American Journal of Plant Sciences
Vol.3 No.7(2012), Article ID:20675,4 pages DOI:10.4236/ajps.2012.37103
In Situ Conservation of Wild Rice Populations: A Targeted Study of Common Wild Rice Oryza rufipogon from China
![]()
1Plant Germplasm and Genomics Center, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China; 2Institute of Crop Germplasm Resources, Guangxi Academy of Agricultural Sciences, Nanning, China; 3Guangxi Agricultural School, Nanning, China; 4Rice Research Institute, Hainan Academy of Agriculture Sciences, Haikou, China; 5Liaozhou Prefecture Institute of Agricultural Sciences, Liaozhou, China.
Email: *lgao@mail.kib.ac.cn
Received July 31st, 2011; revised October 9th, 2011; accepted November 15th, 2011
Keywords: In Situ Conservation; Wild Rice; Oryza rufipogon; China
ABSTRACT
Although great accomplishments of in situ conservation have been made during the last decade throughout the world, there is an urgent need to conduct more targeted research to explore many basic questions about crop wild relatives (CWR) in situ conservation such as how to better identify and manage the target in situ conservation populations of CWR. In this study, we performed an extensive field investigation of 201 natural populations or habitats of O. rufipogon, a seriously endangered wild progenitor of cultivated rice in China. Our updated information suggests that: 1) the majority of the natural populations have been extinct throughout China, which leads to serious fragmentation of the population system as a whole; and 2) the survived populations have become small in size and thus fragmented within the population as a result of the loss of subpopulations. To assess the relationships between the biodiversity education and conservation of wild rice, we employed participatory approaches in the field investigation. Our data indicate that the continuous rapid decline of biodiversity education seemed closely related to the extinction of wild rice germplasm. These findings imply the potential necessity and huge challenge for making in situ conservation plans in the future. By means of our understanding of ecogeography, sociology and culture, and population genetics of the species, we propose a strategy for selecting in situ conservation locations as well as priority sites for establishing in situ conservation practices in China.
1. Introduction
Although the loss of genetic diversity of crop wild relatives (CWRs) resulting from extinction is receiving increasing attention, the threat of extinction for many species grows ever greater as the whole natural ecosystem vanish, human populations proliferate, and human-mediated interference increases. It is apparent that complementary approaches to conserve CWRs are required for effective conservation of a maximum amount of genetic diversity in a given species. Of the two basic conservation strategies, namely in situ and ex situ conservation of CWRs, which could maintain their natural processes of ecology and evolution, has been increasingly recognized by our current agricultural community. Accomplishments of in situ conservation have been made during the last decade throughout the world, however, the majority of these projects focus on field inventories, ecogeographic assessment, site selection, or management recommendations alone [1]. There is a trend to conduct more targeted research to explore many basic questions of CWR in situ conservation, such as how to better identify and manage the target in situ conservation populations of CWRs. To reach such goals, comprehensive studies that will combine different disciplines such as ecogeography, ethnobiology, sociology, and population genetics are particularly needed for those CWRs which occurred in seriously disturbed agricultural ecosystems.
Oryza rufipogon Griff., the progenitor of cultivated rice O. sativa L., is a perennial plant which is widely distributed in the tropics and subtropics of monsoon Asia [2]. The species occurs in eight provinces and/or autonomous regions of China: Guangdong, Guangxi, Hainan, Yunnan, Hunan, Jiangxi, Fujian, and Taiwan [3]. It is a valuable gene pool for rice genetic improvement [4-6]. Unfortunately, our previous field surveys indicated that this species was at the edge of extinction [3,7-9]. In recent years, we conducted population genetic and molecular ecological studies using multiple genetic markers to obtain deeper insights into levels, distribution and dynamics of genetic diversity within and among natural populations of the species from China [3,10-16]. Such investigations of genetic diversity and population structure within the species may not only help to illustrate the evolutionary processes and mechanisms but also provide information useful for developing appropriate and efficient plans for in situ conservation. The causes of genetic erosion seem relatively well identified in the species [3, 7-9]. Nevertheless, for making in situ conservation management, detailed documentation concerning the rate, course and impact of the loss, conditions of maintenance of genetic diversity of the wild species in agroecosystems at various rural societies, and the identification of relatively exact survival conditions of natural populations, is still not sufficient. The integrity of the above-mentioned information undoubtedly forms the basis of selecting in situ conservation sites as well as their further management in natural habitats.
Using our national field inventories which were jointly funded by IFS (International Foundation for Sciences) and IPGRI (International Plant Genetic Resources Institute), we report the extinction or endangered status of the species as well as rapid declines of biodiversity education of wild rice in China. In addition, the rate and course of its extinction was further investigated by comparing with previous field investigations in different time periods. Combined with the population genetic data, we finally propose in situ conservation management strategies of the wild rice species.
2. Materials and Methods
2.1. Field Inventories and Population Sampling
To determine the range of O. rufipogon in China, specimen collections from the four herbaria were used (Herbarium of Institute of Botany, Herbarium of Kunming Institute of Botany, Herbarium of South China Institute of Botany, and Herbarium of Guangxi Institute of Botany). Ecogeographic data including the distribution and natural habitats were from the published literature [17- 20]. A total of 201 natural populations or habitats of O. rufipogon, which were recorded in 1978-1982, were selected and revisited from September to December, 1999. We employed the same methodology as these studies performed in 1994-1995. Field inventories have included extensive representatives of natural habitats of the species in China which cover the broad spectrum of ecogeographic and social and cultural conditions. The basic characterization of each sites (e.g., types of habitats, geographic background, major occurring plant species, etc.) were included in the documentation. Particular attention was paid to the extinct or endangered status of all the populations revisited and their affected biotic, abiotic and human factors. The number of extinct populations was recorded and survival population size for each population was measured. Detailed information about the route and surveyed sites are available on request.
Seed samples and/or young leaves were collected for the extraction of genomic DNA and development of DNA banks. For the purpose of ex situ conservation of the species, live ratoons were sampled and transplanted in three botanical gardens of the Chinese Academy of Sciences (CAS): Xishuangbanna Tropical Botanical Garden (Mengla County, Yunnan Province), Guangxi Botanical Garden (Guiling City, Guangxi Province), and Guangzhou Botanical Garden (Guangzhou City, Guangdong Province). Voucher specimens were collected and preserved at the herbarium (PE) of the Institute of Botany, CAS. All these materials meet the further needs of genetic diversity studies at morphological, allozyme and DNA levels, as well as germplasm preservation.
2.2. Biodiversity Education Surveys
In order to explore the relationships between biodiversity education and the conservation of wild rice, participatory approaches were used in the field investigations. Information related to biodiversity conservation of wild rice germplasm was collected from the four agoecological zones in which the wild rice species is distributed. They are Guangdong, Guangxi, Hainan, and Yunnan provinces. Key informants, including agronomists, local farmers, responsible government officials at different levels, agoenterprise technicians and managers, were interviewed using questionnaires and participatory methods. For each province, at least 150 people were interviewed in terms of biodiversity conservation of wild rice resources.
3. Results
3.1. Rapid Declines in Number and Sizes of O. rufipogon Populations
A total of 201 natural populations or habitats of O. rufipogon were revisited in southern China. Our field survey over the whole range of the species (Table 1) suggests a total of four causes which may result in the extinction and/or declines of natural populations, as were discussed in the previous publications [3,7-9]: 1) the complete loss of the residing habitats; 2) the change of microhabitats after human disturbance; 3) grazing and cutting grasses; 4) the invasion of alien species in the wild rice communities; and 5) the introgression of rice gene flow.
