Open Journal of Synthesis Theory and Applications
Vol. 2 No. 1 (2013) , Article ID: 27200 , 8 pages DOI:10.4236/ojsta.2013.21004
New Method of Generation of Carbon Molecules and Clusters
1Frantsevich Institute for Problems of Materials Science of NASU, Kiev, Ukraine
2Taras Shevchenko National University of Kiev, Kiev, Ukraine
Email: *dep73@ipms.kiev.ua, mebondarenko@ukr.net
Received November 26, 2012; revised December 25, 2012; accepted January 6, 2013
Keywords: Pyrolysis; Benzene; Fullerene; Quasi-Fullerene; Small Molecules; Mass Spectra; Carbon Clusters
ABSTRACT
Firstly the method of joint synthesis of carbon molecules and their hydrides is developed. The stage of high-temperature sublimation of carbon in a new method of generation of carbon molecules is completely excluded. By mass spectrometric method the condensation products of new method of pyrolysis (NMP) benzene are studied. Firstly clusters (C3-C17), typical for carbon vapour, in substances obtained under pyrolysis of hydrocarbons were detected. Fullerene C60 and its hydrides, quasi-fullerenes C48 and C33 in products of benzene pyrolysis are detected also. Firstly it is shown what clusters C3-C5 can be generated at so low (100˚C-200˚C) temperatures of decomposition of substance. Obtained experimental results firstly demonstrate that the small carbon molecules can be generated in reactionary conditions excluding evaporation of carbon. Dehydrogenation and destruction of hydrocarbon molecules is the first stage on a route of the transformation of benzene to carbon molecules.
1. Introduction
It is considered, that precursor of formation of carbon molecules are carbon clusters, generated at high-temperature evaporation processes of carbon or materials on its basis. One year early to discovery of fullerene C60 by Kroto et al. [1], Rofling [2] obtained the unique mass spectrum of carbon clusters that were created at laser evaporation of graphite in a flow of inert gas. In mass spectrum alongside with even and odd carbon clusters of the small sizes Сn (1 < n < 30) were detected even clusters Сn (n > 30), including clusters, appropriate to fullerenes opened later. Kroto et al. [1] have created the special conditions of increased collision (clusterization) of carbon clusters and firstly have obtained mass spectrum of carbon vapour, which contained mainly clusters С60 and С70. Isolation of many others clusters, observable in Rofling mass spectrum [2], from carbon vapour has appeared immeasurably more difficult task. Only after 6 years (in 1990) after detection of fullerenes С60 and С70 by mass spectrometric method, Kretchemer [3] succeeded to create conditions of the arc-discharge, which have allowed to fulfill not only the clusterization of generated carbon vapour with primary formation of С60 and С70, but also to locate them in appreciable amounts in obtained fullerene soot. The research by mass spectrometric method of a powder [3], obtained at evaporation of benzene extract from fullerene soot, has shown presence of positive ions with m/z 60 and 70 in the ratio 10:1 in mass spectrum.
The further researches of benzene extracts from fullerene soot have shown [4] that in carbon vapour generated by the arc-discharge method can be realized raising clusterization with the formation of larger than С60 and С70 molecules. It was possible to allocate fullerenes С76, С84, С86, С90 and С94 [5], and also С78 [6] from o-xylene (or o-dichlorobenzene) extracts of fullerene soot by a chromatography method (Al2O3, toluene). However some of “high” fullerenes such as С74 and С80 is not possible to isolate because of extremely high propensity of the given molecules with not coupled electrons [7] to polymerization.
In [8] carbon clusters, earlier detected only in carbon plasma, were found out in gases of a flame of incomplete combustion of benzene by means of mass spectrometric method. This method has appeared most effective for obtaining of fullerenes [9]. Presence of С60 and С70 is found out also in mass spectra of products of heat treatment of benzene and acetylene [10], naphthalene С10Н6 and corannulene [11-13].
Thus, from large amount clusters generated at super high temperatures of the evaporations and burning of carbon and benzene accordingly are synthesized only fullerene С60 and its homologues as more stable molecules with isolated pentagons. Only С36 was synthesized from smaller carbon molecules with adjacent pentagons, quasi-fullerenes [14]. (Though in [15] there is information about obtaining by an arc-discharge method hydrides (C36H4, C36H) and oxy-hydrides (C36H4O, C36H6O) only, but not molecules С36). Unsuccessful attempts to synthesize quasi-fullerenes are explained by their low stability because of presence of adjacent pentagons at the structure. However quasi-fullerene С20, which molecule consists of only pentagons, is easily formed at an irradiation of polythene by a beam of ions Ar+ [16] and laser аblation of diamond [17]. On the other hand, quasifullerenes С28 and С50 with smaller number of adjacent pentagons are formed only as their derivative: endofullerene M@C28 (М-Ti, Zr, Hf or U) [18] and decahlorofullerene C50Cl10 [19].
As to ions of small clusters Cn (n < 20), which always present in carbon plasma [2] and flame of a benzene/ oxygen stream [8,9], the molecules, appropriate to them, for example С2 and С3, С4 and С5 are found out only in circumstellar medium [20]. From carbon vapour the chains C1-C10 stabilize in solutions of methanol or acetonitrile due to a connection to trailer atoms of carbon H, N or CN with formation of relatively more stable polyynes [21] or cyanopolyynes [22]. The technology of matrix isolation of carbon vapour in solid argon (or neon) at 25 - 14 K [23] allows to keep carbon chains, but time of life of such frozen clusters is extremely small (~10 ms) [24].
The results of study by a mass spectroscopic method of the condensed products of a new method of pyrolysis (NMP) of benzene are presented and discussed in the report. Firstly in mass spectra of several solid products of condensation as and in carbon plasma (or in flame gases) small carbon clusters, new carbon molecules (quasi-fullerenes) as well as fullerene С60 and it hydrides are detected simultaneously. The stage of high-temperature sublimation of carbon in a new method of generation of carbon molecules of the different size is completely excluded.
