Pharmacology & Pharmacy
Vol.06 No.07(2015), Article ID:58412,7 pages
10.4236/pp.2015.67034
In Vitro Anti-HIV Activity of Partially Purified Coumarin(s) Isolated from Fungal Endophyte, Alternaria Species of Calophyllum inophyllum
Melappa Govindappa1*, Kavya C. Hemmanur1, Shrikanta Nithin1, Chandrappa Chinna Poojari1, Gopalakrishna Bhat Kakunje2, Channabasava1
1Endophytic Natural Product Laboratory, Department of Biotechnology, Shridevi Institute of Engineering & Technology, Tumkur, India
2Madhuca, Srinivasa Nagara, Chitpady, Udupi, India
Email: *dravidateja07@gmail.com, endophytessiet@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 June 2015; accepted 25 July 2015; published 29 July 2015
ABSTRACT
5 totally different endophytic fungal species were isolated from bark and leaf parts of Calophyllum inophyllum. Leaf part yielded Trichoderma harzianum and Alternaria species, whereas bark showed the presence of Fusarium species, Aspergillus species and unidentified fungi. Two solvents (hexane and methanol) were used for endophytic fungal extraction and the Alternaria species had shown the presence of coumarin whereas Trichoderma harzianum in methanol extract and Fusarium species in hexane extract had shown the coumarin(s) in all the four methods tested. The total coumarin yield was more in microwave assistance method, the methanol Alternaria species (3.941 ± 0.082) stood first, followed by hexane extract of Alternaria species (3.254 ± 0.082), Fusarium species (2.532 ± 0.082) and Trichoderma harzianum (2.294 ± 0.082), the plant extract showed 4.149 + 0.053. The methanol extract of Alternaria species inhibited the activity of HIV-Reverse Transcriptase (RT) (82.81 ± 1.0), integrase (98%) and protease (78) in maximum level followed by hexane extract of Alternaria species (71.12 ± 0.9, 89, 68), Fusarium species (63.92 ± 1.8, 67, 66) and Trichoderma harzianum (56.69 ± 0.9, 71, 63). The endophytic fungi Alternaria species inhibited all the three viral enzymes at maximum level and it was more than standard drug. However, in order to know possible anti-HIV, it is necessary to isolate active coumarin from the Alternaria species and the mechanism of action will be studied in future studies.
Keywords:
Endophytes, Alternaria Species, HIV-RT, Integrase, Protease, Inhibitory Activity

1. Introduction
Ayurveda is a traditional Indian medicine system being practicing by people to cure various diseases by using plant and plant based products. These practices are still continuing in Indian society. Presently various plant extracts are using in treatments of HIV [1] . Narayan et al. [2] have reported the importance of C. inophyllum in treatment of HIV by inhibiting the activity of HIV-integrase and protease. The HIV-1 encodes three multifunctional enzymes viz. protease, integrase and reverse transcriptase which are responsible for processing of viral proteins into functional enzymes and structural proteins. The RT is the functional enzyme, it transcripts viral RNA into viral DNA, where the integrase is responsible for integration of double standard DNA [3] .
Development of resistant strains and side effects, the synthetic drugs are considering in failure of people interest. To find suitable remedy, the plant and plant based products are now having global importance in developing new drugs which are not having side effects. The drugs are developed for treatments of HIV and are able to inhibit the replication of virus and its replicating enzymes activities.
Calophyllum inophyllum is ornamental plant wood which is hard and strong and has been used in construction or boat building. Among the medicinal uses the oil is used to treat diabetic sores, psoriasis, sun burn and heal blisters. In southern Africa, it is useful for rheumatism, arthritis and lesions due to herpes. It is also used for problems of scalp and hair. The leaves are used for skin care and eye inflammations. The plant has important biological compounds, viz. coumarins, xanthones, truterpenes and it shows antitumor, cytotoxic, antibacterial, analgesic activities. The C. inophyllum also shows antiviral activity, especially the anti-HIV activity by inhibiting antiviral replicating and functional enzymes [4] . For continuous use of plant and plant based drugs, in the future the plants may be vanished. To safeguard the plants and continue to derive medicinal important drugs, endophyte is one of the important organisms to exploit the above same things and we can produce the compounds at higher concentration within short duration. The present work is aimed to isolate different endophytes of Calophyllum inophyllum and evaluated their extracts for anti-HIV activities (by inhibiting three enzymes viz., RT, intrgrase and protease).
