American Journal of Plant Sciences
Vol.3 No.2(2012), Article ID:17541,7 pages DOI:10.4236/ajps.2012.32024
Elevated Carbon Dioxide Alters the Relative Fitness of Taraxacum officinale Genotypes
![]()
Crop Systems and Global Change Laboratory, USDA-ARS, Beltsville Agricultural Research Center, Beltsville, USA.
Email: James.Bunce@ars.usda.gov
Received October 7th, 2011; revised November 9th, 2011; accepted December 5th, 2011
Keywords: Dandelion; Taraxacum officinale; Elevated Carbon Dioxide; Fitness; Growth; Photosynthesis; Leaf Initiation; Adaptation
ABSTRACT
I tested whether elevated [CO2] affected which genotypes of Taraxacum officinale had highest fitness in two field experiments. In one experiment, T. officinale plants which persisted as weeds in alfalfa plots in open top chambers at ambient and elevated [CO2] were compared. In a second experiment, T. officinale seeds collected from local habitats were mixed and scattered in open top chambers at ambient and elevated [CO2], and plants producing seeds after one and two years in monocultures were compared. In both experiments seeds produced in each chamber were collected, and many plants from the seed lot from each chamber were grown in controlled environment chambers to test whether the [CO2] of the chamber of origin affected the mean value of various plant parameters. In both experiments, the results indicated that field exposure to elevated [CO2] altered the relative fitness of genotypes. Elevated [CO2] favored genotypes which produced biomass more rapidly at elevated [CO2] in both experiments, primarily because of faster rates of leaf initiation. The results suggest that genotypes of this species vary widely in fitness at elevated [CO2] whether grown in monocultures or in mixed communities, and that this species could adapt rapidly to rising atmospheric [CO2].
1. Introduction
The concentration of carbon dioxide in the atmosphere ([CO2]) increased from about 280 mol·mol–1 in 1900 to about 390 currently primarily because of increased CO2 emissions caused by human activities, and continues to rise exponentially. Projected levels of atmospheric [CO2] over the current century (e.g. up to 1000 mol·mol–1) usually increase biomass accumulation rates of C3 species, at least in non-competitive situations. Differences among species in the degree of growth stimulation have long been recognized, and have implications for competition and for the species composition and the functioning of future ecosystems.
Intraspecific differences in growth stimulation by elevated [CO2] have also been found in many species [1], including crops e.g. [2-4], trees [5], and Arabidopsis [6,7]. Intraspecific differences in fitness at elevated [CO2] could lead to evolutionary changes within species as atmospheric [CO2] rises. It remains unclear whether evolution at elevated [CO2] would result in increased rates of biomass accumulation at elevated [CO2], which could affect processes as diverse as the global carbon cycle and crop-weed competition. Studies of genotypes growing near CO2 springs have shown that such genotypes sometimes have faster growth rates when grown at elevated [CO2] than local control genotypes, e.g. [8-11]. In contrast, selection at elevated [CO2] did not increase rates of biomass accumulation at elevated [CO2] in Abutilon theophrasti [12], Arabidopsis thaliana [6], Bromus erectus [13], or Sanguisorba minor [14]. However, the studies on Arabidopsis thaliana and Abutilon theophrasti were conducted indoors, and in both Bromus erectus and Sanguisorba minor there was no growth stimulation by elevated [CO2]. It has been suggested that whether or not adaptation to elevated [CO2] would favor faster rates of biomass accumulation might depend on the degree of competition during the selection [12,13]. With the exception of adaptation to elevated [CO2] favoring a shorter life cycle and lower respiration in A. thaliana [15], there is little information about what specific traits are selected for at high [CO2] [16].
In this study, I tested whether elevated [CO2] differentially affected which genotypes of Taraxacum officinale produced viable seeds, and identified morphological and physiological traits associated with fitness at elevated [CO2]. In two separate experiments, selection at elevated [CO2] was examined in plants grown outdoors as a monoculture and as a minor component of a dense herbaceous community. T. officinale is largely apomictic, and no out-crossing flowers, which have a distinct morphology, were observed during the course of these experi ments. Thus seeds produced by each individual would be genetically identical to the parent.
2. Materials and Methods
2.1. Competitive Experiment
In one experiment, the T. officinale L. plants which persisted as weeds and were producing seeds three years after the establishment of alfalfa (Medicago sativa L.) plots in open top chambers at ambient and elevated [CO2] were compared. Alfalfa plots were established in the spring of 2004 in twelve chambers at the South Farm of the Beltsville Agricultural Research Center, Beltsville, Maryland. Half the chambers were flushed with air at the current ambient [CO2] and half were flushed with air 350 ± 50 mol·mol–1 above that of the ambient air. Ambient air averaged 378 mol·mol–1 [CO2] in daytime and 455 at night. The [CO2] treatment was applied at all times except when the ground was covered with snow. Details of chamber design and operation and plot maintenance are given in [17]. Chambers received only natural rainfall. All plant material in the chambers was cut to 5 cm height approximately every 26 days during spring, summer and fall. T. officinale plants became naturally established in all chambers from local seeds, but always constituted less than 15% of the harvested biomass. All seeds of T. officinale which were produced in the chambers during the third year (2007) were collected separately for each chamber, dried for a few days in air and then stored in a refrigerator until testing of the seed lots in indoor controlled environment chambers. There were at least 10 T. officinale plants in each chamber producing flowers in the third year.