Obviously, population numbers of the species in all provinces or regions have been rapidly decreasing since 1978-1982 (Figure 1). Among the 193 populations recorded from 1978 to 1982, only 80 and 75 populations


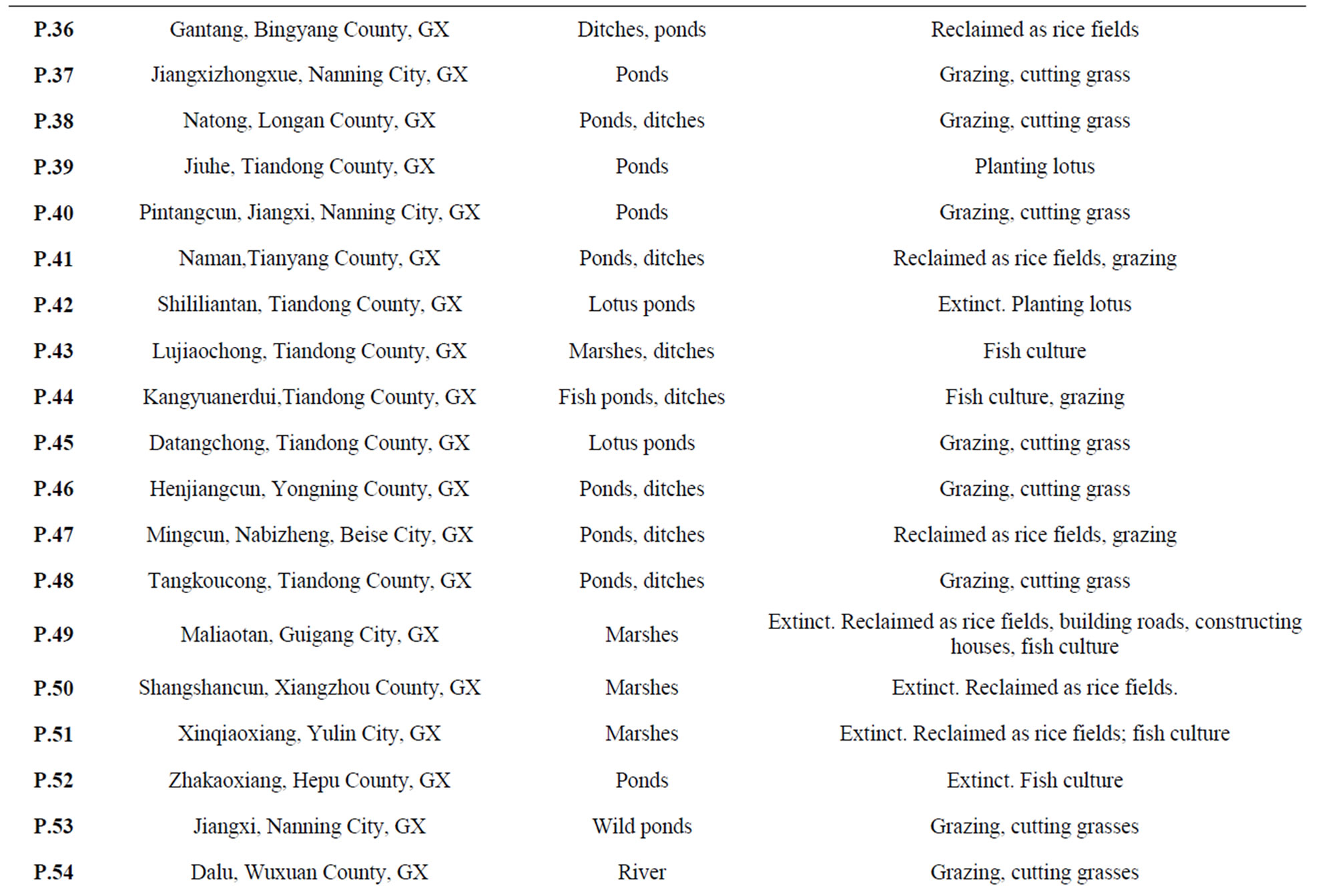
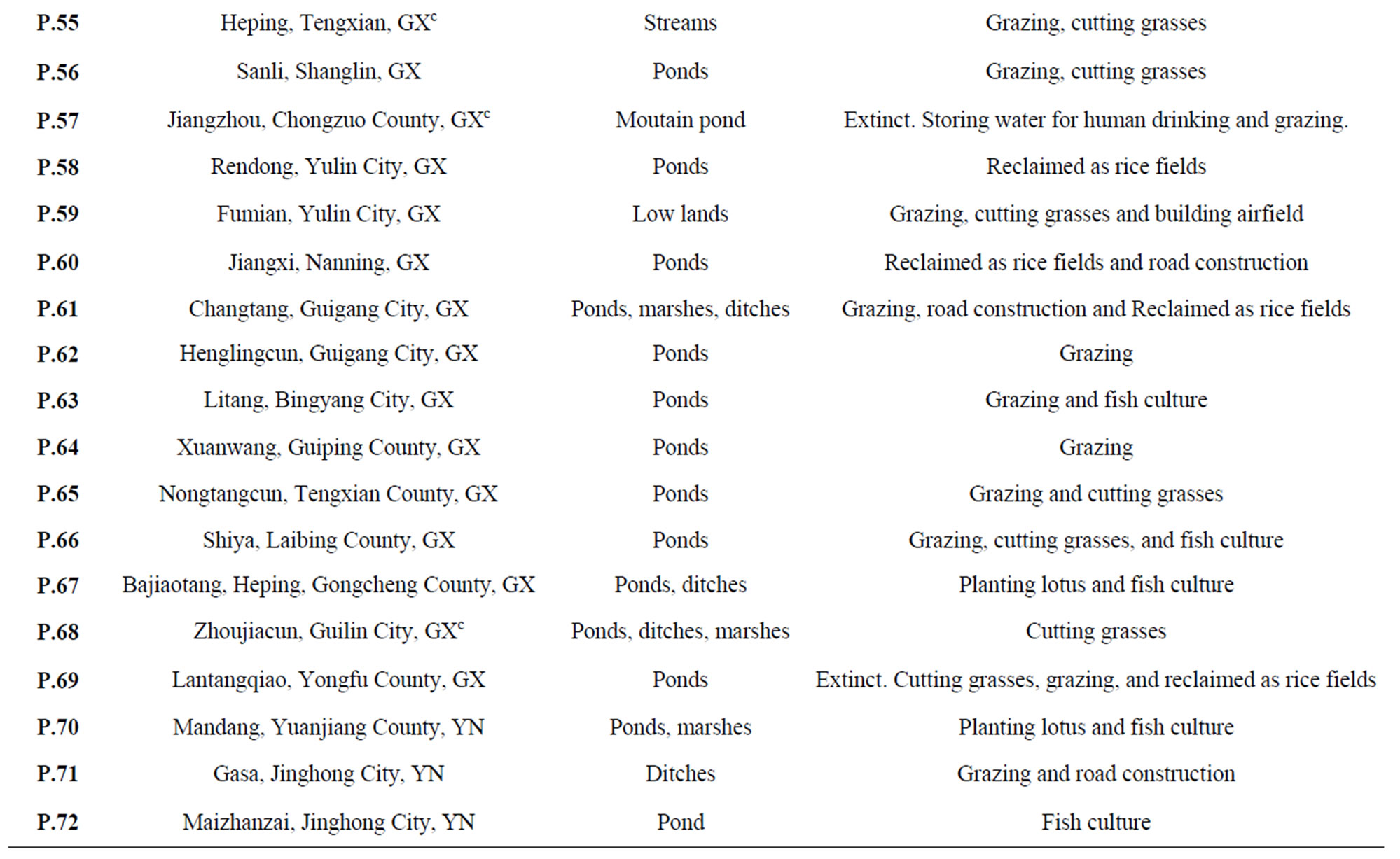


Table 1. Field inventory of natural populations of O. rufipogon from China during 1994-1999 (updated and expanded from Gao et al., 1996; Gao, 1997; Gao et al., 1998)a.