2. Materials and Methods
2.1. Reagents/Chemicals
All the chemicals and media were purchased from Sigma-Aldrich, Merck and all the reagents were of AR grade.
2.2. Collection of Plant Material
Plant Calophyllum inophyllum was collected from Western Ghat region of Udupi, Karnataka during February 2015. Plant was identified with the help of taxonomist, Dr Gopal Krishna Bhat, Udupi.
2.3. Isolation, Identification and Mass Multiplication of Fungal Endophytes
2.3.1. Mass Culture of Fungal Endophytes
Mass cultured the each fungal endophytes using potato dextrose broth for 8 days at room temperature (26˚C ± 2˚C) in separately. After incubation, the fungal mycelium mat was taken for extraction using hexane and methanol. Based on the earlier report of Umashankar et al. [5] , Microwave Assisted Extraction (MAE) method was used, the endophytic fungal mat mixed with methanol was kept for extraction in microwave method at 2 cycles of 5 minutes each at 100˚C and analyzed the percentage of coumarin.
2.3.2. Identification of Coumarin in Isolated Endophytic Fungal Species
Test 1. 3 ml of ethanol extract was evaporated to dryness in a vessel and the residue was dissolved in hot distilled water. It was then cooled and divided into two test portions, one was the reference, the other was the test. To the second test tube, 0.5 ml of 10 NH4OH was added. The occurrence of intense/fluorescence under UV light was a positive test for the presence of coumarins and derivatives. The experiment was carried out for all the experiments in three replicates [6] .
Test 2. 5 ml of the extract was evaporated to dryness and the residue was dissolved in 2 ml of distilled water. The aqueous solution was divided into two equal parts in test tubes. One part was the reference. To the other test tube, 0.5 ml of 10% ammonia solution was added and the test tubes were observed under UV light indicated. The occurrence of a bluish green fluorescence under UV light indicated the presence of coumarin derivatives [6] .
Test 3. To the concentrated alcoholic extract of drug few drops of alcohol FeCl3 solution was added. Development of deep green colour, which turned yellow on addition of conc. HNO3, indicated presence of coumarins.
Test 4. The alcoholic extract was mixed with 1N NaOH solution (one ml each). Development of blue-green fluorescence formation indicated presence of coumarins.
Test 5, detection of phenols. In beakers, 5 ml of each previous filtered extract were taken and 1ml of FeCl3 (1%) and 1 ml K3(Fe(CN)6) (1%) were added. The appearance of fresh radish blue color indicated the presence of polyphenols.
2.4. Anti-HIV Activities
2.4.1. HIV-1 Reverse Transcriptase Inhibition Assay
The HIV reverse transcriptase enzyme inhibition was done with endophytic fungal extracts of C. inophyllum using HIV-RT inhibition method with slight modification of Rege et al. [7] . 25 µl of each endophytic fungal extract (final concentration 5 mg/ml) was added to the reaction mixture. The final mixture was 100 µl (50 mM Tris, pH 7.8), 150 mM KCl, 5 mM MgCl2, 0.05% NP-40, 0.5 mM EGTA, 5 mM DTT, 20 µM dTTP, 0.3 M Glutathione, 2.5 µg/ml BSA, 0.5 µCi (microcurrie) of [3H]TTP, 2.5 µg/ml poly (rA).p(dT). The reaction was started by adding of 0.5 units of recombinant reverse transcriptase enzyme, the mixture was incubated for 3 h at 37˚C and terminated the reaction by adding of 25 µl of 0.1 EGTA by chilling the mixture on ice. 100 µl of each reaction mixture was spotted uniformly onto circular 2.5 cm DE-81 Whatmann filters and kept at ambient temperature for 15 min. The dried filters were washed four times with 5% aqueous Na2HPO4∙7H2O followed by two or three washed with double distilled water. Finally, the filters were thoroughly dried and subjected to scintillation counting. Negative control was set up in parallel and AZT (Azidothymidine/Zidovudine) was used as positive control. Inhibition was calculated as follow,
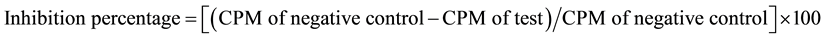 ,
,
where CPM is count per minute.