2.2. Monoculture Experiment
In another experiment, individual seed heads from 40 widely spaced T. officinale plants were collected in the spring of 2005 from each of four local habitats in Beltsville, Maryland. The habitats were a frequently mowed lawn, a field used for annual crops, in which maize was grown the prior season, a meadow which had been mowed about three times per year for many years, and an apple orchard established about ten years prior to collecting seeds. Seeds from all four environments were thoroughly mixed, and after reserving some of the seeds for characterizing the variation within the mixed seed lot in indoor chambers, the seeds were scattered on the surface of recently tilled soil in four open top chambers on May 16, 2005. Each open top chamber covered 1.9 m2 ground. Two of the chambers were flushed continually with air drawn from outside the chamber, and two of the chambers were flushed with air at the same rate to which pure CO2 was added a rate sufficient to maintain the [CO2] at 350 ± 50 mol·mol–1 above that in the ambient [CO2] chambers. The [CO2] treatment was applied at all times except when the ground was covered with snow. Plants other than T. officinale were removed by hand. The chambers were not irrigated. Air samples from each chamber were sequentially pumped through an infrared CO2 analyzer in an adjacent air conditioned shelter for recording chamber [CO2]. Flow rates of CO2 were adjusted daily as necessary. At least 50 plants in each chamber produced flowers in the first year of the experiment, and there were more plants in each chamber in the second year. Seeds were collected from every seed head produced in all chambers during the first year after seeding, and also separately in the second year after seeding. During the first year, approximately half of the seeds from each seed head were collected, and the other half dispersed within the chamber. This was to allow seedling recruitment to occur from the seeds produced in the first year. In the second year, all seeds were collected, since the experiment was to be terminated and there was no need to allow recruitment from the seeds produced. Seeds were pooled within a chamber each year, air dried a few days and stored in a refrigerator for later testing.
2.3. Testing of Seed Lots
For the initial mixed seed lot from the four local habitats and for both of the field experiments, seed lots were tested in controlled environment chambers with 21˚C/15˚C day/night temperatures. There were 13 h per day of light at 1000 mol m–2·s–1 photosynthetically active radiation. The temperatures and day length were selected as representative of spring conditions in Maryland, when T. officinale has its most rapid growth. Plants were grown one per pot in peat moss, watered daily, and fertilized with a slow release complete fertilizer. The chambers were controlled either at 370 ± 10 or 720 ± 10 mol·mol–1 [CO2].
Plants from the initial mixed seed lot were only grown at 370 mol·mol–1 [CO2], in three successive plantings in one chamber. At each planting, there were 32 pots. About 10 seeds were planted in each pot, and emerged seedlings were randomly thinned to one per pot. Plants were grown for up to 45 days, and all remained vegetative. The primary focus was on characterizing leaf physiology and plant morphology, rather than plant growth rates. Plants were therefore measured and destructively harvested as soon as successive leaves were the same size and shape (30 to 45 days). Photosynthesis (A), stomatal conductance (gs) and sub-stomatal carbon dioxide (Ci) were measured inside the growth chamber on intact leaves under the daytime growth conditions of temperature, humidity, [CO2], and at a saturating PPFD of 1500 mol m–2·s–1, using a CIRAS-2 portable leaf gas exchange system (PP Systems, Amesbury, MA). Measurements were conducted on the most recently fully expanded leaves, which tests showed had the highest rates of any leaves on the plants. After measuring leaf gas exchange, the length, maximum width, area and dry mass of that leaf were determined, along with the total plant leaf area and the dry mass of the shoot, tap root, and fibrous roots.
There were three chamber runs at both ambient and elevated [CO2] for each of the two field experiments, with the [CO2] treatments rotated among chambers during the replicate chamber runs. For the “competitive” experiment, in each chamber run six pots were planted with seeds from each of the twelve field chambers in each of the two [CO2] treatment chambers. Pots were thinned to one randomly selected seedling per pot a few days after emergence. Plants were grown for 35 - 37 days from seeding. Leaf photosynthesis and stomatal conductance measurements were conducted as previously described on the day prior to harvest. Dark respiration (R) was also measured on the same leaves, at 15˚C, after 30 to 90 minutes of darkness, but only for plants grown at elevated [CO2]. On day 35, the length, maximum width, area and dry mass of the leaf in which gas exchange had been measured were determined, along with the number of leaves exceeding 0.5 cm in length, and the total leaf area and dry mass of the shoot. For the “monoculture” experiment, fifteen pots were planted with seed of each of the four seed lots in each of two indoor chambers, with the indoor chamber [CO2] treatments rotated among the two chambers in three successive replicate runs. Plants were grown for 35 days from planting, and the same parameters were measured as in the “competitive” experiment described earlier.
2.4. Statistics
In the tests of the mixed seed lot from four local habitats, there were no differences between chamber runs in the mean values of any of the measured plant parameters. Therefore the data from all three chamber runs were combined. Means and standard deviations were compared with those from seed lots collected from the ambient [CO2] monoculture outdoor chambers after one and two years, and grown at 370 mol·mol–1 [CO2] in the indoor chambers. When the hypothesis of homogeneity of variance was rejected using Bartlett’s test for a given parameter, means were compared using Welch’s test, otherwise means were compared using analysis of variance (JMP version 5, SAS Institute, Cary NC).
Statistical tests were conducted on the mean values (of 6 or 15 plants) for each outdoor chamber which were obtained in each indoor chamber run. For comparisons of seed lots from the different field chambers, these mean values were classified by the [CO2] of the field chamber of origin and the indoor growth chamber replication. Two-way analysis of variance was used to test for differences among means of the [CO2] of the field chambers, indoor chamber replication, and the interaction. In no case was the indoor chamber replication or the interaction term significant at the 5% level of probability. In the monoculture experiment, separate analyses were conducted for the two years of seed collection from the field chambers. No direct statistical comparisons were made of indoor chamber runs of 370 and 730 mol·mol–1 [CO2], because the duration of plant growth was not always uniform across these [CO2] treatments.
