Pharmacology & Pharmacy
Vol.3 No.3(2012), Article ID:20635,10 pages DOI:10.4236/pp.2012.33037
Stability Characterization, Kinetics and Mechanism of Degradation of Dantrolene in Aqueous Solution: Effect of pH and Temperature*
![]()
1Division of Product Quality Research, Office of Pharmaceutical Science, Food and Drug Administration, Silver Spring, USA; 2Office of Generic Drugs, Food and Drug Administration, Rockville, USA.
Email: #saeed.khan2@fda.hhs.gov
Received March 27th, 2012; revised April 28th, 2012; accepted May 16th, 2012
Keywords: Dantrolene; UPLC; pH; Impurity; Degradation; Compound B, C
ABSTRACT
The mechanism of degradation of dantrolene in aqueous buffer solutions was studied at various pH values in the range of pH 1.2 - 9.5 and at temperatures ranging from 25˚C to 75˚C to determine the optimum pH and temperature requirements for its stability and eventual product performance over the human gastrointestinal pH range. Dantrolene was analyzed by reversed phase ultra-performance liquid chromatographic (UPLC). Chromatographic separation was achieved on a Waters Acquity UPLC system using a Waters BEH C18 analytical column and Waters BEH C18 guard column. The compounds were eluted with a linear acetonitrile gradient (25% - 75%) over three minutes with a buffer composition of 2.0 mM of sodium acetate at pH 4.5 for degradation studies. The flow rate was maintained at 0.5 mL/min. Column temperature was maintained at 35˚C. Injection volume was 4 µL and the degradation products were detected by a photodiode array (PDA) detector at 375 nm. Degradation products, including compound B and C were analyzed by mass spectroscopy (MS) and nuclear magnetic resonance spectroscopy (NMR) and the degradation pathways were proposed. Degradation of dantrolene followed pseudo first-order kinetics and a V-shaped pH-rate profile over the pH range 1.2 - 9.5. The maximum stability was observed at pH 7.4 and 37˚C. Although the focus of this paper was on the mechanism of hydrolysis of dantrolene, the poor aqueous solubility of dantrolene, the developed understanding can be utilized to improve the quality of the formulation and the risk associated with the extravasation of dantrolene sodium solution in its current form.
1. Introduction
Dantrolene is widely used as a muscle relaxant and for the treatment of malignant hyperthermia [1,2]. Despite the importance of this drug as a powerful muscle relaxant, little is known about the temperature and pH dependence on dantrolene’s degradation in aqueous solution. Dantrolene was reported to undergo hydrolysis in alkaline solution causing it to degrade via hydantoin ring opening to form an open ring compound (designated as compound B) (Figure 1) but no report has been published on a possible catalytic hydrolysis of dantrolene in neutral or acidic media at different temperatures [3]. Dantrolene is in part structurally related to nitrofurantoin and furatadone and also contain an azomethime bond (-N=CH-) [4]. The degradation of drugs containing azomethine bond in aqueous solutions has been reported previously [5,6]. The hydrolysis of dantrolene in acidic condition involves reversible azomethine bond cleavage presumably through a mechanism involving reversible addition of water across the azomethine bond. This leads to the formation of a transient carbinolamine which then rearranges to the compound C. Proposed mechanism of formation of product schemes are shown in Figures 1 and 2.