2. Experimental Results and Discussion
Earlier [25-28] we have been systematically studied the influence of various technological parameters on composition of obtained products and, in particular, condensed substances formed at heat treatment of hydrocarbons. On the basis of the experimental results the new method of pyrolysis (NMP) of organic vapours was developed [28-31]. This method differs from two already known processes of pyrolysis [32]. Flash-pyrolysis (FP) [32,33] is used for obtaining of highly active objects of very small size. Flowing continuous pyrolysis (FCP) [32,34] is applied to obtain carbon nanostructures and polyaromatic hydrocarbons (PAH). NMP allows obtaining simultaneously not only carbon nanostructures but also practically all carbon clusters detected in carbon plasma. Distinctive feature of NMP is an opportunity of partial division of products deposition and condensation. The time of stay of reagents in the most high temperature (~1000˚C) zone A of reaction can be changed in a wide interval that allows generating of intermediate products. A part of condensed substances and pyrolytic soot are taken out from a zone A and are located in more lowtemperature zones B and D. Vapour-like products (sometimes together with traces of soot) also is condensed in the special zone C of cooled reactionary space. Products of several (8 - 10) experiences obtained at given temperature taken from zones B, C and D were blended. Results of study of the condensed products B, C and D of the heat treatment of benzene vapours by a method of matrix-assisted laser (nitrogen, 337 nm) desorption/ionization (MALDI) (Bruker Daltonics Flex Analysis) are submitted here. The extract (ethanol, toluene or water) was located on a metal substrate and after evaporation of the solvent was exposed to a laser irradiation.
2.1. Products of Zone B
From a product, located in a zone B, condensed substances were extracted serially by toluene В1 and then ethanol В2. After evaporation of ethanol from a solution В2 a deposit В3 have obtained as conglomerates from transparent white crystals. The deposit В3 is easily dissolved in water В4. In mass spectrum of negative ions of a water solution В4 (Figure 1) there are peaks which correspond to values m/z (48, 60, 72, 84, 96, 108, 120 and 132) differing precisely on 12 units (Figure 1, inset). The similar periodicity is connected to different number of atoms of carbon in detected clusters: С4, С5, С6, С7, С8, С9, С10 and С11. The spectrum of cations contains some peaks of small intensity with m/z: 429 and 219.
Mass spectra of cations and anions of a sample В2 contain two general peaks with m/z 72 (cluster С6) and m/z 144 (cluster С12). In mass spectrum of anions (Figure 2) the periodicity already marked for В4 is observed among the most intensive peaks: 8 clusters with consecutive (in 12 units) increasing of number of carbon atoms from С3 up to С14 (Figure 2, inset). Also peaks with m/z 219 and 429 are contained in a spectrum of cations, those were found out in a spectrum of a product В4. Therefore it is possible to assume that in ethanol and water the substance (or substances) is dissolved which is exposed to destruction with formation of small carbon clusters from С3 up to С12 under action of a laser beam. Though, probably that separate small carbon molecules are stabilized in ethanol and in water as well. Earlier a similar range of carbon clusters was detected only in carbon plasma [2], in gases of benzene combustion [9] and at a
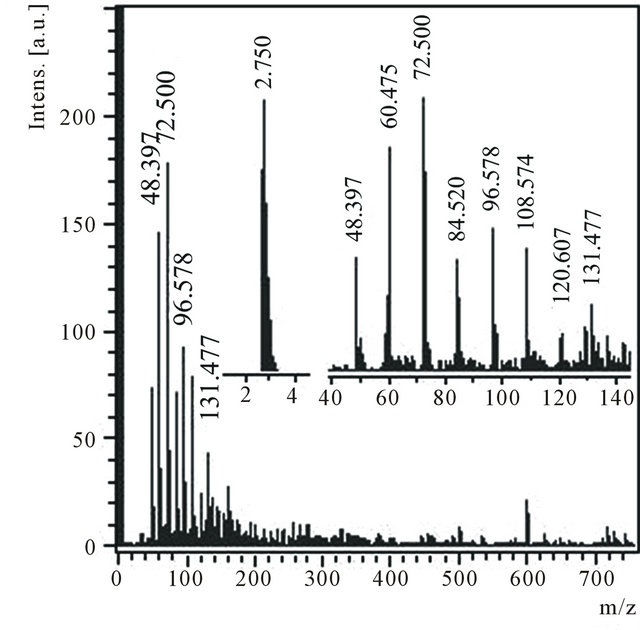
Figure 1. Anions mass spectrum of a water solution of the product В4 with m/z 40 - 150 and 2 - 4 regions in the insets.

Figure 2. Anions mass spectrum of the ethanol solution of the product B2 with m/z 45 - 200 and 2 - 4 regions in the insets.
laser irradiation of fullerene soot [35]. Probably, in fullerene soot formed at оligomerization of carbon clusters not only soluble in toluene С60 and С70 can be condensed as products by their increased clusterization. Precursors of С60 and С70 also can be condensed in this soot with formation of fixed (or otherwise deactivated) radicals (molecules), mainly soluble in alcohol and water.