3. Results
3.1. Competitive Experiment
Mean dry mass of seeds was 0.52 mg per seed for seeds collected from the ambient [CO2] field chambers, and 0.53 mg per seed for seeds collected from the elevated [CO2] chambers. These means did not differ significantly at P = 0.05. Plants grown from seed lots collected from the elevated [CO2] chambers had significantly more total leaf area and shoot dry mass at the final harvest than plants from seed lots from the ambient [CO2] chambers, when grown in indoor test chambers at 720 mol·mol–1 [CO2] (Table 1). There were no significant differences between plants from ambient and elevated field chambers in leaf photosynthesis, stomatal conductance, Ci, dry mass per unit of area, or respiration rates expressed either on an area or dry mass basis (Table 1). The length and area of the leaves measured for gas exchange also did not differ with the [CO2] of the field chamber, but plants from elevated [CO2] chambers had more leaves longer than 0.5 cm than did those from ambient [CO2] chambers.
When seed lots from the ambient and elevated field chambers were tested in the indoor chambers at 370 mol·mol–1 [CO2], plants from the elevated [CO2] chambers had significantly higher shoot dry mass and leaf mass per area, but not total leaf number or total leaf area (Table 1).
3.2. Monoculture Experiment
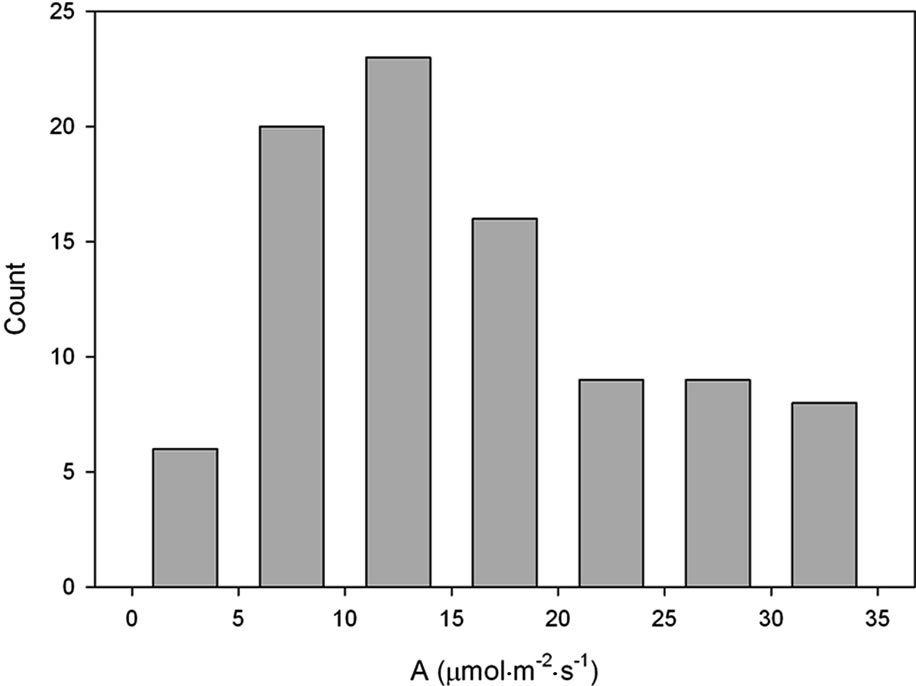
The plants developed from the seeds collected from the four local habitats were extremely variable in physiology and in morphological characteristics when grown in the indoor controlled environment chambers at ambient [CO2]. This is illustrated by the broad frequency distributions of plant traits (Figure 1). After growth in monocultures in the field chambers at ambient CO2, the mean values of most traits differed from the initial population, and the variance was significantly lower for several of the traits (Table 2). Mean values of photosynthesis, stomatal conductance and leaf length also differed between years in the monoculture experiment (Table 2).
Mean dry mass of seeds was 0.51 mg per seed for seeds
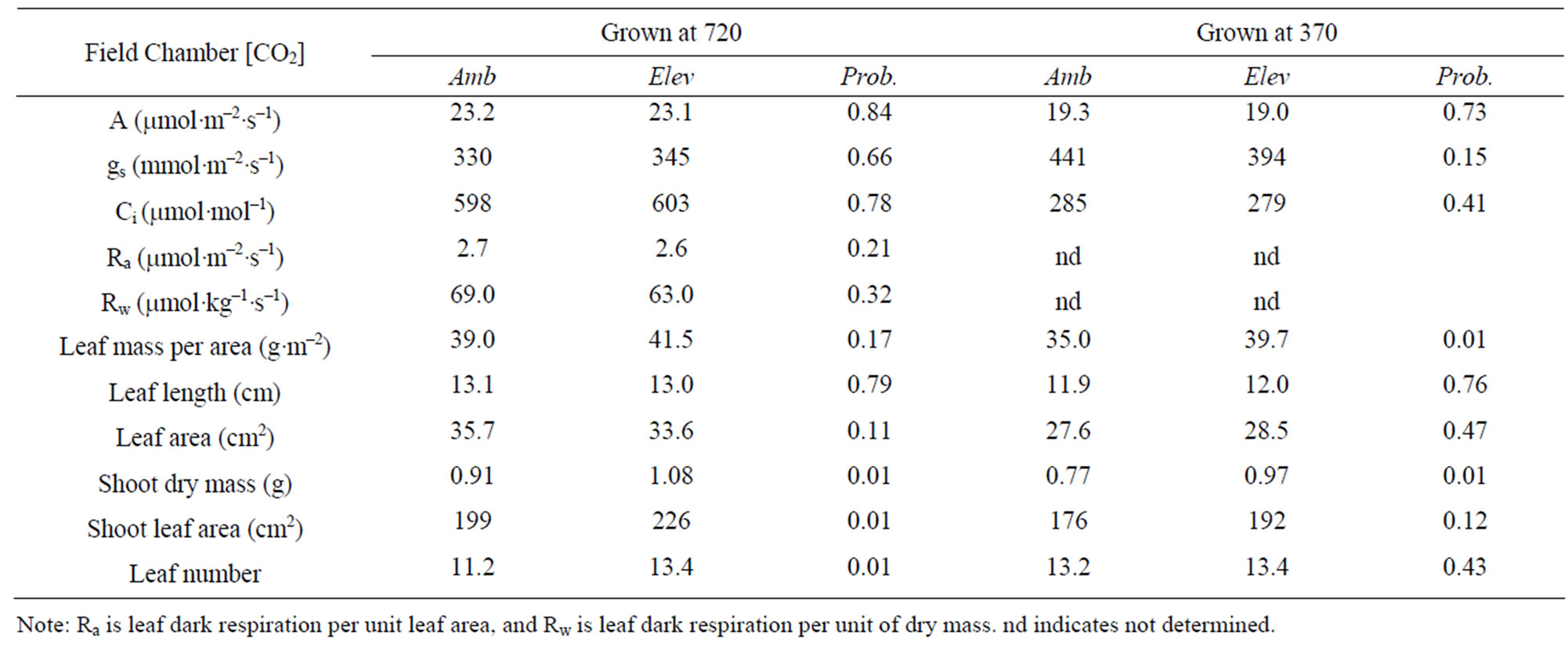
Table 1. Mean characteristics of T. officinale plants grown from seeds collected from ambient or elevated [CO2] field chambers with alfalfa, and grown at either 720 or 370 mol·mol–1 [CO2] in indoor chambers, and the probability of a greater F value under the null hypothesis of no difference between ambient and elevated [CO2] field chambers.
