspects,” Journal of Pharmacy and Pharmacology, Vol. 43, 1991, pp. 486-491. doi:10.1111/j.2042-7158.1991.tb03519.x
- R. Haartmann and H. Meisel, “Food-Derived Peptides with Biological Activity: From Research to Food Applications,” Current Opinion in Biotechnology, Vol. 18, No. 2, 2007, pp. 163-169. doi:10.1016/j.copbio.2007.01.013
- P. A. Sarafidis, N. Khosla and G. L. Bakris, “Anti-Hypertensive Therapy in the Presence of Proteinuria,” American Journal of Kidney Diseases, Vol. 49, No. 1, 2007, pp. 12-16. doi:10.1053/j.ajkd.2006.10.014
- R. Briante, F. Febbraio and R. Nucci, “Antioxidant Properties of Low Molecular Weight Phenols Present in the Mediterranean Diet,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 1, 2003, pp. 6975-6981. doi:10.1021/jf034471r
NOTES
*Corresponding author.