In mass spectra of anions and cations of an extract В1 there are groups of peaks with m/z 720, 696, 672, 648 and 624 (Figure 3(a)), which, as it is accepted to consider, are characteristic for fullerene С60 and clusters С58,
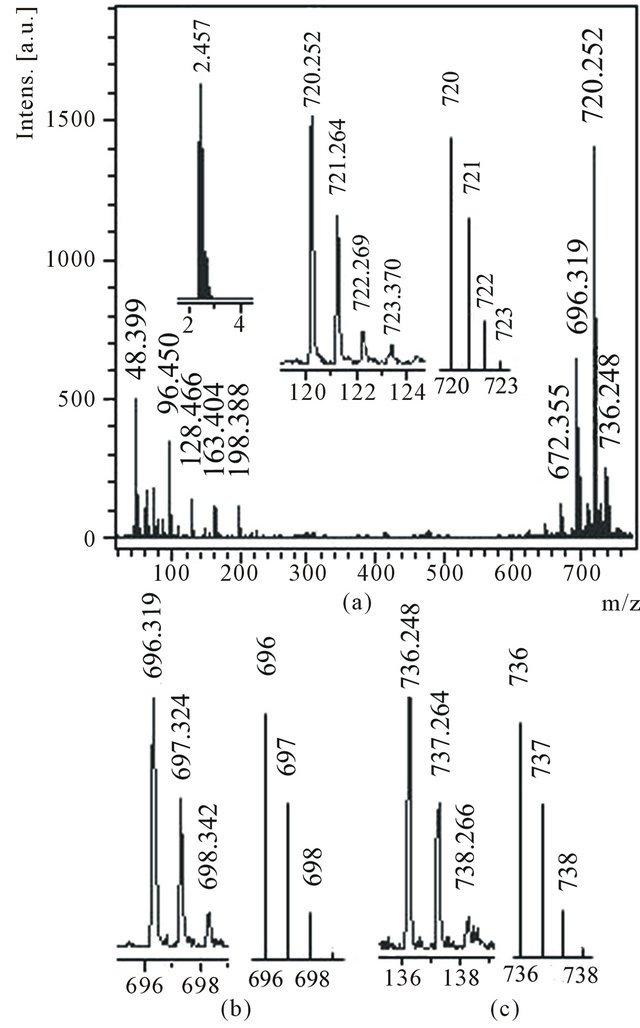
Figure 3. Anions mass spectrum of the toluene solution of the product B1 (a) with the expansions of the experimental and theoretical isotope distributions for C60 in the inset, the expansions around the (b) m/z 696, (c) m/z 736 peaks and the calculated isotope mass ratios for C58, С60H16 molecules respectively.
С56, С54 and С52. Really, from thin structure of peaks С60 (Figure 3(a), inset), and С58 (Figure 3(b)) follows that the isotope distribution in these peaks completely corresponds to natural distribution of isotopes of carbon in molecules С60 and С58. In a spectrum of anions distinctly (against to a spectrum of cations) the periodicity in a range of peaks with m/z from 48 up to 120 also is visible which was observed in mass spectra of negative ions of products soluble in water and ethanol. It is accepted to consider that clusters group closest on the size to С60 and group of smallest clusters are formed at destruction С60 or its derivatives (La@C60, C60O [36]) only at powerful laser irradiation. It is possible that substances soluble in water and ethanol are dissolved as well in toluene. Hydrogenated fullerenes С60Н6, С60Н16 and С60Н20 which peaks distinctly are visible in spectra of both anions and cations are dissolved as well in toluene. According to thin structure of peak with m/z 736 (Figure 3(c)) the isotope distribution in it completely corresponds to calculated ratio of isotopes for a molecule С60Н16.
2.2. Products of Zone С
Product C contains mainly transparent light particles. According to the data of the X-ray microanalysis (X-ray microanalyzer Camebax SX-50) the product consists only of carbon. The product C is practically completely dissolved in ethanol. Mass spectrum of anions (Figure 4) of an ethanol solution С1 contains a group of the most intensive peaks with m/z 36, 48, 60, 72, 84, 96, 108, 120, 132, 144 and 156, which can correspond to anions of small carbon molecules from С3 up to С13 (Figure 4, inset). Peaks with m/z 169 and 181, it is possible, correspond to hydrogenated molecules. According to thin structure of peak with m/z 576 isotope distributions in it correspond to a molecule С48.
Mass spectrum of cations (Figure 5) contains a group of peaks which the values m/z (85, 97, 109, 133 and 193) can correspond to protonated molecules C7, C8, C9, С11 and C16 (Figure 5(b)). Three distinct peaks with m/z 72, 180 and 396 can correspond to molecules С6, С15 and С33. Though, from thin structure of peak with m/z 396 (Figure 5(a), inset) follows that a part of molecules С33 are partially hydrogenated. Hence, the molecules С7, С8 and С9 are detected either as anions, or as protonated clusters. Only molecule С6 is detected in both spectra.
2.3. Products of Zone D
Soluble in toluene substances from a product D were extracted and deposited by ethanol. Deposited red-brown powder D1 was dissolved in acetone. Mass spectra of anions (Figure 6) and cations (Figure 7) of an acetone extract D1 essentially differ. First of all, in a spectrum of anions a peak with m/z 576 is distinctly seen which, as is marked earlier, presents in mass spectrum of a product С1 dissolved in ethanol. In a spectrum the peak with m/z 576 is distinctly seen as well, the isotope distribution in which ((Figure 6(a), inset) differs from natural isotope distribution of carbon in a molecule С48. It is probably, molecule С48 is partially hydrogenated (up to С48Н2). Hence, quasi-fullerene С48 is located in different zones of reactionary space and is easily dissolved both in ethanol and in acetone.
In a spectrum of anions (Figure 6(b)) there is a large group of very intensive peaks with relatively small values m/z: 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, 168 and 180. Periodicity of occurrence of these peaks is 12 units that can demonstrate the belonging of these peaks to clusters from С3 up to С15. The similar structure of mass spectrum of anions was found out and for a product С1 (Figure 4, inset), dissolved in ethanol. The very intensive peak with m/z 255 can correspond to hydrogenated molecule С21Н3 (or С20Н15). Just the peak with m/z 255
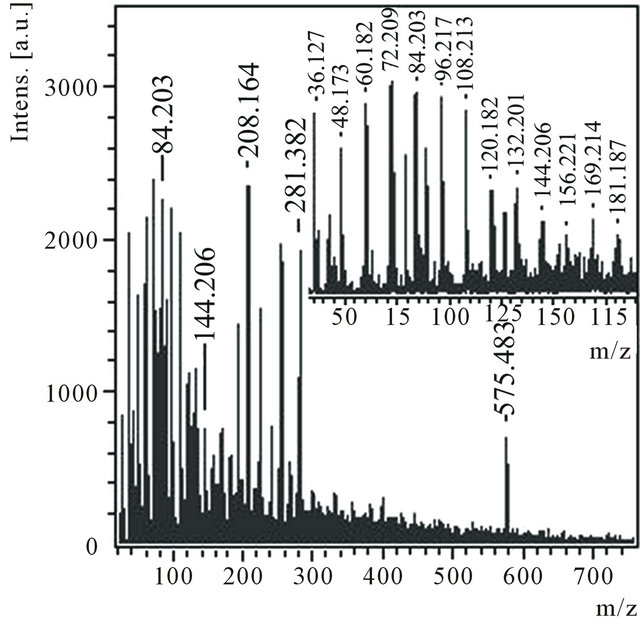
Figure 4. Anions mass spectrum of the ethanol solution of the product C1 with m/z 45 - 185 region in the inset.