 (a)
(a)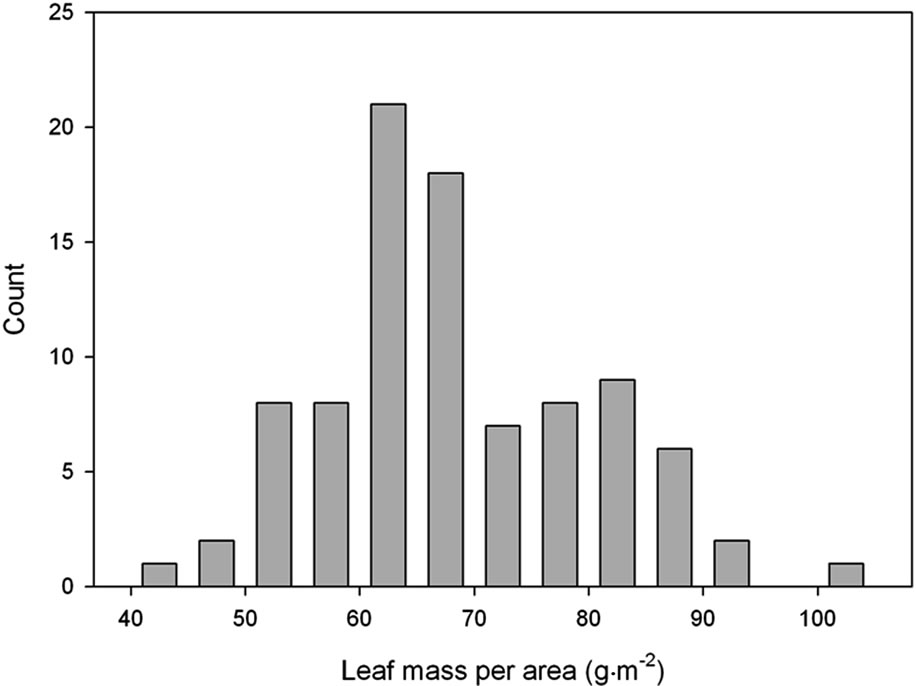 (b)
(b)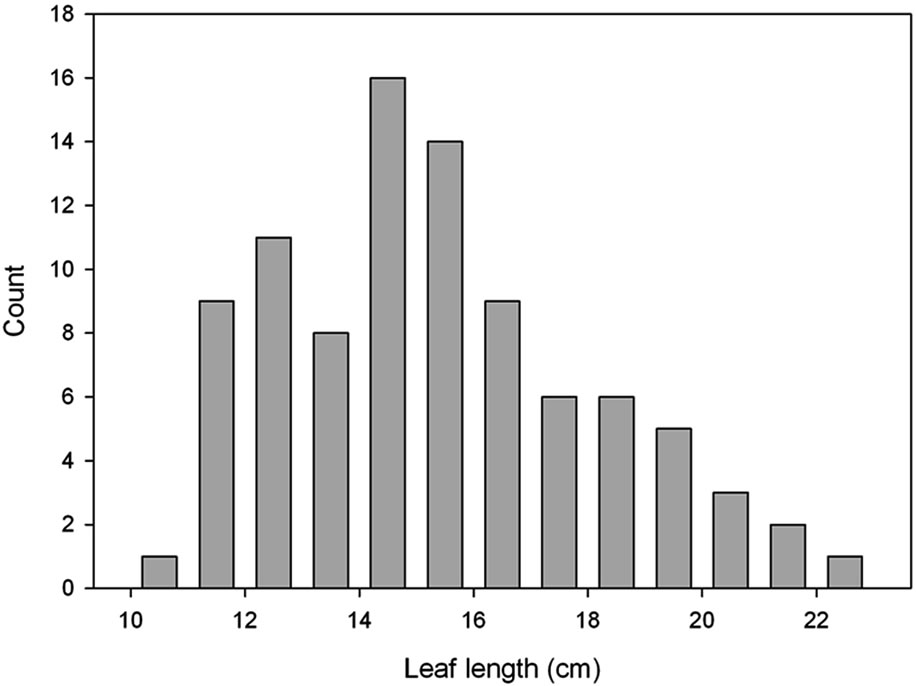 (c)
(c)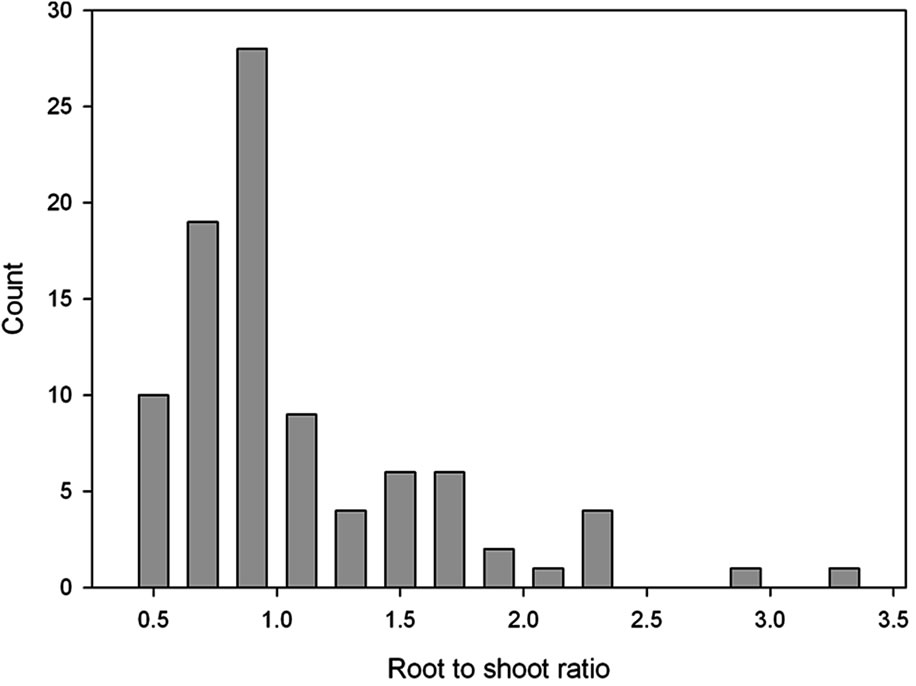 (d)
(d)
Figure 1. Frequency distributions of (a) photosynthetic carbon dioxide assimilation rate; (b) Leaf mass per unit of leaf area; (c) Root to shoot ratio, and (d) Length of fully expanded leaves of Taraxacum officinale plants grown from mixed seeds collected from four local habitats. A total of 91 plants were sampled after growth under uniform controlled environment conditions at ambient [CO2].
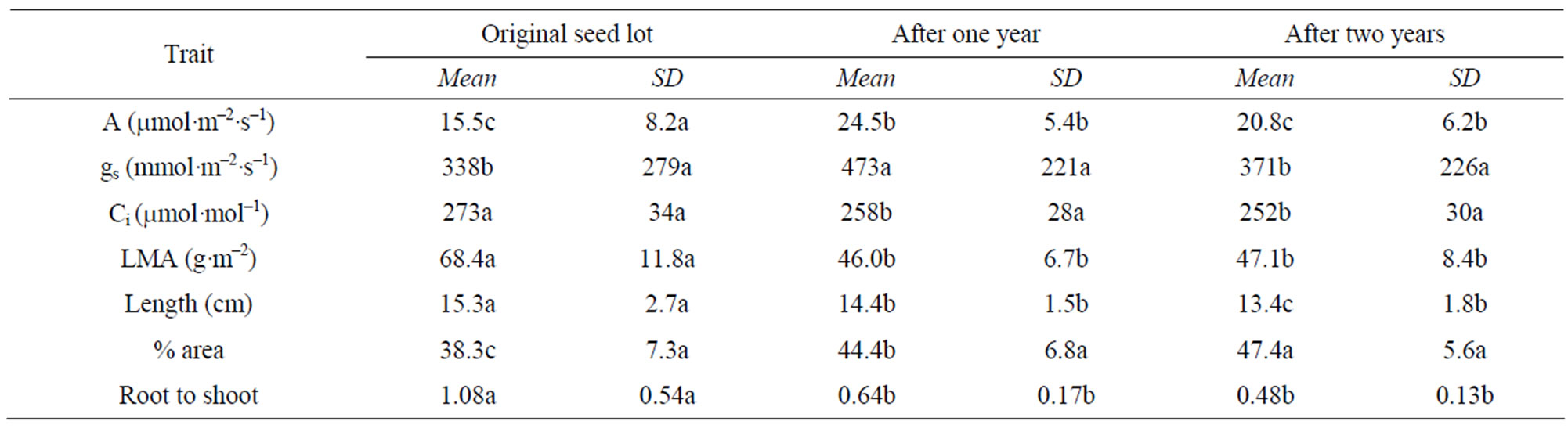
Table 2. Mean and standard deviation (SD) of characteristics of T. officinale plants grown from seeds in the original seed lot, and collected from plants grown in field monoculture chambers at ambient [CO2] for one or two years, after growth at ambient [CO2] in indoor controlled environment chambers.
collected from the ambient [CO2] field chambers, which was not significantly different from the 0.52 mg per seed for seeds collected from the elevated [CO2] chambers. Seeds produced in the first year of the monoculture experiment produced plants which differed significantly in the shoot dry mass, total leaf area, and leaf number, when plants were grown at elevated [CO2] in the indoor chambers (Table 3), with faster growth in plants from elevated [CO2] field chambers. There were no significant differences between seed lots from the ambient and elevated chambers in other traits measured, and no differences were found in any measured traits when plants were grown at ambient [CO2] (Table 3).
Seeds collected from the elevated [CO2] field chambers in the second year of the monoculture experiment also produced plants with significantly higher shoot dry mass, leaf area, and leaf number than from the ambient chambers when grown at 720 mol·mol–1 [CO2] (Table 4). The plants from the elevated [CO2] field chambers also had longer leaves, with larger area and higher leaf mass per unit of area. When these seed lots were grown at 370 mol·mol–1, the only trait that differed significantly between ambient and elevated [CO2] field chambers was leaf number (Table 4).