Figure 1. The extinction rate of O. rufipogon estimated by the numbers of natural populations. GX: Guangxi Province; GD: Guangdong Province; HN: Hainan Province; YN: Yunnan Province; JX: Jiangxi Province; HN: Hunan Province; FJ: Fujian Province; TW: Taiwan Province.
were found from 1994 to 1995 and in 1999, respectively. Different provinces or regions, however, exhibited various extinction rates. In Yunnan Province, for instance, the species was once widely distributed in Jinhong County. At least twenty-six populations were formerly reported, but only three existed in 1994 and by 1999 only two survived. In Guangdong and Hainan provinces, 1182 populations were previously found. Of the ninety-three populations revisited from 1994 to1995, sixty-five populations had disappeared. For instance, the Yanglan Population of Sanya City, Hainan Province, in which Yuan and his colleagues [5] first found a Wild Abortive individual to successfully have bred hybrid rice, was seriously endangered in 1995 and finally extinct in 1999. A surprising situation was also discovered in Guangxi Province. Among the sixty-eight populations reported from 1978 to1982, only forty-six populations existed in 1994. Our most recent collecting mission in 1999 found that the extinction is accelerating and even becoming worse within the region. For example, the Maliaotang Population (P49) of Guixian County, the largest population (approximately 28 ha) in China, which was seriously endangered in 1994, was extinct in 1999. The extinction of the population in Caling County, Hunan Province, was reported before 1994 [21]. Kiang (1979) [22] reported that the only two localities of common wild rice O. rufipogon, in Xinzu and Taoyun, Taiwan Province, disappeared due to the rapid urban development.
The decline in population size showed that those surviving populations have been seriously threatened. Comparing historical records, for example, we did observe that several populations (such as P7 and P8) become larger in size in 1999 than during 1978-1982 under light and/or moderate human disturbance. Our field inventory also suggested that moderate extent of human disturbance seem favorable for the development and expansion of the populations. However, the majority of the remaining populations were swiftly falling into decay under the strong pressure of human disturbance. For example, P25 was about 41 acres in size during 1978-1982, but there were only a few individuals left due to reclaiming the area into rice fields and fish ponds in 1999. Genetic erosion has been documented by gradual extinction of subpopulations formerly recorded in the most northern population of the species located in Dongxiang County, Jiangxi Province from 9 to 5 to 2 in 1980, 1985 and 1994, respectively [11].
3.2. Rapid Decline in Biodiversity Education of Wild Rice Germplasms
Ethnobotanical investigations revealed a considerable decline of biodiversity education of wild rice germplasms. Field investigations and collection missions of wild rice germplasm were fully funded and nationally organized from 1978 to 1982. A considerable participation has widely involved by farmers, agricultural scientists of local, regional and national institutions, and relevant officers at different levels of governments. This became an efficient way to conduct very extensive biodiversity education of the germplasm during that period. We may conservatively presume the percentages of persons who were aware of agricultural values and conservation practices important for wild rice were at least 60% in those target regions where ethnobotanical investigations were conducted under the present study. Compared to the estimated level (60%) for Guangxi, Guangdong and Hainan, and Yunnan provinces from 1978 to 1982, where extremely quick decline of biodiversity education was found from 1994 and 1995. Of the three regions surveyed from 1994 to 1995, Yunnan showed the relatively highest level (30.5%) while Guangdong and Hainan had the lowest level (10.1%) of biodiversity education. A slightly higher level (13.2%) was found in Guangxi than in Guangdong and Hainan. Our data in these regions in 1999 further indicate levels of biodiversity education continuously decreased at different rates (Figure 2). Although the fastest rate of decline occurred in Yunnan, the region still kept the highest level (25.6%) in 1999.
4. Discussion
4.1. Why O. rufipogon Is Seriously Endangered?
One of the most important aspects that make a species endangered is the loss or weakness of its reproductive abilities. Although our recent investigation were alarming, there is no evidence to directly support the extremely endangered status of O. rufipogon in China is a genetically endangered species due to the weakness of propagation abilities. The species has a mixed breeding system, which adaptively changes the proportion of sexual and asexual reproduction when going from lower to higher latitudes [23]. Our field observation suggested that it possess strong vegetable reproduction as well as colonizing growth ability [8]. In terms of efficient dissemination due to diverse breeding system, it is implausible that the species is endangered because of the weakness of reproduction weakness. Moreover, O. rufipogon is a predominantly wind-pollinated perennial herb, grows in a wide geographic range of monsoon Asia and forms a large population system. Population genetic data showed that this species has high levels of genetic variability in comparison with other wild rice species in the genus [21]. The biological characteristics of the species may be favorable for maintaining the high extent of genetic diversity within the species and thus lead to a considerable
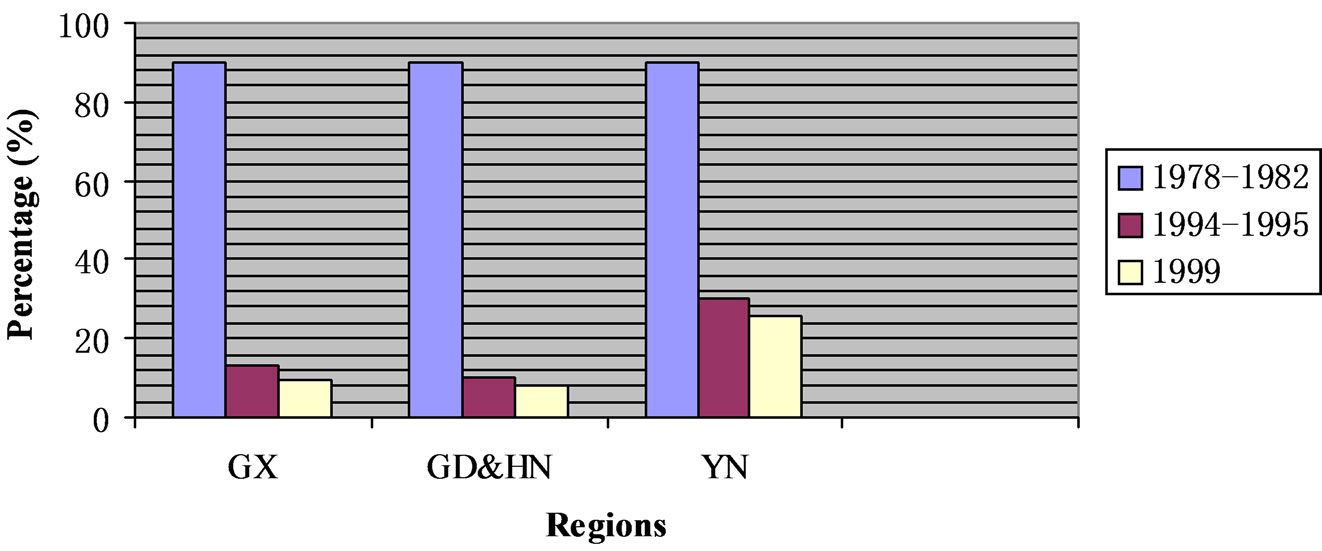
Figure 2. The decline of biodiversity education of wild rice germplasm in China based on ethnobotanical investigations. GX: Guangxi Province; GD: Guangdong Province; HN: Hainan Province; YN: Yunnan Province.
adaptation potential [11-13,16,24]. However, it would be of great interest to understand the endangered causes and status of the wild rice throughout its entire range by expanding the investigation out of China and comparing the data collected from other worldwide geographical regions.
The destruction of natural habitats is the most possible cause of the endangered status of the species, as was reported in other Asian countries [25-27]. It usually occurs in permanent shallow water habitats of about 10 - 30 cm depth. Any direct or indirect destruction of the specific condition will quickly drive these populations and/or subpopulations into extinction in the forms of a loss of genotypes and alleles. However, population declines and habitat destruction will eventually force it to become a genetically endangered species. Once most of the former large populations have been divided into small local populations, which only consist of few individuals, the extinction of the populations and/or subpopulations makes surviving ones grow in seriously fragmented and thus isolated habitats in the changing agro-ecosystem. Theoretically, such small populations are subject to genetic drift and inbreeding and thus lead to a reduced genetic variation. In a predominantly outcrossing perennial species like O. rufipogon, mating events among relatives and intra-clones may frequently take place and will bring about a loss of genetic variability as well as a deficit of heterozygotes [28]. The consequences of genetic drift are also heightened by the loss of natural selection within small populations. The potential to adapt to the changing environment may be seriously diminished as a result of the high extent of homozygosity in the long run. Our study on a northern marginal population of the species provided the evidence that the population genetic structure was changed due to fewer subpopulations of smaller size [11].