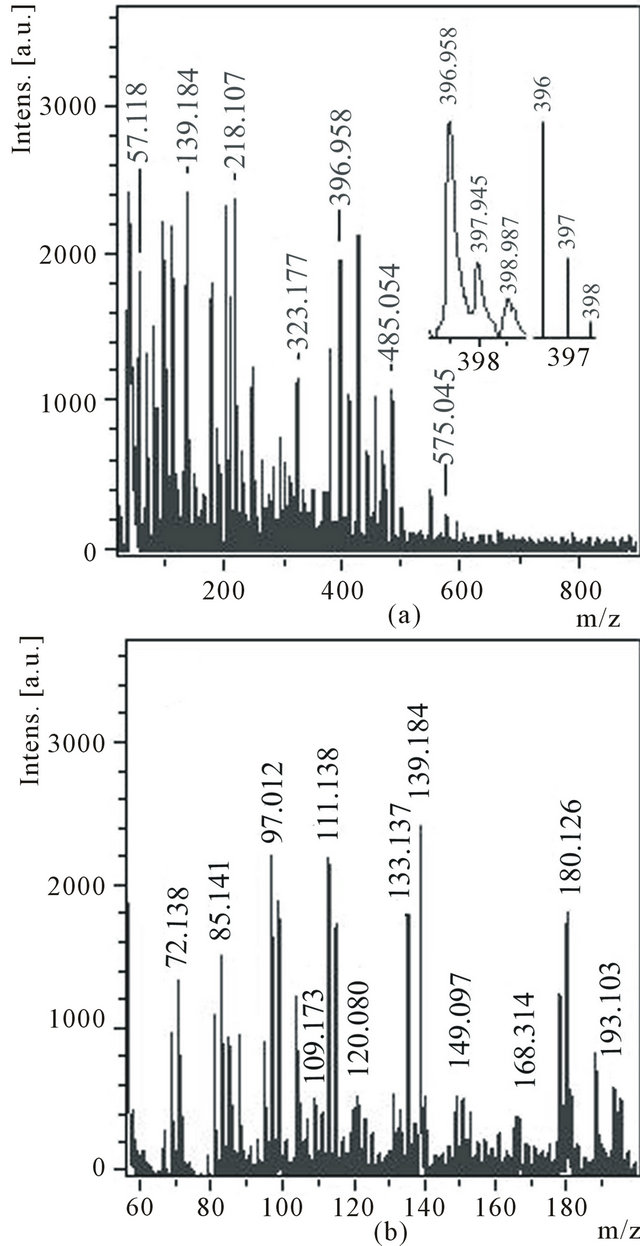
Figure 5. Cations mass spectrum of the ethanol solution of the product C1 with the expansions of the experimental and theoretical isotope distributions for C33 in the inset (a), m/z 55 - 200 region (b).
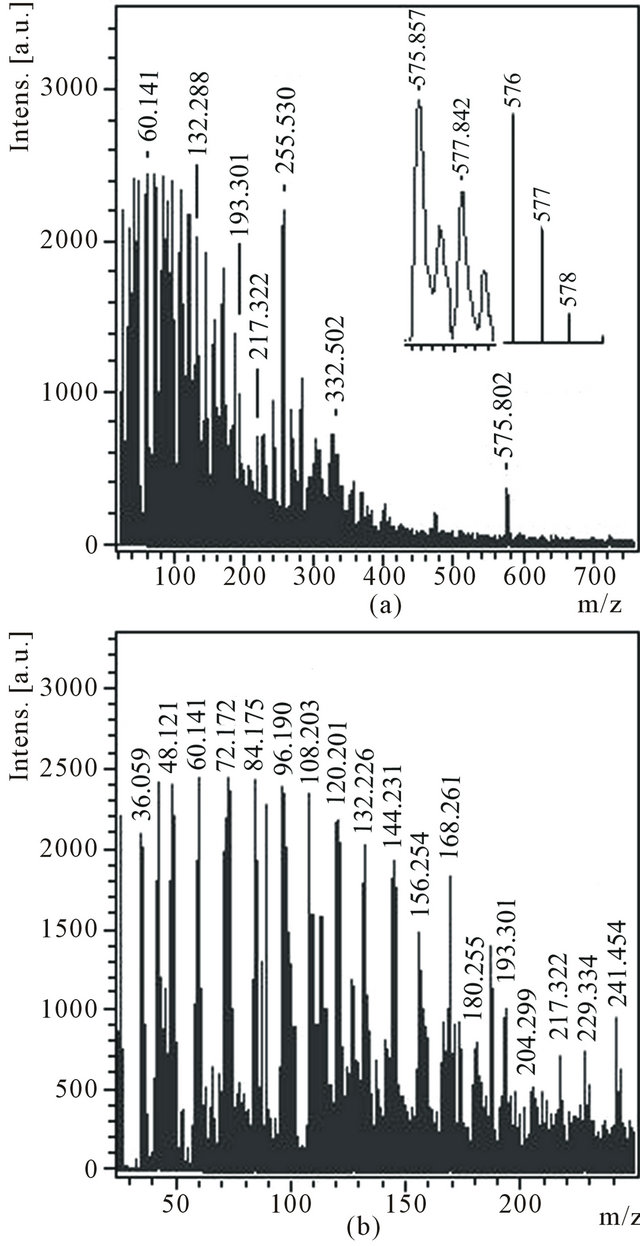
Figure 6. Anions mass spectrum of the acetone solution of the product D1 with the expansions of the experimental and theoretical isotope distributions for C48 in the inset (a), m/z 45 - 250 region (b).