4. Discussion
Besides ranging over a large geographical area, Taraxacum officinale is a very variable species even within small geographic areas. Some of the variation is assorted by local habitat conditions, such as light and soil water [18]. The plants grown from the samples of seeds collected from the four local habitats in this study showed remarkable variation in many traits when grown indoors under uniform conditions. The variation in T. officinale was much larger than in Plantago lanceolata [19,20]) or Anthoxanthum odoratum [20] collected from a range of habitats in Durham, NC. Seeds produced by plants grown in monocultures in the ambient [CO2] field chambers had different mean values than the initial seed lot, and smaller variation for many plant traits. This indicates that certain genotypes in the original seed lot were better able to produce viable seeds under these field conditions than were other genotypes. The selection pressure of the field chamber environment was evidently not constant over years, because mean values of some parameters differed significantly between the first and second year in the field chambers. For example, mean leaf CO2 assimilation rates were lowest in plants from the initial seed lot, highest after one year in the ambient [CO2] field chambers, and intermediate in the second year (Table 2). It is not known whether such changes over time resulted from differences in weather between years or from increased plant density over time. This species seems to be ideally suited for population and physiological studies of what plant traits are favored in which environments.
Seed production was favored in different genotypes at elevated than at ambient [CO2] in both the monoculture and the competitive experiments. This indicates that [CO2] was a selective agent for this species. Comparing plants grown from seeds eliminates the potential problem of virus infection of vegetatively propagated material [20]. The most consistent characteristic of the genotypes selected for at elevated [CO2] was faster leaf area and biomass production, and an increased rate of leaf initiation, when grown at elevated [CO2]. Selection at elevated [CO2] also resulted in plants with greater numbers of leaves when grown at elevated [CO2] in Sanguisorba minor [14]. Similarly, higher relative growth rates were found in Plantago asiatica plants from sites with higher [CO2] when grown in a common environment, due partly to a genetically determined higher leaf to root mass ratio [11]. The faster growth and leaf initiation in T. officinale could not be attributed to differences in leaf photosynthesis or respiration rates or to seed size differences. Transgenerational effects of growth at elevated [CO2] on seedling growth were found in three species [21], but with slower growth for seeds developed at elevated [CO2]. Transgenerational effects were probably not involved in the observations reported here, based on the
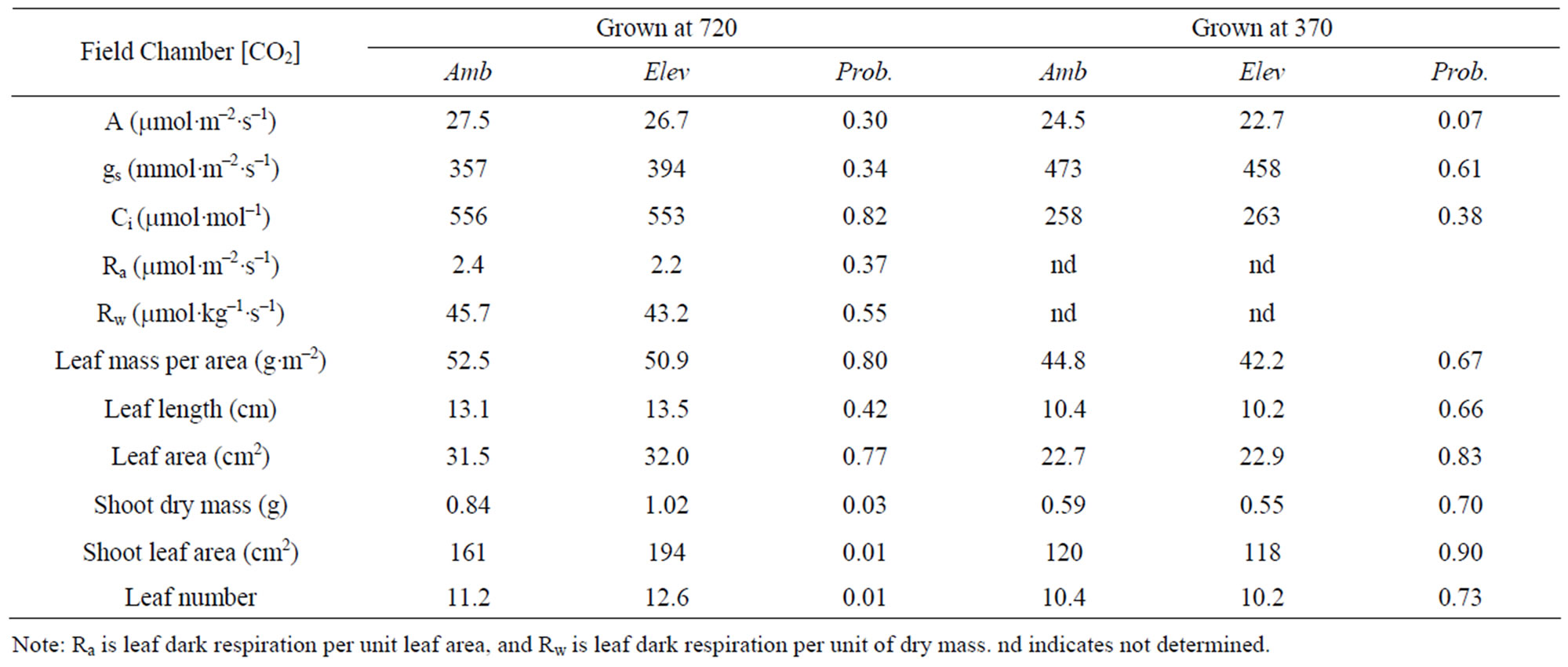
Table 3. Mean characteristics of T. officinale plants grown from seeds collected from ambient or elevated [CO2] field monoculture chambers after one year, and grown at either 720 or 370 mmol·mol−1 [CO2] in indoor chambers, and the probability of a greater F value under the null hypothesis of no difference between ambient and elevated [CO2] field chambers.