Our field inventories showed that the disappearance of wild populations might also come from slow elimination of other aggressive weeds. This was reported previously in India, Thailand, and other Asian countries [8,27,29, 30]. Two examples of the recovery and/or in situ conservation populations of Taiwan, Hunan, and Jiangxi provinces, China, strongly suggested that population ecological studies are of importance to better understand the structure and dynamics of the plant communities in which wild populations will be conserved [31-34].
The genetic assimilation stemming from the introgression of rice gene flow may bring about the extinction of wild populations. Based on the observation of frequent introgression of rice gene flow in the populations of O. rufipogon in Taiwan, China, Oka and Chang [35] proposed that disadvantageous effects of genes of cultivated rice might tend toward consequent extinction of wild populations. In light of the fact that the majority of the populations of O. rufipogon are under strong gene flow from the surrounding rice fields, more studies on monitoring the influence of genetic assimilation due to rice gene flow may provide a prerequisite for making in situ conservation plans [10].
Finally, the decline of biodiversity education has potential impacts on the habitat destruction of wild rice populations, and thus further lead to their loss. While the biodiversity education may be helpful to accelerate or hamper this destruction, one should realize that the tremendous economic & social changes which have been going through in China seem to be the major reasons for the loss of wild rice populations. Our preliminary investigation provided the evidence that, to some extent, the conservation education of rice biodiversity may be related to the loss or declines of natural populations in given geographic regions. It is likely that the ignorance of biodiversity conservation often determines the government policies, CWRs management and local farmers’ activities. However, the detailed investigation and evaluation of the impacts of biodiversity education on the conservation efficiency are needed before we could make a clear argument regarding how and why the decline in biodiversity education has had a negative effect on the conservation of wild rice. The efficiency of conservation management calls for an extensive participation in the conservation activities by creating a system of positive incentives to involve local communities, governments, and so on, as partners [15]. Therefore, enhancing the education training of rice biodiversity conservation may be of significance in the future conservation management.
4.2. Current Conservation Status and Necessities of in Situ Conservation
Genetic resources of the Chinese O. rufipogon are currently preserved by means of: 1) conventional gene banks; 2) field gene banks; 3) botanical gardens; 4) DNA banking; and 5) in situ conservation. In China, ex situ conservation is still a major method to conserve wild rice genetic resources from loss by means of the gene banks and field gene banks [8,10,15]. Up to 4480 seed samples of O. rufipogon have been collected and preserved in the gene banks [33]. About 8933 accessions of rhizome samples have been grown and preserved in two national field gene banks of wild rice located at Nanning City (Guangxi Province) and Guangzhou City (Guangdong Province) (most of them are O. rufipogon) [36]. From 1994, we first transplanted and maintained a number of collections of O. rufipogon populations at three botanical gardens of the Chinese Academy of Sciences. These collections are inadequate in terms of the numbers of accessions, but they are of significance in diversity education rather than germplasm conservation and utilization. Moreover, the DNA bank of this species was developed by using 1245 individuals of 47 natural populations in China [24]. This bank provides the hope to re-introduce DNA materials into related genotypes or species. However, the regeneration of whole organism or even the expression of given genes in related genotypes/species remains extremely difficult [37]. Thus, such a frozenpreserved DNA banking may be used for research purposes of evolution and conservation rather than germplasm preservation. Based on ecogeographic inventories, some targeted populations are proposed for in situ conservation (Table 2). However, only two sites (Dongxiang, Jiangxi Province; Zhengcheng, Guangdong Province) were well preserved [20] (Donghai Song, personnel communication). Therefore, the development of an in situ conservation management plan is an unsolved practice in China.
Complementary strategies and technologies are essential to conserve gene resources of CWRs [38], however, in situ conservation needs particular attention because of several confronted limitations and problems in the species. First, low seed production limits the collection of sufficient grains from natural populations and ex situ grown populations or plants for the regeneration of the gene banks. We found that populations located at low latitude regions mainly rely on asexual production and the proportion of sexual reproduction tends to decrease as the latitude increase [23]. Similar observations tend to conclude that vegetable reproduction may be more important than seed reproduction in this species [39,40]. In addition, it is usually difficult to adequately collect genetically representative samples for ex situ conservation because of the easily shattering habit of this species. Very poor seed production was also observed in two ex situ populations preserved at Xishuangbanna Tropical Botanical Garden and South China Botanical Garden (Gao, 1994-1999, personnel observations). Therefore, it is a time-consuming task to harvest sufficient seed samples from ex situ grown individuals which produce a small amount of seeds, even if the panicles are carefully bagged before shattering. The species may have partial incompatibility, which was similarly reported in another wild rice species O. longistaminata [41]. Second, genetic erosion occurs and continues in the gene bank and field gene banks due to improper storage, confused management, mechanical mixture, or unexpected accidents [8,9]. Because O. rufipogon is mainly an outcrossing species, fewer used individuals will result in genetic drift and subsequently the loss of genetic variability when the preserved accessions are renewed. Maintaining the heterogeneous genetic structure of wild populations is undoubtedly a challenge via ex situ conservation. Finally and the most important problem is that most natural habitats of the species were seriously destructed. This study shows that human activities have led to the extinction of about 38.86% of the populations since 1978 in China, and thus in situ conservation plans are urgently required to prevent genetic diversity from further loss.
4.3. Setting Priorities for in Situ Conservation
Although the necessity and significance for making in situ conservation of agricultural biodiversity are evident, the scientific bases of in situ conservation are still under discussion and need to be strengthened. The endangered Oryza species may be generally identified as being endangered at the population and species levels. For example, O. schlechteri, which exists in only 3 to 4 populations and was rediscovered in Papua New Guinea, is in the endangered status at the level of species because of its extreme rarity in comparison with other species [27]. A species like O. rufipogon in this study, however, should be regarded as the endangered species at the level of populations owning to serious population declines, although it is widely distributed, genetically heterogeneous


Table 2. List of O. rufipogon populations having priorities for in situ conservation in China.
and potentially adaptive. By means of our understanding of ecogeography, sociology and culture, and population genetics of the species, we proposed a strategy for selecting in situ conservation locations (Figure 3) as well as priority sites for establishing in situ conservation practices in China (Table 2). These sites were selected by employing the following criteria.