Figure 7. Сations mass spectrum of the acetone solution of the product D1 with the expansion around the m/z 219.
allows to see some difference in mass spectra of аnions of products С1 (transparent light particles) and D1 (red - brown powder). However, mass spectra of cations testify to essential distinctions of these products (Figure 5, Figure 7). In mass spectrum D1 most intensive peak with m/z 133 as well as rather intensive peaks with m/z 85 and 219 is contained. It is possible to believe, that peaks with m/z 85 and 133 correspond to minimally hydrogenated molecules. The peak with m/z 219 can correspond also to protonated molecule С18Н3 (Figure 7, inset). It is possible, that the product D1 contains stabilized by other products of benzene pyrolysis molecules С7 and С11, which are decomposed on clusters mainly of smaller size С3-С5 under laser beam.
Thus, clusters С3-С15 as well as С60 or С48 are detected as anions in all three products of benzene pyrolysis. In a spectrum of cations the molecules С6-С9 and С11 as well as С15 and С33 are detected only.
2.4. Products 1B of Zone B
It is necessary to note that the composition of products of benzene pyrolysis, in particular, located in a zone B essentially depends on a regime of synthesis. From a product 1В obtained at lower temperature of pyrolysis were extracted by toluene the condensed substances and some of them deposited at addition of ethanol. The obtained deposit 2В as red (wax-similar) film was again dissolved in toluene and its mass spectra are submitted in Figure 8. Mass spectrum of anions (Figure 8(a)) contains the most intensive peak with m/z 168 as well as less intensive peaks with m/z 96, 132 and 216. From thin structure of peaks with m/z 96 and 168 follows, that the distribution of isotopes of carbon in them corresponds to molecules С8 and С14. At the same time, from the extended spectrum it is possible to see that some of small carbon molecules, for example С13, are hydrogenated essentially. The spectrum of cations (Figure 8(b)) testifies a high degree of hydrogenation of a product 2В. The most intensive peak with m/z 139 according to its thin structure corresponds to a hydrogenated molecule С11Н7. The peaks with the large values m/z also correspond to hydrogenated molecules of carbon or thermostable polyaromatic hydrocarbons (PАHs) which can be intermediate at the formation С60 and its hydrides.
According to the chemical analysis a red product 2В consists of carbon, hydrogen (up to 4.2% mass.) and oxygen (up to 3.1% mass.). The composition of volatile products of thermal decomposition 2В was investigated by a method temperature-programmed desorbtion mass spectrometry (TPDMS). Тhermodesorption measurement was carried out on monopole mass spectrometer МХ- 7304А (Sumy, Ukraine) with impact electron ionization (EI) [37]. A sample 2В at the bottom of molibdeniumquarts ampoule was evacuated at room temperature up to
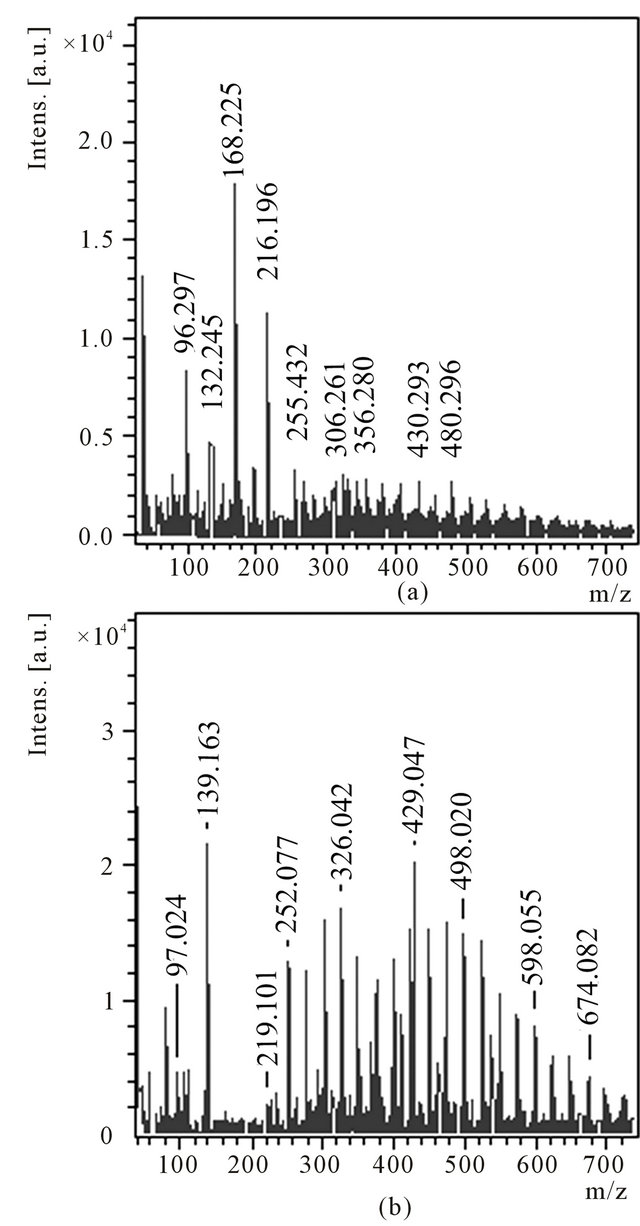
Figure 8. Anions (a) and cations (b) mass spectra of the toluene solution of the product 2B.