Physicochemical properties including solubility, lipophilicity and ionization of dantrolene have been reported and the pharmacokinetics of dantrolene has been described [1,7-9]. Yet very limited information on the effect of pH on maximum stability has been published [7]. Previous reports of dantrolene hydrolysis in aqueous solution have not discerned conclusively whether dantrolene exhibits: 1) sharply defined pH dependence for maximum stability or 2) whether it shows a broad pH range effect on maximum stability. In order to understand the degradation kinetics of dantrolene, aqueous
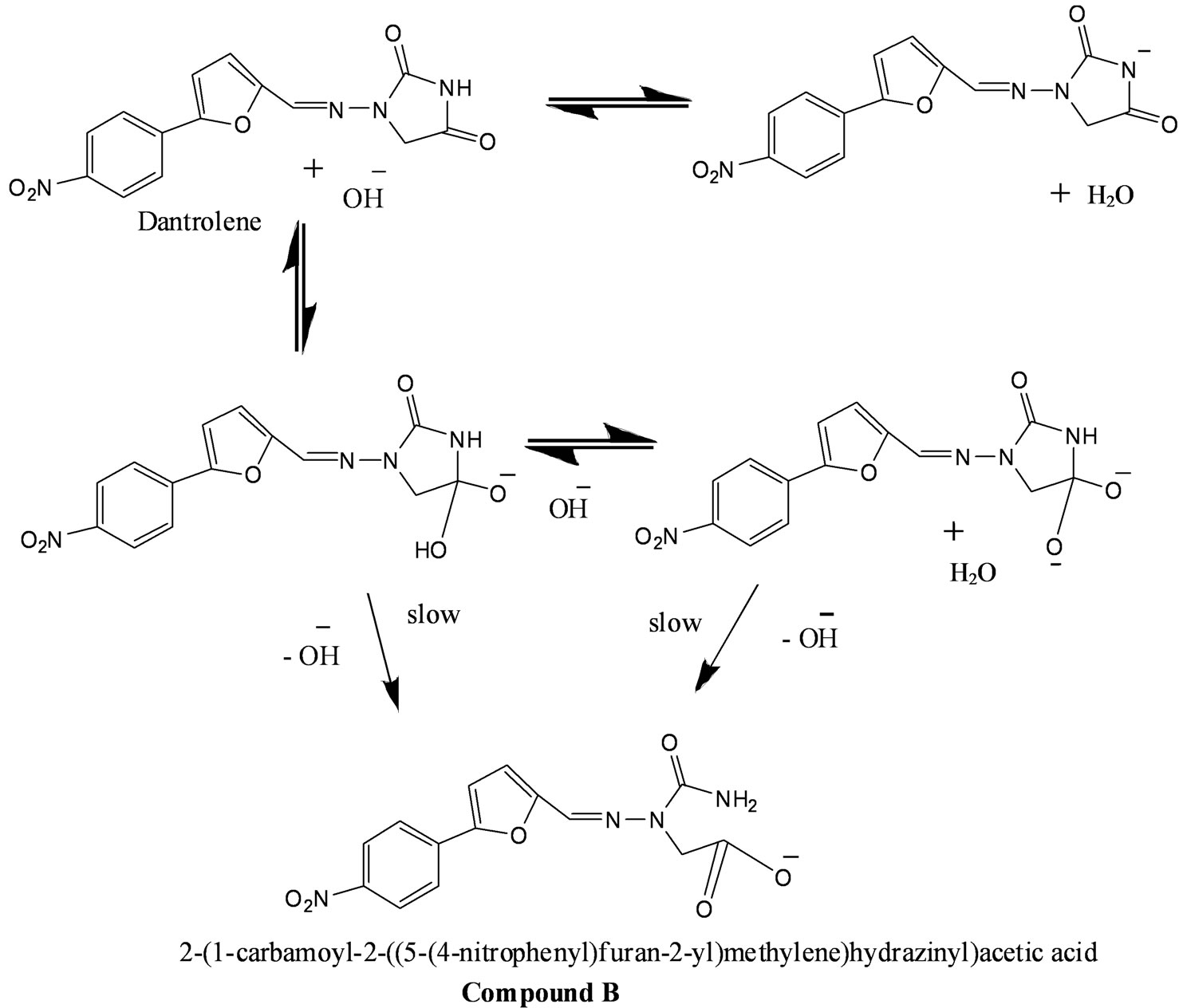
Figure 1. Degradation pathway for dantrolene in alkaline media.
stability studies were carried out, because such studies on dantrolene have not been reported in the literature.
The objectives of the present study were to investigate the stability of dantrolene in aqueous solution as a function of pH and temperature; and to identify the pathways of chemical degradation in the aqueous solution for mechanistic understanding using stability indicating UPLC method [10].
2. Experimental
2.1. Materials
Dantrolene and its related impurities reference standards (A, B and C) were purchased from the United States Pharmacopeia (Rockville, MD). Nylon syringe filters were purchased from Sun-SRI (Rockwood, TN). Sodium acetate was purchased from Sigma-Aldrich (St. Louis, MO). Boric acid salt was purchased from JT Baker (Philipsburg, NJ). N, N-dimethylformamide (DMF) was purchased from Acros Organics (Geel, Belgium). HPLCgrade acetonitrile was purchased from Burdick and Jackson (Muskegon, MI). Certified ACS-Grade HCl solution, HPLC-grade monobasic potassium phosphatepotassium chloride and potassium biphthalate was purchased from Fisher Scientific (Fairlawn, NJ). NaOH solution was purchased from LabChem Inc (Pittsburg, PA) HPLC-ready 18 megohm water was obtained, in-house, from a Milli-Q Gradient A-10 water purification system, Millipore Corp. (Bedford, MA).