1) Assignment of conservation priorities to larger populations that harbored abundant gene diversity. The goal of any in situ conservation management aims to conserve the utmost amount of genetic diversity and heterogenous population genetic structure. Assessment of population genetic structure detected by microsatellite markers suggested that the greatest amount of the total genetic variation reside within populations (FST = 0.246) (Table 3). Therefore, large populations such as P5 and P7 proposed in this study, which possess high levels of genetic diversity and are located in the center of genetic variation, should be more attractive for in situ conservation plans;
2) In order to capture genetic differences that reside among different populations, the setting of target sites
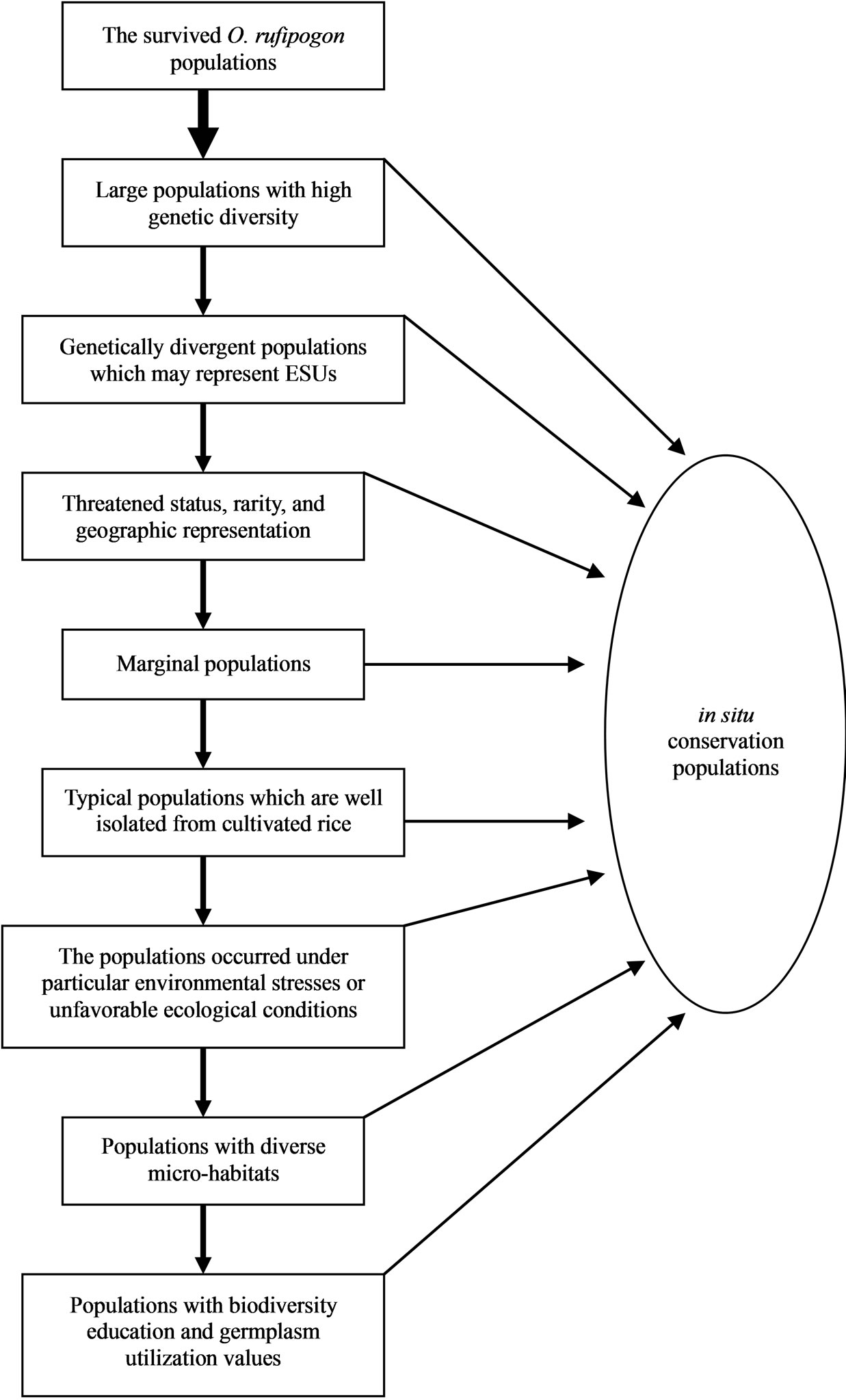
Figure 3. A proposed strategy for identifying in situ conservation sites of O. rufipogon.
should focus on genetically variable populations that cover the entire ecological spectrum of the species. Therefore, the large remnant populations with high levels of genetic variation, such as SLGX (P25), LCGX (P30), ZXGX (P37), PTGX (P40), KEGX (P44), QSHN (P16), HFGD (P6), HYGD (P5), LDHN (P15) and GZGD (P11), from Guangxi, Guangdong and Hainan provinces, should be more attractive for in situ conservation actions;