5 × 10−5 Pa. The linear heating of a sample up to 650˚C was carried out with speed 0.15 K∙s−1 [37]. The volatile thermolysis products passed through a high-vacuum valve (5.4 mm in diameter) into the ionization chamber of the mass spectrometer The ion currents of the desorption and thermolysis products were recorded with a secondary-electron multiplier VEU-6. Mass spectra were Registered in a range 1 - 210 amu. The hydrogen as can see from a curve of thermodesorption (Figure 9(a)) begins allocation from a sample 2В already at room temperature (in vacuum) and in enough large amount. It is improbable that PAHs in a similar way can be decomposed. It should be noted that the intensive peaks of hydrogen is also observed in the MALDI mass spectra of water, ethanol and toluene solutions of the product B4 (Figure 1, inset), B2 (Figure 2, inset) and B1 (Figure 3(a), inset) respectively. Mass spectrum EI at 200˚C on Figure 9(b) is presented. Intensive peaks with 18, 28 and 44 amu correspond to molecular ions desorbed water, nitrogen and carbon dioxide, respectively. Ions with 31
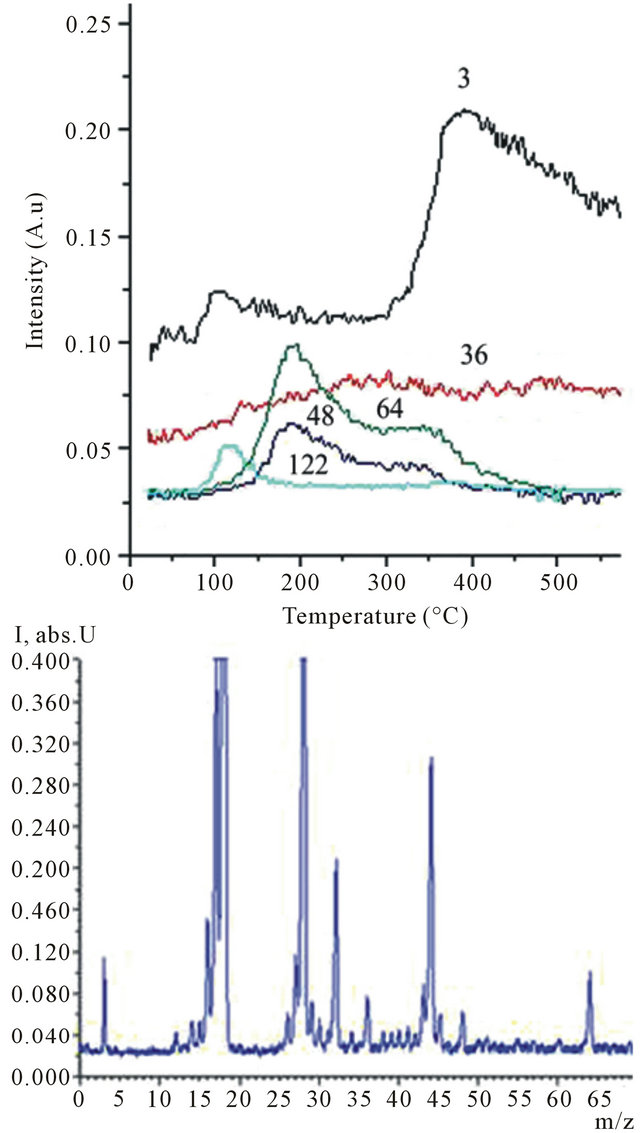
Figure 9. Experimental thermogram for selected m/z 3, 36, 48, 64 and 122 peaks (a) and representative mass spectrum at 200˚C (b) for the product 2B.
and 45 amu as fragments of decomposition of molecules of the solvent (ethanol) are characteristic for EI mass spectra. The basic products of thermodesorption are carbon clusters С3, С4 and С5Н2 with molecular mass 36, 48 and 64 accordingly. It is possible that detected on MALDI mass spectra carboneous (С8 and С14) and hydrogenated molecule (С11Н7) are thermo unstable already at low temperatures.
Thus, firstly clusters, distinctive for carbon plasma, are generated at destruction of substances obtained at hydrocarbon pyrolysis. Pyrolysis is carried out at temperatures excluding evaporation of carbon therefore small carbon clusters (С3-С5) can be formed only due to dehydrogenation and destruction of benzene molecules. The destruction of a molecule С6Н6 can be proceeded its complete dehydrogenation with formation of a linear or ring molecules С6 of polyynic or cumulenic structure. Clusters С7-С11 and С15 can be products of clusterization С3- С6. The formation of molecules С60, С48 and С33 can be realized owing to polycondensation of molecules С6Н6, while hydrides (С60Н6, С60Н16, С60Н20) are formed because of reactions of polymerization of molecules С6Н6 or hydrogenation of the formed molecules С60. It is possible, that the radicals, for example С6Н5, are formed at partial dehydrogenation of molecules С6Н6 which further, as well as С6, can be precursors of carbon molecules and clusters. However absence of biphenyl (С6Н5-С6Н5) in products of benzene pyrolysis as product of the first stage of reaction of polycondensation С6Н6, but presence of clusters С12 (m/z 144) and С18 (m/z 216) as dimer and trimer С6 accordingly, can testify to preferable formation С60 and С48 from carbon clusters, instead of from hydrocarbons radicals.
Very important question connected to the detailed mechanism of the formation С60 and С48, remains, open: whether clusters С6 of an initial molecule С6Н6 accept participation in formation of molecules С60 and С48? Or these large carbon molecules are formed only due to increase of clusterization of fragments (С3-С5) of disintergration С6?
Pyridine (C5NH5) is heterocyclic analogue of benzene (С6Н6) therefore from precursors C5N or С3-С5 should be formed accordingly heteroatomic or monoatomic fullerenes and quasi-fullerenes. Our preliminary researches [29,30] have shown that at pyridine pyrolysis large nitrogen-carbon containing molecules are formed which further study represents not only scientific but also practical interest.
3. Conclusion
New method of organics pyrolysis for generation of carbon clusters as an alternative powerful laser (or arc-discharge) evaporation of graphite is developed. Condensation products obtained at new method of pyrolysis of benzene vapours by mass spectrometric method are studied. In products of all kinds of carbon molecules and some hydrides are detected. Obtained experimental results firstly demonstrate that the small carbon molecules can be generated in reactionary conditions excluding evaporation of carbon. The first stage of the transformation of benzene molecules to carbon molecules is their dehydrogenation and destruction. Firstly fullerene С60 and quasifullerenes С48 and С33 as well as small carbon molecules and some hydrides molecules in different substances are detected simultaneously.