2.2. Instrumentation and Chromatographic Conditions
The quantitative analyses were performed on a Waters Acquity UPLC system (Waters, Milford, MA), equipped with binary solvent manager, auto sampler, column compartment, and PDA detector controlled by Waters Empower 2 software. Separation was achieved on an Acquity UPLC BEH C18 column (50 mm × 2.1 mm i.d., 1.7 μm). The standards and samples were separated using a gradient mobile phase consisting of 75:25 (2.5 mM sodium acetate buffer pH 4.5 (A) and acetonitrile (B)). The column temperature was set at 35˚C, and the injection volume was 4 μL. Samples were analyzed over a wavelength range of 210 - 410 nm and a λmax of 375 nm was used for characterization. The flow rate was 0.5 ml/min. The initial linear gradient conditions of 75% of A and 25% of B was then changed to 25% of A and 75% of B at 3 min and remained at plateau condition to 5.16 min and then reversed to its original composition afterwards.
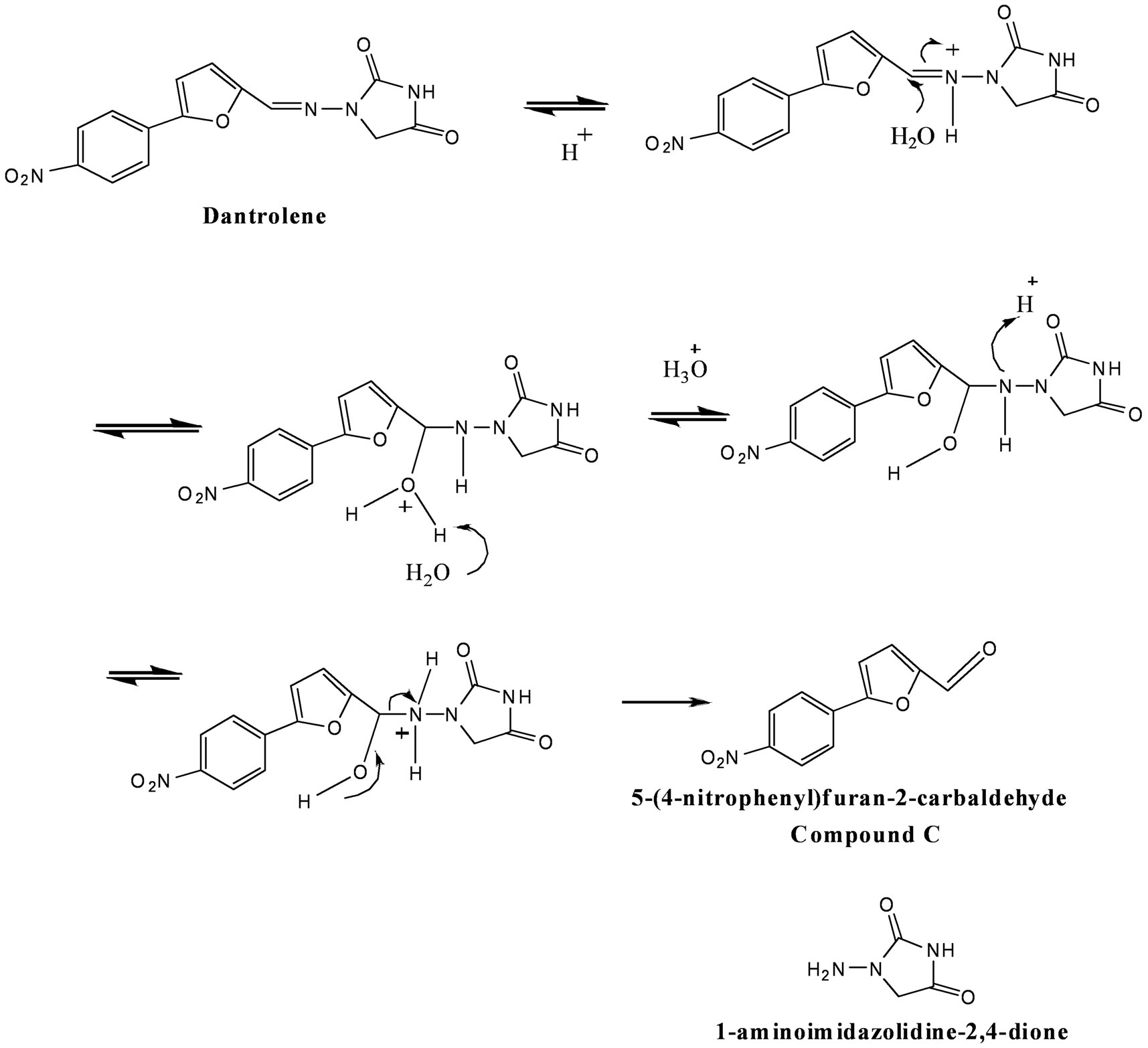
Figure 2. Scheme of proposed degradation pathway for dantrolene in acidic media.