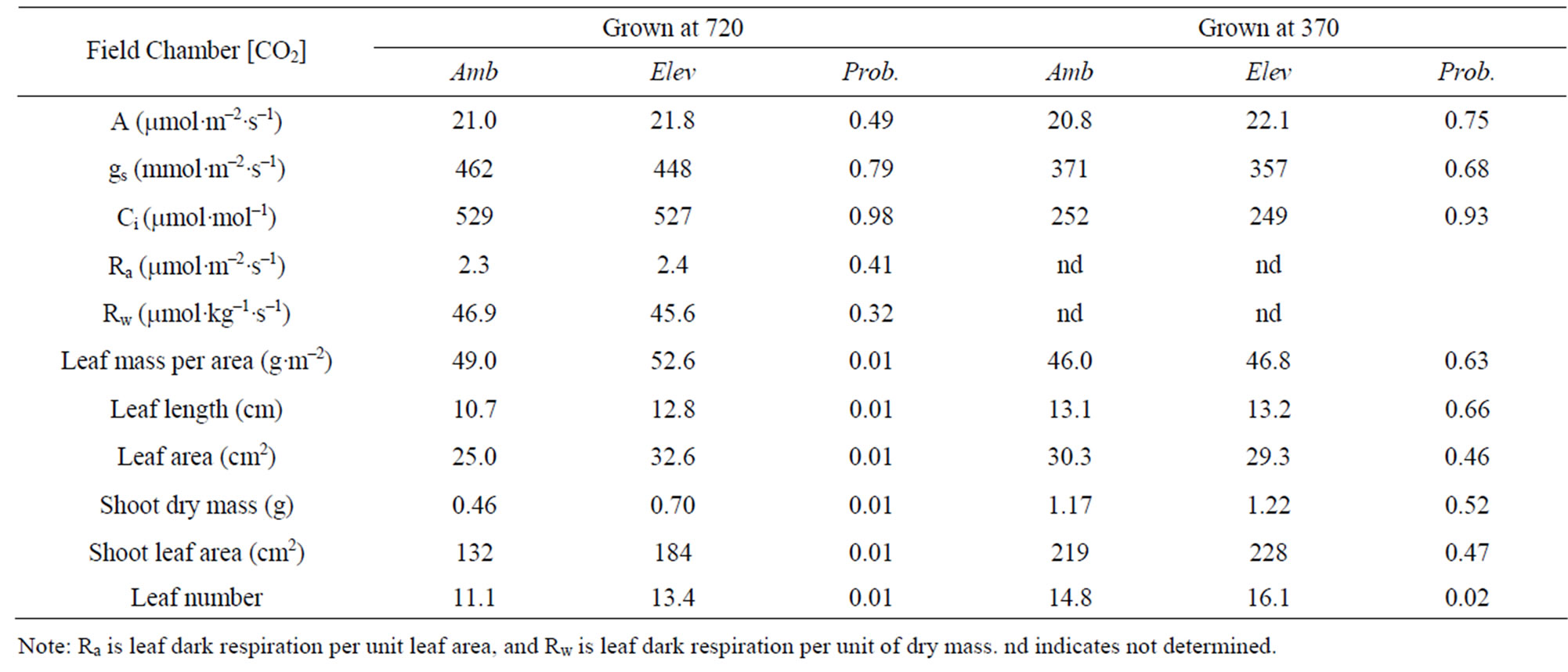
Table 4. Mean characteristics of T. officinale plants grown from seeds collected from ambient or elevated [CO2] field monoculture chambers after two years, and grown at either 720 or 370 mmol·mol–1 [CO2] in indoor chambers, and the probability of a greater F value under the null hypothesis of no difference between ambient and elevated [CO2] field chambers.
lack of differences in seed size, and the general absence of differences observed when the seed lots were assayed at ambient [CO2] [22]. The faster growth of genotypes selected at elevated [CO2] resulted in 19% to 52% higher mean shoot mass after 35 - 37 days of growth at elevated [CO2], depending on the experiment. This suggests that adaptation of T. officinale to elevated [CO2] is likely to increase its rate of biomass accumulation. Indeed, the biomass of T. officinale harvested over the three years of alfalfa culture was 2.3 times as high in the elevated chambers as in the ambient chambers, despite a simultaneous average 32% increase in biomass of the alfalfa plas, which dominated the system [17].
It is noteworthy that in most cases the differences in traits of the plants adapted to elevated [CO2] were not evident when plants were grown at lower [CO2]. It seems that traits adapting these plants to elevated [CO2] have a plastic response to [CO2], perhaps related to utilizing the stimulation of photosynthesis to accelerate leaf production. While it is perhaps not surprising that some adaptive traits are only evident in the environment where they are adaptive, this has potentially important implications for efforts to adapt crop species to rising atmospheric [CO2] [10].
Overall, the results suggest that genotypes of T. officinale vary widely in fitness at elevated [CO2] whether grown in monocultures or in mixed communities, indicating that this species could adapt rapidly to rising atmospheric [CO2].