2.4.2. Assay of HIV-1 Protease Inhibitory Activity
This assay was modified from the previously reported method [8] . In brief, the recombinant HIV-1 PR solution was diluted with a buffer composed of a solution containing 50 mM of sodium acetate (pH 5.0), 1 mM ethylenediamine disodium (EDTA.2Na) and 2 mM 2-mercaptoethanol (2-ME) and mixed with glycerol in the ratio 3:1. The substrate peptides Arg-Val-Nle-NH2 was diluted with a buffer solution of 50 mM sodium acetate (pH 5.0). Two microliters of plant extract and four microliters of HIV-1 PR solution (0.025 mg/ml) were added to a solution containing 2 μl of substrate solution (2 mg/ml) and the reaction mixture of 10 μl was incubated at 37˚C for 1 h. A control reaction was performed under the same condition, but without the plant extract. The reaction was stopped by heating the reaction mixture at 90˚C for 1 min. Subsequently, 20 μl of sterile water was added and an aliquot of 10 μl was analyzed by HPLC using RP-18 column (4.6 mm × 150 mm i.d., Supelco 516 C-18-DB 5 μm, USA). Ten microliters of the reaction mixture was injected to the column and gradiently eluted with acetonitrile (15% - 40%) and 0.2% trifluoroacetic acid (TFA) in water, at a flow rate of 1.0 ml/min. The elution profile was monitored at 280 nm. The retention times of the substrate and p-NO2-Phe-bearing hydrolysate were 4.709 and 2.733 min, respectively. The inhibitory activity of HIV-1 PR was calculated as follows,
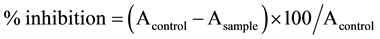
whereas A is the relative peak of the product hydrolysate. Acetyl pepstatin was used as a positive control.
2.4.3. Assay of HIV-IN Inhibitory Activity
Oligonucleotide substrates. Oligonucleotides of long terminal repeat donor DNA (LTR-D) and target substrate (TS) DNA were purchased from QIAGEN Operon, USA and stored at −25˚C before use. The sequence of biotinylated LTR donor DNA and its unlabelled complement were 5'-biotin-ACCCTTTTAGTCAGTGTGGA AAATCTCTAGCAGT-3'(LTR-D1) and 3'-GAAAATCAGTCACACCTTTTAGAGATCGTCA-5' (LTR-D2), respectively. Those of the target substrate DNA (digoxigenin-labelled target DNA, TS-1) and its 3'-labelled complement were 5'-TGACCAAGGGCTAATTCACT-digoxigenin and digoxigenin-ACTGGTTCCCGATTAA GTGA-5' (TS-2), respectively.