3) Assignment of conservation priorities to the populations on the basis of threatened status, rarity, and geographic representative. Taking in situ conservation actions for those seriously threatened and/or rare populations are urgent, because they are apt to being extinct shortly (e.g., P2, 37 and 67);
4) Assignment of conservation priorities to marginal populations and those populations under particular environmental stresses or ecological conditions. In O. rufipogon, the differences of genetic structure between marginal and central populations, such as fewer heterozygotes and lower genetic diversity in marginal populations,
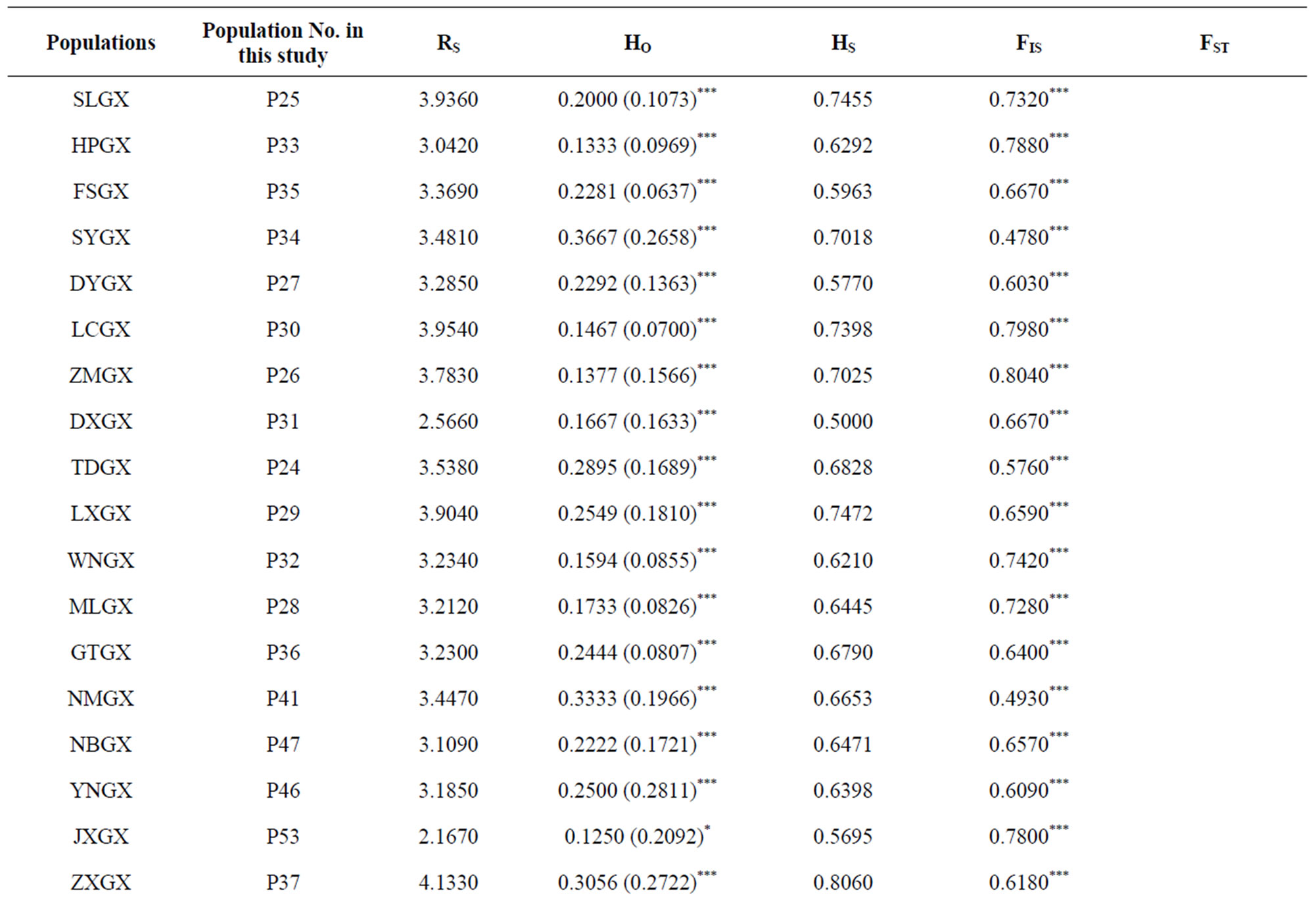
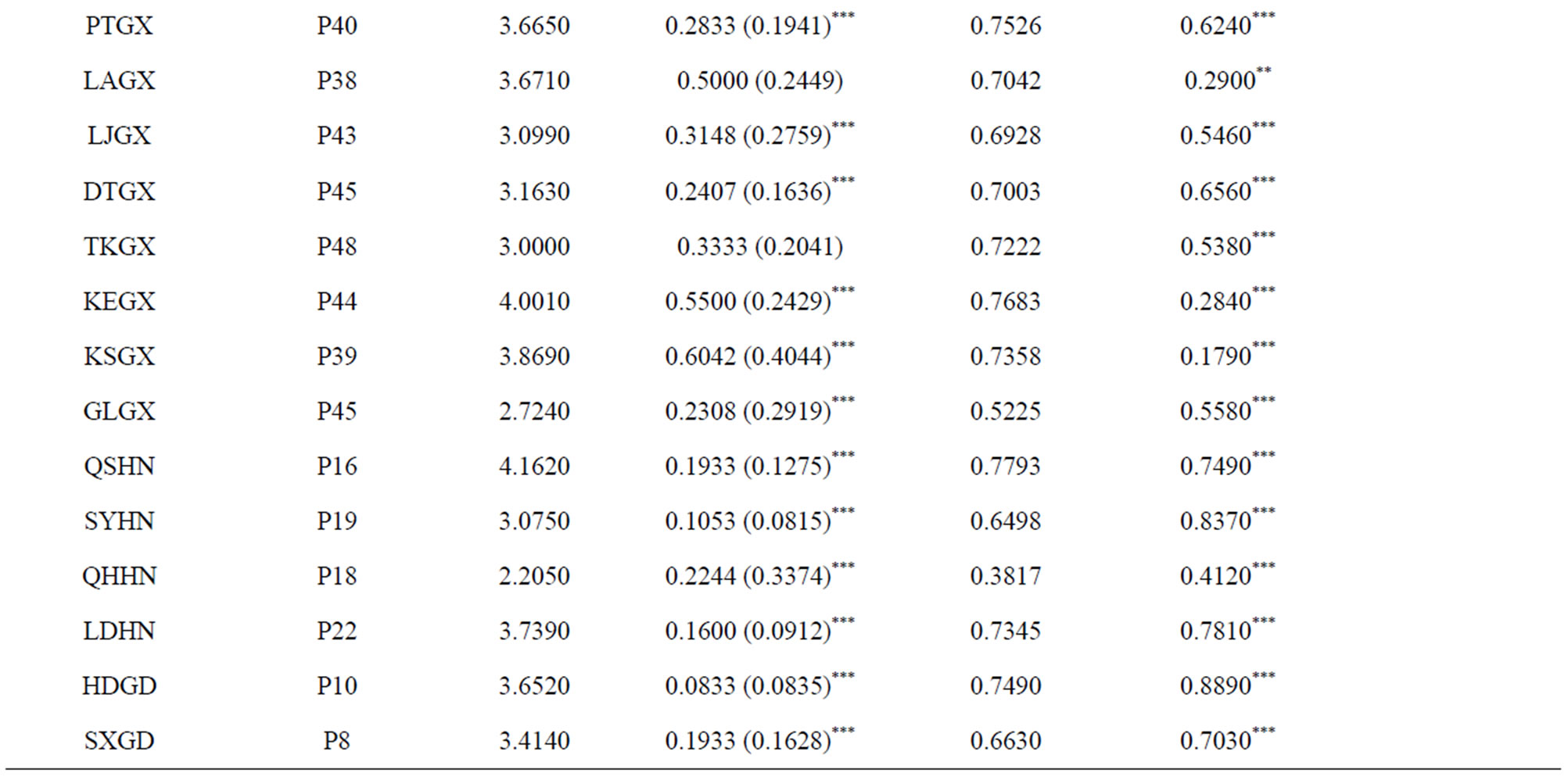
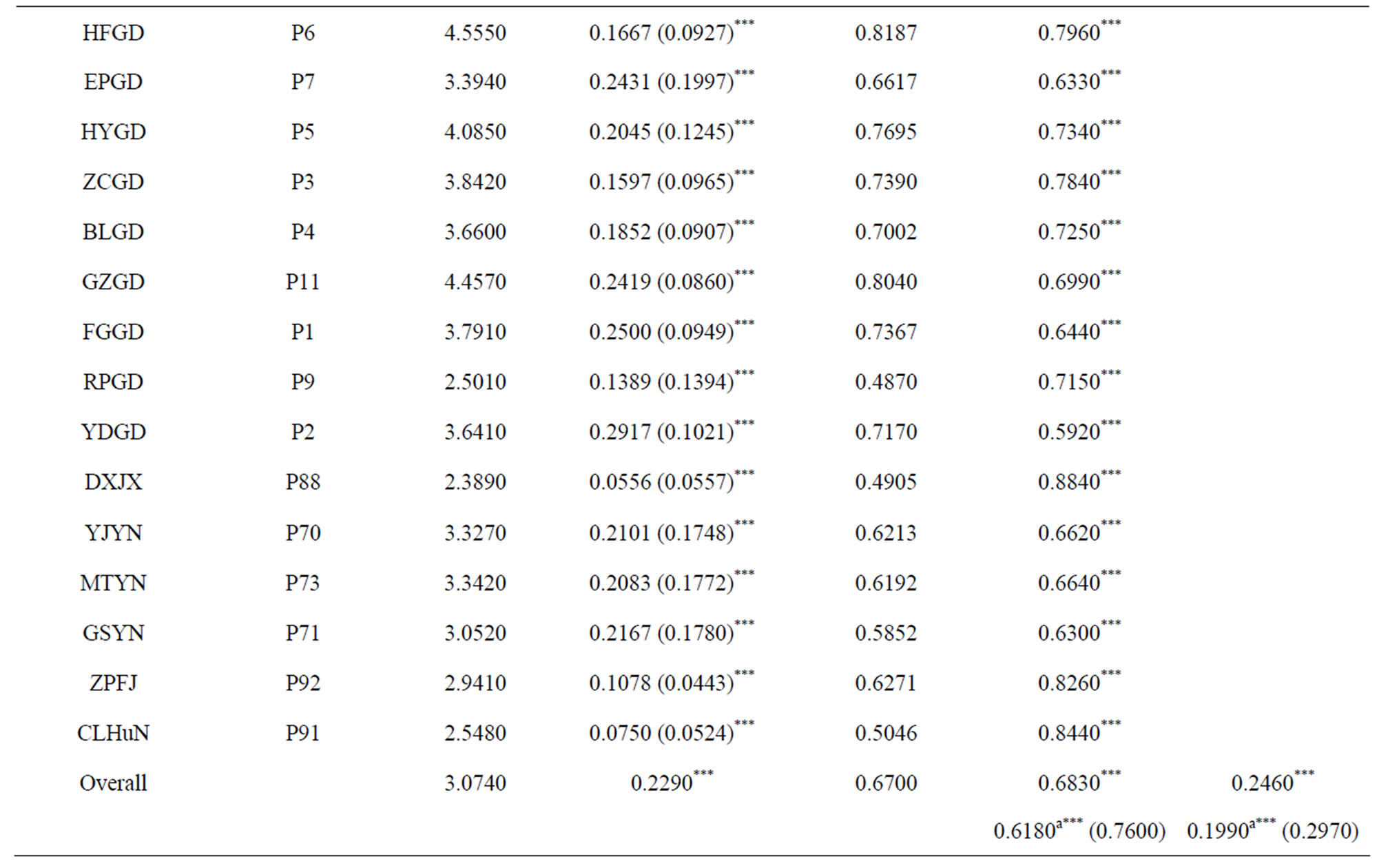
Table 3. Mean values of RS (allelic richness), HO (observed heterozygosity), HS (the within sample gene diversity), FIS (the heterozygote deficit within populations), and FST (the fixation of different alleles in different populations) for each studied population, different geographic region and general system of the species. Weir & Cockerham’s F-statistic overall values of FST and FIS for each population are also shown. Statistically significant deviations from Hardy-Weinberg expectations are indicated by *(P < 0.05), **(P < 0.01) and ***(P < 0.001) (modified from Gao 2004). aBootstrapping over loci at 95% confidence interval.