REFERENCES
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl and R. E. Smalley, “C60: Buckminsterfullerene,” Nature, Vol. 318, No. 6042, 1985, pp. 162-163. doi:10.1038/318162a0
- E. A. Rohlfing, D. M. Cox and A. Kaldor, “Production and Characterization of Supersonic Carbon Cluster Beams,” Chemical Physics, Vol. 81, No. 7, 1984, pp. 3322-3330. doi:10.1063/1.447994
- W. Kratschmer, L. D. Lamb, K. Fostiropoulos and D. R. Huffman, “Solid C60: A New Form of Carbon,” Nature, Vol. 347, No. 6291, 1990, pp. 354-358. doi:10.1038/347354a0
- R. Taylor, J. P. Hare, A. K. Abdul-Sada and H. W. Kroto, “Isolation, Separation and Characterisation of the Fullerenes C60 and C70: The Third Form of Carbon,” Journal of the Chemical Society, Chemical Communications, Vol. 20, No. 20, 1990, pp. 1423-1425. doi:10.1039/c39900001423
- F. Diederich, R. Ettl, Y. Rubin, R. L. Whetten, R. Beck, M. Alvarez, S. Anz, D. Sensharma, F. Wudl, K. C. Khemani and A. Koch, “The Higher Fullerenes: Isolation and Characterization of C76, C84, C90, C94, and C70O, an Oxide of D5h-C70,” Science, Vol. 252, No. 5005, 1991, pp. 548- 551. doi:10.1126/science.252.5005.548
- F. Diederich, R. L. Whetten, C. Thilgen, R. Ettl, I. Chao and M. M. Alvarez, “Fullerene Isomerism: Isolation of C2v, -C78 and D3-C78,” Science, Vol. 254, No. 5039, 1991, pp. 1768-1770. doi:10.1126/science.254.5039.1768
- M. D. Diener and J. M. Alford, “Isolation and Properties of Small-Bandgap Fullerenes,” Nature, Vol. 393, No. 6686, 1998, pp. 668-671. doi:10.1038/31435
- Ph. Gerhardt, S. Löffler and K. H. Homann, “Polyhedral Carbon Ions in Hydrocarbon Flames,” Chemical Physics Letters, Vol. 137, No. 4, 1987, pp. 306-310. doi:10.1016/0009-2614(87)80889-8
- J. B. Howard, J. T. McKinnon, Y. Makarovsky, A. L. Lafleur and M. E. Johnson, “Fullerenes C60 and C70 in Flames,” Nature, Vol. 352, No. 6331, 1991, pp. 139-141. doi:10.1038/352139a0
- G. M. Jenkins, L. R. Holland, H. Maleki and J. Fisher, “Continuous Production of Fullerenes by Pyrolysis of Acetylene at a Glassy Carbon Surface,” Carbon, Vol. 36, No, 12, 1998, pp. 1725-1727. doi:10.1016/S0008-6223(97)00220-0
- R. Taylor, G. J. Langley, H. W. Kroto and D. R. M. Walton, “Formation of C60 by Pyrolysis of Naphthalene,” Nature, Vol. 366, No. 6457, 1993, pp. 728-731. doi:10.1038/366728a0
- C. J. Crowley, R. Taylor, H. W. Kroto, D. R. M. Walton, P. C. Cheng and L. T. Scott, “Pyrolytic Production of Fullerenes,” Synthetic Metals, Vol. 77, No. 1-3, 1996, pp. 17-22. doi:10.1016/0379-6779(96)80048-8
- J. Osterodt, A. Zett and F. Vögtle, “Fullerenes by Pyrolysis of Hydrocarbons and Synthesis of Isomeric Methanofullerenes,” Tetrahedron, Vol. 52, No. 14, 1996, pp. 4949- 4962. doi:10.1016/0040-4020(96)00103-2
- C. Piskoti, J. Yarger and A. Zettl, “A New Carbon Solid, C36,” Nature, Vol. 393, No. 6687, 1998, pp. 771-774. doi:10.1038/31668
- A. Koshio, M. Inakuma, T. Sugai and H. Shinohara, “A Preparative Scale Synthesis of C36 by High Temperature Laser Vaporization: Purification and Identification of C36H6 and C36H6O,” Journal of the American Chemical Society, Vol. 122, No. 2, 2000, pp. 398-399. doi:10.1021/ja9934347
- Z. Wang, X. Ke, Z. Zhu, F. Zhu, M. Ruan, H. Chen, R. Huang and L. Zheng, “A New Carbon Solid Made of the World’s Smallest Caged Fullerene C20,” Physics Letters A, Vol. 280, No. 5-6, 2001, pp. 351-356. doi:10.1016/S0375-9601(00)00847-1
- Z. Iqbal, Y. Zhang, H. Grebel, S. Vijayalakshmi, A. Lahamer, G. Benedek, M. Bernasconi, J. Cariboni, I. Spagnolatti, R. Sharma, F. J. Owens, M. E. Kozlov, K. V. Rao and M. Muhammed, “Evidence for a Solid Phase of Dodecahedral C20,” European Physical Journal B, Vol. 31, No. 4, 2003, 509-515. doi:10.1140/epjb/e2003-00060-4
- T. Guo, M. D. Diener, Y. Chai, M. J. Alford, R. E. Haufler, S. M. McClure, T. Ohno, J. H. Weaver, G. E. Scuseria and R. E. Smalley, “Uranium Stabilization of C28: A Tetravalent Fullerene,” Science, Vol. 257, No. 5077, 1992, pp. 1661-1664. doi:10.1126/science.257.5077.1661
- Z. Chen, “The Smaller Fullerene C50, Isolated as C50Cl10,” Angewandte Chemie International Edition, Vol. 43, No. 36, 2004, pp. 4690-4691. doi:10.1002/anie.200401764
- F. Cataldo, “Cyanopolyynes: Carbon Chains Formation in a Carbon Arc Mimicking the Formation of Carbon Chains in the Circumstellar Medium,” International Journal of Astrobiology, Vol. 3, No. 03, 2004, pp. 237-246. doi:10.1017/S1473550404002149