2.4.4. Multiplate Integration Assay (MIA)
The integration reaction was evaluated according to the method previously described [9] . Briefly, a mixture (45 μl) composed of 12 μl of IN buffer [containing 150 mM 3-(N-morpholino) propane sulfonic acid, pH 7.2 (MOPS), 75 mM MnCl2, 5 mM dithiothritol (DTT), 25% glycerol and 500 μg/ml bovine serum albumin], 1 μl of 5 pmol/ml digoxigenin-labelled target DNA and 32 μl of sterilized water were added into each well of a 96 well plate. Subsequently, 6 μl of sample solution and 9 μl of 1/5 dilution of integrase enzyme was added to the plate and incubated at 37˚C for 80 min. Wells were washed with PBS four times, 100 μl of 500 mU/ml alkaline phosphatase (AP) labelled anti-digoxigenin antibody were added and incubated at 37˚C for 1 h. The plate was washed again with washing buffer containing 0.05% Tween 20 in PBS four times and with PBS four times. Then, AP buffer (150 μl) containing 100 mM Tris-HCl (pH 9.5), 100 mM NaCl, 5 mM MgCl2 and 10 mM p-nitrophenyl phosphate was added to each well and incubated at 37˚C for 1 h. Finally, the plate was measured with a microplate reader at a wavelength of 405 nm. A control composed of a reaction mixture, 50% DMSO and an integrase enzyme, while a blank is buffer-E containing 20 mM MOPS (pH 7.2), 400 mM potassium glutamate, 1 mM ethylenediamine tetra acetate disodium salt (EDTA.2Na), 0.1% Nonidet-P40 (NP-40), 20% glycerol, 1 mM DTT and 4 M urea without the integrase enzyme. Suramin, a polyanionic HIV-1 IN inhibitor was used as a positive control.
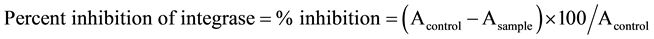
where A is the optical density detected from each well.
2.4.5. Assay of Gp120 Binding Inhibition
Binding of Gp120 to CD4 was analysed using a commercially available Gp120 capture ELISA kit using standard procedure of Rege et al. [7] . To check, our fungal endophytic extracts could interfere with the binding of CD4 to Gp120 by interaction with soluble Gp120, each fungal extract (5 mg/ml) was mixed with 25 ng of purified Gp120 in a total volume of 100 µl and incubated at room temperature (26˚C ± 2˚C) for 1h. Each mixture was added to microtiter plate wells separately coated with CD4 ligand and incubated at room temperature for 1 h. The solutions were aspirated and the wells were washed 3 times with buffer. The Gp120 binding to CD4 was assessed by using detector reagent provided in the kit. Negative and positive control (heparin) was set up in parallel.
The percent inhibition was calculated by,
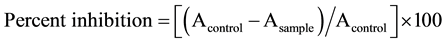 ,
,
A is the optical density.
3. Results and Discussion
Table 1 predicts that two parts (leaf and bark) of Calophyllum inophyllum yielded five different endophytic fungal species. The leaf part exhibited only two fungi (Trichoderma harzianum and Alternaria species) and Alternaria species, Fusarium species and unidentified fungi were noticed from the bark part. The Alternaria species was identified in two parts.
The coumarin(s) identification tests were conducted to all endophytic fungi using separate two different solvent. The coumarin presence was observed in methanol extracts of T. harzianum and in hexane extract of Fusarium species. Both hexane and methanol extract of Alternaria species had shown the presence of coumarin (Table 2). The methanol extract of Alternaria species had shown highe activity of integrase followed by hexane extract of Alternaria species. The total coumarin yield was more in microwave assistance method, the methanol
Table 1. Presence of fungal endophytes in different parts of Calophyllum inophyllum.
+: presence, −: not presence, data based on three replicates of each.
Table 2. Identification of coumarin(s) in two solvent extracts of endophytic fungal species of C. inophyllum.
+: presence, −: not presence, data based on three replicates of each.
Alternaria species (3.941 ± 0.082) stood first, followed by hexane extract of Alternaria species (3.254 ± 0.082), Fusarium species (2.532 ± 0.082) and Trichoderma harzianum (2.294 ± 0.082), the plant extract showed 4.149 ± 0.053 (Table 3). The highest amount of coumarins was obtained from microwave assistance method, 2 cycles of 5 min at 100˚C. Similar results were noticed by Umashankar et al. (2012) by using same method.