may be unfavorable to strong selection pressures and thus they are apt to become extinct (Gao unpublished). Thus, marginal populations such as P2, P6, P9, P19, P88, P90 and P92 of the species, which were found in China and are under strong ecological disadvantages and human disturbance, should be given favorable consideration for in situ conservation management to maintain the integrity of the gene pool of the species. In addition to marginal populations, the populations grown under particular environmental stresses or ecological conditions are interesting for in situ conservation as well because they may provide untapped special sources of resistance genes to biotic and abiotic stresses to enlarge the genetic basis of rice breeding programs (such as P22);
5) Assignment of conservation priorities to the typical wild populations which are well isolated and remote from rice gene flow. The best-known evolutionary consequence of gene flow of cultivated crops is the tendency to homogenize population structure [42], and further increases the likelihood of extinction of wild populations [41,43,44]. These isolated populations where unique alleles and allele combinations are predominant, are of particular importance in the conservation. Indeed, typical specimens of O. rufipogon are now rarely found throughout Asia because of extensive hybridization with the crop [45]. In defining conservation units, the first step is to identify and protect historically isolated sets of populations within a species [46]. Therefore, several typical populations well preserved in China such as P1, P43, P68, P70 and P88 which are isolated out of rice gene flow, have high in situ conservation values for the purposes of evolutionary studies and genetic breeding uses;
6) Assignment of conservation priorities to the entire ecosystem including microgeographical structure. Genetic diversity and differentiation correlates with microgeographic heterogeneities, suggesting that conservation strategies must consider not only global and regional, but also, most importantly, local ecological heterogeneities across a species range [47]. In the case of O. rufipogon, we found that genetic differentiation predominantly occurs within a local population [14]; and 7) Assignment of conservation priorities to the populations which have values of biodiversity education and germplasm utilization. For example, P4 (Changling, Boluo County, GD) is the first wild rice population of the species reported by E. D. Merrill in 1917 in China [48], and is the location where Ting [49] later collected the precious wild rice strains that led to the birth of a series of rice varieties of “Zhongshan 1 Hao”, which were widely grown in southern China for at least thirty years. Therefore, making in situ conservation of such a population should have potentials for promoting conservation education and germplasm utilization.
4.4. Conclusion and Perspectives for the in Situ Conservation Management
O. rufipogon is seriously endangered in China, as shown by three observations in the present study. First, the majority of natural populations have been extinct throughout China, leading to serious fragmentation of the population system as a whole; second, the survived populations have been becoming smaller in size and thus fragmented within the population as a result of the loss of subpopulations; and finally, the quick continuous decline of biodiversity education seems closely related to the extinction of wild rice germplasm. These findings imply a potential necessity and huge challenge for making in situ conservation plans in the future. Therefore, more specific research such as in situ conservation management as well as CWRs policy is urgently needed. Moreover, it is equally important to enhance biodiversity education and create systems of positive incentives to involve local communities including governments, academic institutions, and farmers as active partners in the efforts to conserve the species.
5. Acknowledgements
We thank all persons who assisted with the collection and investigation of the germplasms in the field during our trips to Guangxi, Guangdong, Hainan, Yunnan, Hunan, Fujian and Jiangxi during the past six years. We are most appreciative anonymous reviewers of helpful suggestions for improving the manuscript, and Sara Barton for her careful reading and valuable comments. Financial supports were obtained from various sources including the International Foundation for Sciences (Grant No.: C/2738-1, 2), the 1999 Vavilov-Frankel Fellowship from the International Plant Genetic Resources Institute (IPGRI), Key Project of Natural Science Foundation of Yunnan Province (2010CC011), Hundreds Oversea Talents Program of Yunnan Province, Hundreds Talents Program of Chinese Academy of Sciences (CAS) and Talents Program of Yunnan Province (20080A009) to L. Z. GAO.
REFERENCES
- B. A. Meilleur and T. Hodgkin, “In Situ Conservation of Crop Wild Relatives: Status and Trends,” Biodiversity and Conservation, Vol. 13, No. 4, 2003, pp. 663-684. doi:10.1023/B:BIOC.0000011719.03230.17
- D. A. Vaughan, “The Wild Relatives of Rice: A Genetic Resources Handbook,” International Rice Research Institute, Philippines, 1994.
- L. Z. Gao, “Studies on Genetic Variation of Three Wild Rice (Oryza spp.) and Their Conservation Biology in China,” PhD Thesis, Institute of Botany, Chinese Academy of Sciences, Beijing, 1997.
- T. T. Chang, “Conservation of Rice Genetic Resources: Luxury or Necessity?” Science, Vol. 224, No. 4646, 1984, pp. 251-256. doi:10.1126/science.224.4646.251
- L. P. Yuan, S. S. Virmani and C. X. Mao, “Hybrid Rice— Achievement and Outlook,” In: Progress in Irrigated Rice Research, International Rice Research Institute, Manila, 1989, pp. 219-235.
- J. Xiao, J. Li, S. Grandillo, S. N. Ahn, S. R. McCouch, S. D. Tanksley and L. P. Yuan, “A Wild Species Contains Genes That May Significantly Increase the Yield of Rice,” Nature, Vol. 384, No. 6606, 1996, pp. 223-224. doi:10.1038/384223a0
- L. Z. Gao, S. Z. Zhang, Y. Zhou, S. Ge and D. Y. Hong, “A Survey of Current Status of Wild Rice in China,” Biodiversity Science, Vol. 4, 1996, pp. 162-166.
- L. Z. Gao, Y. Zhou, S. Ge, D. Y. Hong, Y. M. Liang, D. H. Lin, C. B. Chen, M. S. Wu and D. A. Huang, “Current Status of the Genetic Resources of Oryza rufipogon Griff. and Its Conservation Strategies in Guangxi Province,” Acta Agricultura Sinica, Vol. 31, 1998, pp. 32-39.
- L. Z. Gao, B. A. Schaal, C. H. Zhang, J. Z. Jia and Y. S. Dong, “Assessment of Population Genetic Structure of Common Wild Rice Oryza rufipogon Griff. Detected by Microsatellite DNA and Allozyme Loci,” Theoretical and Applied Genetics, Vol. 106, No. 1, 2002, pp. 173-180.
- L. Z. Gao and D. Y. Hong, “Advances in the Studies on the Genus Oryza in China,” Scientia Agricultura Sinica, Vol. 32, No. 6, 1999, pp. 40-46.
- L. Z. Gao, S. Ge, D. Y. Hong, W. Chen, W. Z. Jiang and X. K. Wang, “Genetic Erosion in Northern Marginal Population of Common Wild Rice Oryza rufipogon Griff. and Its Conservation, Revealed by Allozyme Analysis,” Hereditas, Vol. 133, No. 1, 2000, pp. 47-53. doi:10.1111/j.1601-5223.2000.00047.x
- L. Z. Gao, S. Ge and D. Y. Hong, “Allozymic Diversity and Genetic Structure of Common Wild Rice Oryza rufipogon Griff., China,” Theoretical and Applied Genetics, Vol. 101, No. 3, 2000, pp. 494-502. doi:10.1007/s001220051508
- L. Z. Gao, S. Ge and D. Y. Hong, “Intra-Population Genetic Structure and Gene Flow of Typical Population of Common Wild Rice Oryza rufipogon Griff.,” Journal of Plant Research, Vol. 114, 2001, pp. 107-113. doi:10.1007/PL00013973
- L. Z. Gao, S. Ge and D. Y. Hong, “Low Levels of Allozyme Diversity and Conservation Genetics of Common Wild Rice Oryza rufipogon Griff. from Yunnan, China,” Euphytica, Vol. 124, No. 3, 2001, pp. 273-281. doi:10.1023/A:1015740331079
- L. Z. Gao, “The Conservation of Rice Biodiversity in China: Significance, Genetic Erosion, Ethnobotany and Prospect,” Genetic Resources and Crop Evolution, Vol. 50, 2002, pp. 17-32. doi:10.1023/A:1022933230689
- L. Z. Gao, “Population Structure and Conservation Genetics of Wild Rice Oryza rufipogon (Poaceae): A Region-Wide Perspective from Microsatellite Variation,” Molecular Ecology, Vol. 13, No. 5, 2004, pp. 1009-1024. doi:10.1111/j.1365-294X.2004.02108.x
- National Exploring Group of Wild Rices, “Investigation of Resources of Wild Rice in China,” Acta Agricultura Sinica, Vol. 6, 1984, pp. 1-8.