2.3. System Suitability Procedures
The stock and system suitability solutions of dantrolene and its related impurities are described as follows: 1 mg/mL of dantrolene stock solution and its related impurities were prepared separately by dissolving in DMF. The separate stock solutions of dantrolene and its two impurities, Related Compound B and C were further diluted to 100 µg/mL of dantrolene, Rel B & Rel C, separately in DMF. These 100 µg/mL solutions were labeled as working solutions. All working solutions in 20 mL scintillation vials were stored in refrigerated conditions at 4˚C. The system suitability mixture comprised of working standard solutions of dantrolene, dantrolene Related Compound, B and C diluted with 75:25 pH 4.5 sodium acetate buffer:acetronitile to concentrations of 10 µg/mL, 2 µg/mL, 2 µg/mL, respectively. Six replicate injections of the system suitability standard were made at both the start and end of the sequence runs of particular acidic or alkaline dantrolene samples at a particular temperature.
2.4. Kinetics Procedures at Different pH and Temperatures
Prior to creating acidic or alkaline dantrolene samples for kinetic studies, 7 different pH buffer solutions were made at a constant ionic strength of 0.2. The ionic strength was adjusted with sodium chloride. The pH ranged from low acidic range of 1.2 to most basic at 9.5. All of the pH buffers were prepared in 200 mL volumetric flasks with purified DI water and the final pH was adjusted. The pH 1.2 was prepared by adding 50 mL of 0.2 M KCl solution and 85 ml of 0.2 M HCl solution and then adding water to volume. The pH 2.2 was prepared by adding 50 mL of 0.2 M KCl solution, 7.8 mL of 0.2 M HCl solution and then adding water to volume. The pH 4.6 was prepared via adding 50 mL of 0.2 M potassium biphthalate solution, 11.1 mL of 0.2 M NaOH solution and then water to volume. The pH 6.6 was prepared via adding 50 mL of monobasic potassium phosphate solution, 16.4 mL of 0.2 M NaOH solution and then water to volume. The pH 8.0 was prepared via adding 50 mL of 0.2 M Boric acid and KCl solution, 3.9 mL of 0.2 M NaOH solution and then water to volume. The pH 9.5 buffer solution was prepared by adding 50 mL of 0.2 M Boric acid and KCl solution, 32.1 mL of 0.2 M NaOH solution and then water to volume. The procedures were obtained from USP 32 chapter on Reagents: Buffer Solutions. The solutions were freshly prepared and pH’s were measured at room temperature of 25˚C by Oakton pH 11 Standard Portable pH meter (Vernon Hills, IL).
The preparation of kinetic samples: The stock solution of 100 µg/ml of dantrolene in DMF was diluted to 2 µg/mL in the following solutions, at pH 1.2, 2.2, 4.6, 6.6, 7.4, 8.0 or 9.5. The kinetic samples at a particular pH were tested at the following temperatures: 25˚C ± 0.5˚C, 45˚C ± 0.5˚C, 60˚C ± 0.5˚C and 75˚C ± 0.5˚C. For each pH samples at a specific temperature, two separate 10 mL solutions of 2 µg/mL of dantrolene were prepared. After the dilution to 2 µg/mL with a specific pH buffer solution, two 10 mL solutions in 20 mL scintillation vials (clear glass wrapped with aluminum foil) were stored at a specific pH in the appropriate oven with the set temperature settings except for 25˚C which was conducted under room temperature. The following time points were used to collect the samples from the vials: 5, 10, 15, 20, 25, 30 and 1 hour. At each time point, 1 mL of the 2 µg/mL samples were extracted and placed into one of the two UPLC vials corresponding to one of the two 10 mL 2 µg/mL solutions in 20 mL scintillation vials at a specific pH. The UPLC vials were immediately closed with a cap and stored in a freezer at –85˚C in order to quench the reaction. When it was time for samples analysis under UPLC gradient conditions of mobile phase composition of 75:25 (acetate buffer pH 4.5: acetronitrile), the UPLC vials were removed from freezer storage and stored at room temperature for an hour before testing. Each of the injected UPLC vials was injected thrice. Kinetics data analysis was then conducted by utilizing the absorbance data collected and the validated calibration curve of dantrolene in DMF ranging from 0.5 to 10 µg/mL.