Evaluated all the two solvent endophytic fungal extracts against HIV-RT, the highest activity was observed with methanol extract of Alternaria species (82.81 ± 1.0) followed by hexane extract of Alternaria species (71.12 ± 0.9), Fusarium species (54.32 ± 1.4) and Trichoderma harzianum extracts (48.81 ± 1.3) were compared with standard heparin (74.54 ± 0.8) (Table 4). The methanol and hexane extract of Alternaria species inhibited the RT at maximum level, it was more than standard (heparin). The more inhibition of integrase was observed in methanol extract of Alternaria species (98), it was greater than the standard drug followed by same endophytic hexane extract (89) (Figure 1). Pepsin or Gp 120 binding assay was also greatly inhibited by methanol extract of Alternaria species (86.38 ± 1.9), the activity was more than to standard drug (72.51 ± 1.4) (Table 5). The protease activity was strongly inhibited by methanol extract of Alternaria species (78) followed by hexane extract of Alternaria species (68) followed by Fusarium species (66) and Trichoderma harzianum (63) and these data was compared with standard drug acetylpepstain (81). The plant extract had shown 73% of inhibition of the enzyme protease (Figure 2). Similar results are reported by Narayan et al. [2] by using plant C. inophyllum different solvent extract. Our results were also confirmatory with the findings of Rege et al. [7] , Govindappa et al. [10] , Estari et al. [11] and Rege and Chowdhury [12] . Some of endophytic crude extracts or isolated compounds possessed the anti-HIV activity by inhibiting these enzymes [13] -[15] . This is the first report of endophytic fungal species of C. inophyllum, presence coumarin(s) and their in vitro anti-HIV activity and further research has to be carried out to find out exact anti-HIV coumarin molecule.
Table 3. Yield of total coumarin(s) from different endophytic fungal species in microwave assistance method.
Table 4. Effect of endophytic fungal coumarin(s) on HIV-RT inhibition.
HE: Hexane extract, ME: Methanol extract, data based on three replicates of each. Note: inhibition > 50% is considered as significant.
Table 5. Effect of endophytic fungal coumarin(s) extracts on Gp120 binding inhibition.
HE: Hexane extract, ME: Methanol extract, data based on three replicates of each. Note: inhibition > 50% is considered as significant.
Figure 1. Effect of endophytic extracts in inhibition of integrase enzyme.
Figure 2. Inhibition of protease activity by different endophytic fungal extracts.
4. Conclusion
Five different endophytic fungal species were identified from bark and leaf part of Calophyllum inophyllum and the methanol extract of Alternaria species yielded more coumarin compared to other endophytic fungal species and solvent extract. The methanol extract of Alternaria species inhibited the activity of HIV-RT, integrase and protease in maximum level by inhibiting their activity. The coumarin(s) present in the endophytic fungi, Alternaria species can be identified in further research and the exact coumarin their role role in inhibiting the HIV replicating enzymes can also be identified.
Acknowledgements
The authors wish to thank, SERB-DST, New Delhi and Dr. Amitava Roy, Scientist “F”, SERB, for supporting the financial assistance for the project (SB/MEO-355/2013 dated 29-10-2013).
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
Cite this paper
MelappaGovindappa,Kavya C.Hemmanur,S.Nithin,ChandrappaChinna Poojari,GopalakrishnaBhat,K.Channabasava, (2015) In Vitro Anti-HIV Activity of Partially Purified Coumarin(s) Isolated from Fungal Endophyte, Alternaria Species of Calophyllum inophyllum. Pharmacology & Pharmacy,06,321-328. doi: 10.4236/pp.2015.67034
References
- 1. Jassim, S.A.A. and Naji, M.A. (2003) Novel Antiviral Agents: A Medicinal Plant Perspective. Journal of Applied Microbiology, 95, 412-427.
http://dx.doi.org/10.1046/j.1365-2672.2003.02026.x - 2. Narayan, C.L. and Rai, R.V. (2011) A Screening Strategy for Selection of Anti-HIV Integrase and anti-HIV Protease Inhibiotors from Plant Extracts of Indian Medicinal Plants. International Journal of Phytomedicine, 3, 312-318.