- Yunnan Exploring Group of Rice Germplasm Resources, “Exploring Report of Rice Germplasm Resources from Yunnan,” 1988, p. 358.
- M. S. Wu, “A Preliminary Survey of Wild Rices in Guangxi,” Hereditas (Beijing), Vol. 3, No. 3, 1981, pp. 36-37.
- C. S. Ying, “Collection, Documentation and Catalogue of the Rice Germplasm Resources in China,” In: C. S. Ying, Ed., Rice Germplasm Resources in China, Chinese Agricultural Technology & Science Press, Beijing, 1993, pp. 29-45.
- J. Zhou, J. K. Chen, X. M. Wang and Y. Zhong, “A Numerical Taxonomic Study on Variation in Populations of Common Wild Rice (Oryza rufipogon Griff.) in Hunan and Jiangxi provinces, China,” Journal of Wuhan Botanical Research, Vol. 10, 1992, pp. 235-242.
- Y. T. Kiang, J. Antonovics and L. Wu, “The Extinction of Wild Rice (Oryza perennis formosana) in Taiwan,” Journal of Asian Ecology, Vol. 1, 1979, pp. 1-9.
- L. Z. Gao, S. Ge and D. Y. Hong, “A Preliminary Study on Ecological Differentiation within Common Wild Rice Oryza rufipogon Griff.,” Acta Agronomica Sinica, Vol. 26, 2000, pp. 210-216.
- L. Z. Gao, “Population Structure and Conservation Genetics of Wild Rice Oryza rufipogon (Poaceae): A Region-Wide Perspective from Microsatellite Variation,” Molecular Ecology, Vol. 13, No. 5, 2004, pp. 1009-1024. doi:10.1111/j.1365-294X.2004.02108.x
- D. A. Vaughan, “In Situ Conservation of Wild Rices in Asia,” Rice Genetic Newsletter, Vol. 7, 1990, pp. 90-91.
- D. A. Vaughan and L. A. Sitch, “Gene Flow from the Jungle to Farmers: Wild-Rice Genetic Resources and Their Uses,” BioScience, Vol. 41, No. 1, 1991, pp. 22-28. doi:10.2307/1311537
- D. A. Vaughan and T. T. Chang, “In Situ Conservation of Rice Genetic Resources,” Economic Botany, Vol. 46, No. 4, 1992, pp. 368-383. doi:10.1007/BF02866507
- H. Morishima and P. Barbier, “Mating System and Genetic Structure of Natural Populations in Wild Rice Oryza rufipogon,” Plant Species Biology, Vol. 5, 1990, pp. 31- 39. doi:10.1111/j.1442-1984.1990.tb00190.x
- H. Morishima, Y. Sano and H. I. Oka, “Observation on Wild and Cultivated Rices and Companion Weeds in the Hilly Area of Nepal, India and Thailand,” Report of Study Tour in Tropical Asia 1979 Rep, Natl. Inst. Genetics, Mishima, 1980, p. 97.
- IBPGR-IRRI Rice Advisory Committee, “Descriptors for Rice (Oryza sativa L.),” International Rice Research Institute, Manila, 1980.
- J. Zhou, “Studies on Conservation Biology of Common Wild Rice (Oryza rufipogon Griff.) Populations at Its Northern Range Limit,” PhD Thesis, Wuhan University, Wuhan, 1995.
- H. I. Oka, “Ecology of Wild-Rice Planted in Taiwan. 3. Differences in Regenerating Strategies among Genetic Stocks,” Botanical Bulletin of Academia Sinica, Vol. 33, 1992, pp. 133-140.
- H. I. Oka, “Ecology of Wild-Rice Planted in Taiwan. 1 Sequential Distribution of Species and Their Interactions in Weed Communities,” Botanical Bulletin of Academia Sinica, Vol. 33, 1992, pp. 287-293.
- H. I. Oka and C. S. Lu, “Competition, Density Response and Self-Thinning Observed in Rice,” Botanical Bulletin of Academia Sinica, Vol. 36, 1995, pp. 113-120.
- H. I. Oka and W. T. Chang, “Hybrid Swarms between Wild and Cultivated Rice Species, Oryza perennis and O. sativa,” Evolution, Vol. 15, 1961, pp. 418-430. doi:10.2307/2406310
- H. H. Pang, Q. W. Yang and J. Zhao, “The Progress in Investigation, Identification and Preservation of Chinese Wild Rice Genetic Resources,” Journal of Plant Genetic Resources, Vol. 1, 2000, pp. 52-56.
- K. Miller, M. H. Allegretti, N. Johnson and B. Jonsson, “Measures for Conservation of Biodiversity and Sustainable Use of Its Components,” In: V. H. Heywood and E. T. Watson, Eds., Global Biodiversity Assessment, United Nations Environment Programme (UNEP), Cambridge University Press, Cambridge, 1995, pp. 915-1061.
- M. Dulloo, L. Guarino, F. Engelmann, N. Maxted, J. Newbury, F. Attere, et al., “Complementary Conservation Strategies for the Genus Coffea: A Case Study of Mascarene Coffea Species,” Genetic Resources and Crop Evolution, Vol. 45, No. 6, 1998, pp. 565-579. doi:10.1023/A:1008621028343
- National Institute of Genetics, “Trip to Indonesia and Thailand for the Ecological Genetic Study in Rice,” Report of a Study Tour in 1985/86, NIG Contribution 1729, Mishima, Japan, 1987.
- H. I. Oka, “Origin of Cultivated Rice,” Japan Scientific Societies Press, Tokyo, 1988.
- Y. E. Chu, H. Morishima and H. I. Oka, “Reproductive Barriers Distributed in Cultivated Rice Species and Their Wild Relatives,” Japanese Journal of Genetics, Vol. 44, 1969, pp. 207-223. doi:10.1266/jjg.44.207
- M. Slatkin, “Gene Flow and the Geographic Structure of Natural Populations,” Science, Vol. 236, No. 4803, 1987, pp. 787-792. doi:10.1126/science.3576198
- N. C. Ellatrand, H. C. Prentice and J. F. Hancock, “Gene Flow and Introgression from Domesticated Plants into Their Wild Relatives,” Annual Review Ecology Systematics, Vol. 30, 1999, pp. 639-563.
- Y. E. Chu and H. I. Oka, “Introgression across Isolating Barriers in Wild and Cultivated Oryza Species,” Evolution, Vol. 24, 1970, pp. 344-355. doi:10.2307/2406809
- T. T. Chang, “Rice,” In: J. Smartt and N. W. Simmonds, Eds., Evolution of Crop Plants, 2nd Edition, Longman, Harlow, 1995, pp. 147-155.
- C. Moritz, “A Molecular Perspective of Biodiversity,” In: S. Kato, Ed., The Biology of Biodiversity, Springer Verlag, Tokyo, 1999.
- E. Nevo, “Genetic Diversity in Wild Cereals: Regional and Local Studies and Their Bearing on Conservation ex Situ and in Situ,” Genetic Resources and Crop Evolution, Vol. 45, 1999, pp. 355-370. doi:10.1023/A:1008689304103
- Y. Ting (Ed.), “Chinese Rice Cultivation,” Agricultural Press, Beijing, 1961.
- Y. Ting, “Wild Rice in Guangdong Province and the New Varieties Bred by Wild Rice Strains,” Bulletin of Chinese Agricultural Society, 1933, p. 114.
NOTES
*Corresponding author.