2.5. Determination of Degradation Constant
The observed pseudo first-order degradation rate constants, kobs, were calculated from the slopes of semilogarithmic plots of the drug fraction remaining versus time in accordance with Equation (1).
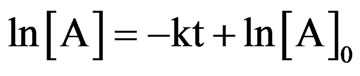 (1)
(1)
A plot of ln[A] vs. time t gives a straight line with a slope of −k.
Where A0 was the initial concentration and A was the remaining concentration of dantrolene at time, t. The effect of temperature on the rate of dantrolene degradation was determined at pH 1.2, 7.4 and 9.5 in mobile phase composition of 75:25 (acetate buffer pH 4.5: acetronitrile). The observed pseudo first-order degradation rate constants (kobs), were calculated using Equation (1). Summary of rate constant for degradation of dantrolene in aqueous solution at pH and temperature is given in Table 1.
3. Results and Discussion
3.1. Hydrolysis of Dantrolene as a Function of pH
The hydrolysis of dantrolene was studied in the pH range 1.2 - 9.5 and at an initial concentration of approx 2 µg/mL. This concentration of dantrolene was chosen because of limited solubility of Dantrolene at higher concentration of 5 µg/mL or 10 µg/mL and the more limited column loading capacity of sub 2 micron particles used
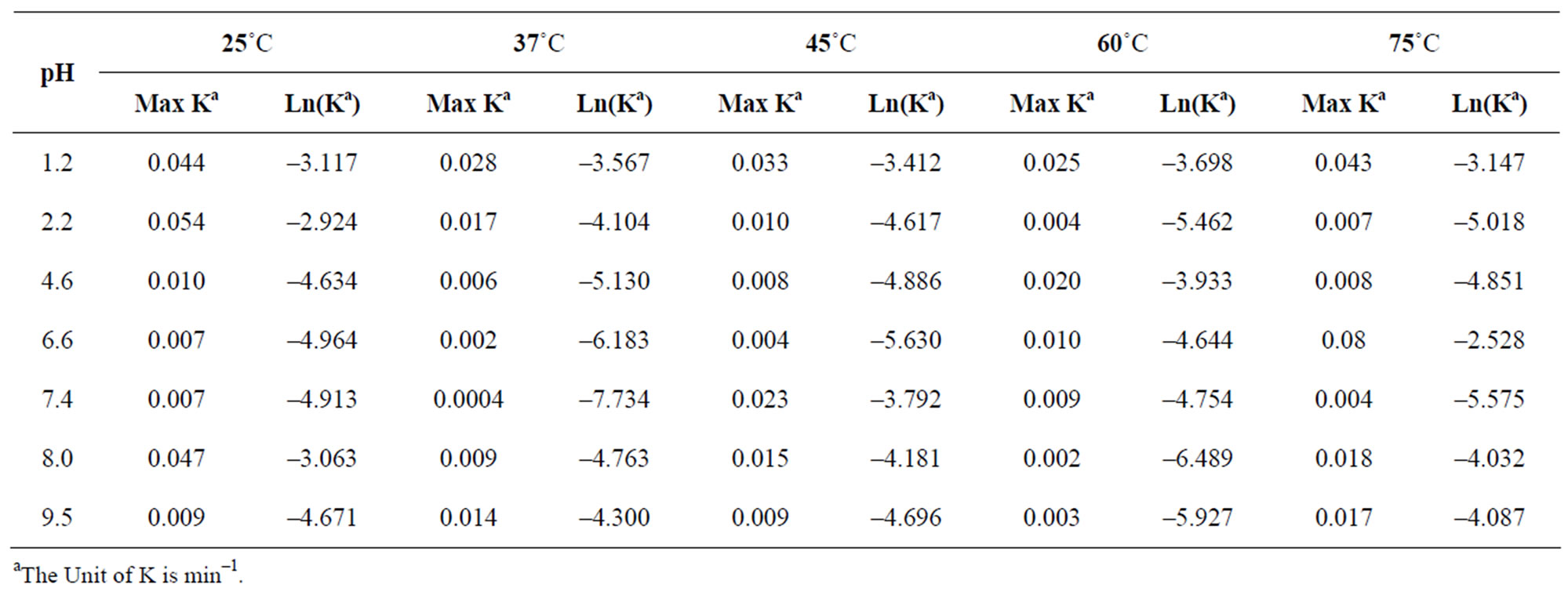
Table 1. Summary of rate constants for degradation of dantrolene in aqueous solution at pH and temperatures.