- 3. Tewtrakul, S., Nakamura, N., Hattori, M., Fujiwara, T. and Supavita, T. (2002) Flavanone and Flavonol Glycosides from the Leaves of Thevetia peruviana and Their HIV-1 Reverse Transcriptase and HIV-1 Integrase Inhibitory Activities. Chemical and Pharmaceutical Bulletin, 50, 630-635.
- 4. Sundur, S., Shrivastava, B., Sharma, P., Raj, S.S. and Jayasekhar, V.L. (2014) A Review Article of Pharmacological Activities and Biological Importance of Calophyllum inophyllum. International Journal of Advanced Research, 2, 599-603.
- 5. Umashankar, T., Govindappa, M. and Ramachandra, Y.L. (2014) In Vitro Antioxidant and Antimicrobial Activity of Partially Purified Coumarins from Fungal Endophytes of Crotalaria pallid. International Journal of Current Microbiology and Applied Sciences, 3, 58-72.
- 6. Jagessar, R.C. and Cox, M. (2010) Phytochemical Screening of the CHCl3 and CH3CH2OH Extract of Stems, Twigs, Roots and Barks of Conocarpus erectus L. International Journal of Academic Research, 2, 36-45.
- 7. Rege, A.A., Ambaye, R.Y. and Deshmukh, R.A. (2010) In Vitro Testing of Anti-HIV Activity of Some Medicinal Plants. Indian Journal of Natural Products and Resources, 1, 193-199.
- 8. Tewtrakul, S., Subhadhirasakul, S. and Kummee, S. (2003) HIV-1 Protease Inhibitory Effects of Medicinal Plants Used as Self Medication by AIDS Patients. Songklanakarin Journal of Science and Technology, 25, 239-243.
- 9. Tewtrakul, S., Miyashiro, H., Hattori, M., Yoshinaga, T., Fujiwara, T., Tomimori, T., et al. (2001) Inhibitory Effects of Flavonoids on Human Immunodeficiency Virus Type-1 Integrase. Journal of Traditional Medicines, 18, 229-238.
- 10. Govindappa, M., Kumar, N.V.A. and Santoyo, G. (2011) Crotalaria pallida Extracts as a Putative HIV-Protease Inhibitors. Journal of Research in Biology, 4, 285-291.
- 11. Estari, M., Venkanna, L., Sripriya, D. and Lalitha, R. (2012) Human Immunodeficiency Virus (HIV-1) Reverse Transcriptase Inhibitory Activity of Phyllanthus emblica Plant Extract. Biology and Medicine, 4, 178-182.
- 12. Rege, A.A. and Chowdhury, A.S. (2014) Evaluation of Ocimum sanctum and Tinospora cordifolia as Probable HIV Protease Inhibitors. International Journal of Pharmaceutical Sciences Review and Research, 25, 315-318.
- 13. Xiang, Z.C., Jiang, Y. and Guo, S.X. (2006) In Vitro Anti-HIV Activity of a Chinese Fungus Extract. Biomedical and Environmental Sciences, 19, 169-172.
- 14. Umashankar, T., Govindappa, M. and Ramachandra, Y.L. (2012) In Vitro Antioxidant and Anti-HIV Activity of Endophytic Coumarin from Crotalaria pallida Aiton. Planta Medica, 78, 102.
http://dx.doi.org/10.1055/s-0032-1307610 - 15. Wellensiek, B.P., Ramakrishnan, R., Bashyal, B.P., Eason, Y., Gunatilaka, A.A.L. and Ahmad, N. (2013) Inhibition of HIV-1 Replication by Secondary Metabolites from Endophytic Fungi of Desert Plants. The Open Virology Journal, 7, 72-80.
http://dx.doi.org/10.2174/1874357920130624002
NOTES

*Corresponding author.