REFERENCES
- J. K. Ward and J. K. Kelly, “Scaling up Evolutionary Responses to Elevated CO2: Lessons from Arabidopsis,” Ecology Letters, Vol. 7, No. 5, 2004, pp. 427-440. doi:10.1111/j.1461-0248.2004.00589.x
- L. H. Ziska, J. A. Bunce and F. Caulfield, “Intraspecific Variation in Seed Yield of Soybean (Glycine max) in Response to Increased Atmospheric Carbon Dioxide,” Australian Journal of Plant Physiology, Vol. 25, No. 7, 1998, pp. 801-807. doi:10.1071/PP98058
- J. A. Bunce, “Contrasting Responses of Seed Yield to Elevated Carbon Dioxide under Field Conditions within Phaseolus vulgaris,” Agriculture, Ecosystems and Environment, Vol. 128, No. 4, 2008, pp. 219-224. doi:10.1016/j.agee.2008.06.003
- H. Shimono, M. Okada, Y. Yamakawa, H. Nakamura, K. Kobayashi and T. Hasegawa, “Genotypic Variation in Rice Yield Enhancement by Elevated CO2 Relates to Growth before Heading, and Not to Maturity Group,” Journal of Experimental Botany, Vol. 60, No. 2, 2009, pp. 523-532. doi:10.1093/jxb/ern288
- A. M. Rae, P. J. Tricker, S. M. Bunn and G. Taylor, “Adaptation of Tree Growth to Elevated CO2: Quantitative Trait Loci for Biomass in Populus,” New Phytologist, Vol. 175, No. 1, 2007, pp. 69-79. doi:10.1111/j.1469-8137.2007.02091.x
- J. K. Ward, J. Antonovics, R. B. Thomas and B. R. Strain, “Is Atmospheric CO2 a Selective Agent on Model C3 Annuals?” Oecologia, Vol. 123, 2000, pp. 330-341. doi:10.1007/s004420051019
- P. Li, A. Sioson, S. P. Mane, A. Ulanov, G. Grothaus, L. S. Heath, T. M. Murali, H. J. Bohnert and R. Grene, “Response Diversity of Arabidopsis Thaliana Ecotypes in Elevated [CO2] in the Field,” Plant Molecular Biology, Vol. 62, 2006, pp. 593-609. doi:10.1007/s11103-006-9041-y
- M. Fordham, J. D. Barnes, I. Bettarini, A. Polle, N. Slee, C. Raines, F. Miglietta and A. Raschi, “The Impact of Elevated CO2 on Growth and Photosynthesis in Agrostis canina L. ssp. montelucci Adapted to Contrasting Atmospheric CO2 Concentrations,” Oecologia, Vol. 110, 1997, pp. 169-178. doi:10.1007/s004420050146
- A. Polle, I. McKee and L. Balschke, “Altered Physiological and Growth Responses to Elevated [CO2] in Offspring from Holm Oak (Quercus ilex L). Mother Trees with Lifetime Exposure to Naturally Elevated [CO2],” Plant, Cell and Enviornment, Vol. 24, 2001, pp. 1075- 1083. doi:10.1046/j.1365-3040.2001.00752.x
- P. C. D. Newton and G. E. Edwards, “Plant Breeding for a Changing Environment,” In: P. C. D. Newton, R. A. Carran, G. R. Edwards and P. A. Niklaus, Eds., Agroecosystems in a Changing Climate, Taylor and Prancis Publishers, London, 2007, pp. 309-319.
- I. Nakamura, Y. Onoda, N. Matsushima, J. Yokoyama, M. Kawata and K. Hikosaka, “Phenotypic and Genetic Differences in a Perennial Herb across a Natural Gradient of CO2 Concentration,” Oecologia, Vol. 165, 2011, pp. 809-818. doi:10.1007/s00442-010-1900-1
- F. A. Bazzaz, M. Jasienski, S. C. Thomas and P. Wayne, “Microevolutionary Responses in Experimental Populations of Plants to CO2-Enriched Environments: Parallel Results from Two Model Systems,” Proceedings of the National Academy of Science USA, Vol. 92, No. 18, 1995, pp. 8161-8165. doi:10.1073/pnas.92.18.8161
- R. Steinger, A. Stephan and B. Schmid, “Predicting Adaptive Evolution under Elevated Atmospheric CO2 in the Perennial Grass Bromus erectus,” Global Change Biology, Vol. 13, No. 5, 2007, pp. 1028-1039. doi:10.1111/j.1365-2486.2007.01328.x
- S. Wieneke, D. Prati, R. Barndl and J. Stocklin, “Genetic Variation in Sanguisorba minor after 6 years in Situ Selection under Elevated CO2,” Global Change Biology, Vol. 10, No. 8, 2004, pp. 1389-1401. doi:10.1111/j.1365-2486.2004.00813.x
- M. A. Gonzalez-Meler, E. Blanc-Betes, C. E. Flower and J. K. Ward, “Plastic and Adaptive Responses of Plant Respiration to Changes in Atmospheric CO2 Concentration,” Physiologia Plantarum, Vol. 137, 2009, pp. 473- 484. doi:10.1111/j.1399-3054.2009.01262.x
- Y. Onoda, T. Hirose and K. Hikosaka, “Does Leaf Photosynthesis Adapt to CO2-Enriched Environments? An Experiment on Plants Originating from Three Natural CO2 Springs,” New Phytologist, Vol. 182, No. 3, 2009, pp. 698-709. doi:10.1111/j.1469-8137.2009.02786.x
- J. A. Bunce, “Effects of Elevated Carbon Dioxide on Photosynthesis and Productivity of Alfalfa in Relation to Seasonal Changes in Temperature,” Physiology and Molecular Biology of Plants, Vol. 13, 2007, pp. 243-252.
- J. A. Bunce, “Relationships between Maximum Photosynthetic Rates and Photosynthetic Tolerance of Low Leaf Water Potentials,” Canadian Journal of Botany, Vol. 59, No. 5, 1981, pp. 769-774. doi:10.1139/b81-108
- A. H. Teramura and B. R. Strain, “Localized Populational Differences in the Photosynthetic Response to Temperature and Irradiance in Plantago lanceolata,” Canadian Journal of Botany, Vol. 57, No. 22, 1979, pp. 2559-2563. doi:10.1139/b79-304
- D. A. Sims and S. Kelley, “Somatic and Genetic Factors in Sun and Shade Population Differentiation in Plantago lanceolata and Anthoxanthum odoratum,” New Phytologist, Vol. 140, No. 1, 1998, pp. 75-84. doi:10.1046/j.1469-8137.1998.00248.x
- J. A. Lau, J. Peiffer, P. B. Reich and P. Tiffin, “Transgenerational Effects of Global Environmental Change: Long-Term CO2 and Nitrogen Treatments Influence Offspring Growth Response to Elevated CO2,” Oecologia, Vol. 158, No. 1, 2008, pp. 141-150. doi:10.1007/s00442-008-1127-6
- T. M. McPeek and X. Wang, “Reproduction of Dandelion (Taraxacum officinale) in a Higher CO2 Environment,” Weed Science, Vol. 55, No. 4, 2007, pp. 334-340. doi:10.1614/WS-07-021