- F. Cataldo, “Simple Generation and Detection of Polynes in an Arc Discharge between Graphite Electrodes Submerged in Various Solvents,” Carbon, Vol. 41, No. 13, 2003, pp. 2653-2689. doi:10.1016/S0008-6223(03)00345-2
- F. Cataldo, “Synthesis of Polyynes in a Submerged Electric Arc in Organic Solvents,” Carbon, Vol. 42, No. 1, 2004, pp. 129-142. doi:10.1016/j.carbon.2003.10.016
- W. Jr. Weltner, P. N. Walsh and C. L. Angell, “Spectroscopy of Carbon Vapor Condensed in Rare-Gas Matrices at 4˚ and 20˚ K. I,” Journal of Chemical Physics, Vol. 40, 1964, pp. 1299-1305. doi:10.1063/1.1725312
- I. Cermak, M. Förderer, S. Kalhofer, H. Stopka-Ebeler and W. Krätschmer, “Laser-Induced Emission Spectroscopy of Matrix-Isolated Carbon Molecules: Experimental Setup and New Results on C3,” Journal of Chemical Physics, Vol. 108, No. 24, 1998, pp. 10129-10142. doi:10.1063/1.476472
- A. I. Kharlamov, S. V. Loythenko, N. V. Кirillova, S. V. Kaverina and V. V. Fomenko, “Toroidal Nanostructures of Carbon. Single-Walled 4-, 5- and 6-Hadrons and Nanorings,” Reports of the National Academy of Sciences of Ukraine, No. 1, 2004, pp. 95-100. https://www.etde.org/etdeweb/details_open.jsp?osti_id=20465861
- A. I. Kharlamov, L. N. Ushkalov, N. V. Кirillova, V. V. Fomenko and N. I. Gubareny, “Synthesis of Onion Nanostructures of Carbon at Pyrolysis of Aromatic Hydrocarbons,” Reports of the National Academy of Sciences of Ukraine, No. 3, 2006, pp. 97-103.
- G. Kharlamova, A. Kharlamov, N. Kirillova and A. Skripnichenko, “Novel Transparent Molecular Crystals of Carbon,” In: A. Vaseashta and I. Mihailescu, Eds., Functionalized Nanoscale Materials, Devices and Systems, Springer, Dordrecht, 2008, pp. 373-379. doi:10.1007/978-1-4020-8903-9_34
- A. I. Kharlamov and N. V. Kirillova, “Fullerenes and Hydrides of Fullerenes as Products Transformation (Polycondensation) of Molecules of Aromatic Hydrocarbons,” Reports of the National Academy of Sciences of Ukraine, No. 5, 2009, pp. 110-118. http://www.nbuv.gov.ua/portal/all/reports/2009-05/09-05-19.html
- A. Kharlamov, G. Kharlamova, O. Khyzhun and N. Kirillova, “New Substances: Red Carbon Suboxide, Red N-Doped Fullerene (C50N10)O3H10 and Red Carbon,” In: S. Zaginaichenko, D. Schur, V. Skorokhod, Eds., Carbon Nanomaterials in Clean—Energy Hydrogen Systems, Springer, Dordrecht, 2011, pp. 257-268. doi:10.1007/978-94-007-0899-0_24
- O. Kharlamov, G. Kharlamova, N. Kirillova, O. Khyzhun and V. Trachevskii, “Synthesis of New Carbon Compounds: N-Doped Fullerene (C50N10)O3H10 and ‘Pyridine’ Nanocarbon,” In: A. Vaseashta, E. Braman and P. Susmann, Eds., Technological Innovations in Sensing and Detection of Chemical, Biological, Radiological, Nuclear Threats and Ecological Terrorism, Springer, Dordrecht, 2012, pp. 245-253. doi:10.1007/978-94-007-2488-4_27
- А. I. Kharlamov, M. E. Bondarenko and N. V. Kirillova, “New Method for Synthesis of Fullerenes and Fullerene Hydrides from Benzene,” Russian Journal of Applied Chemistry, Vol. 85, No. 2, 2012, pp. 233-239. doi:10.1134/S1070427212020127
- R. F. C. Brown, “Pyrolytic Methods in Organic Chemistry: Application of Flow and Flash Vacuum Pyrolytic Techniques,” Academic Press, New York, 1980.
- M. J. Plater, M. Praveen and D. M. Schmidt, “Buckybowlsynthesis: A Novel Application of Flash Vacuum Pyrolysis,” Fullerene ScienceandTechnology, Vol. 5, No. 4, 1997, pp. 781-800. doi:10.1080/15363839708012231
- N. R. Conley and J. J. Lagowski, “On an Improved Pyrolytic Synthesis of - and [70]-Fullerene,” Carbon, Vol. 40, No. 6, 2002, pp. 949-953. doi:10.1016/S0008-6223(01)00227-5
- M. A. Khodorkovsky, T. O. Artamonova, S. V. Murashov A. L. Shahmin, A. A. Belyaevа, L. P. Rakcheeva, I. M. Fonseca and S. B. Lubchik, “The Study of the Higher Fullerenes by Ablation of Carbonaceous Materials,” Technical Physics, Vol. 75, No. 10, 2005, pp. 51-54. http://www.ioffe.rssi.ru/journals/jtf/2005/10/p51-54.pdf
- Q. Kong, L. Zhao, J. Zhuang, J. Xu, S. Qian, Y. Li, R. Cai, H. Hou and J. Wang, “Formation of Odd-Numbered Fullerene-Related Species and Its Relation to the Formation of Metallofullerenes,” International Journal of Mass Spectrometry, Vol. 209, No. 1, 2001, pp. 69-79. doi:10.1016/S1387-3806(01)00477-8
- T. V. Kulik, V. N. Barvinchenko, B. B. Palyanitsa, O. V. Smirnova, V. K. Pogorelyi and A. A. Chuiko, “A Desorp- tion Mass Spectrometry Study of the Interaction of Cin- namic Acid with a Silica Surface,” Russian Journal of Physical Chemistry A, Vol. 81, No. 1, 2007, pp. 83-90.doi:10.1134/S0036024407010165
NOTES
*Corresponding author.