in UPLC columns. The degradation of dantrolene in buffers over pH 1.2 - 9.5 at 35˚C resulted in appearance of two degradation product compounds B and C with a retention time equivalent to 1.1 min and 2.1 min in the UPLC chromatogram (Figure 3). All the correlation coefficients (r) of the semi-logarithmic plots of drug concentration versus time were >0.99, indicating the degradation of dantrolene followed pseudo first-order kinetics. Pseudo first order plots showing the degradation of dantrolene at pH 1.2, 7.4 and 9.5 and at various temperatures are shown in Figures 4-6. An increase in concentration was observed during first 10 min of the stability profile. The reason for the observed phenomenon is not well understood. It is speculated that complexation or a dimer product is formed in the initial time period. For the sake of clarity the data from first 10 min was omitted in the plot. Figures 7(a)-(c) show the change in concentration of degradation products; compound B and C with time in acidic and alkaline pH. The concentration of B and C were calculated from the peak areas in UPLC chromatograms and standard curves.
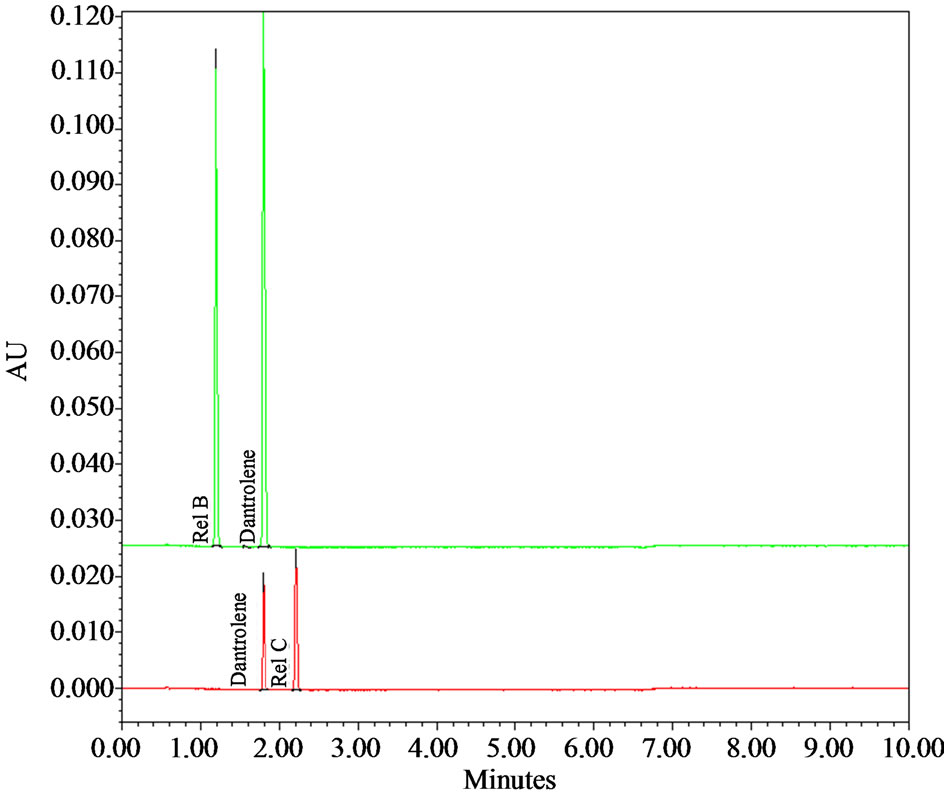
Figure 3. UPLC Chromatograms of dantrolene at pH 1.2 (red) and pH 9.5 (green). Note the disappearance of dantrolene peak and formation of relative compounds B (alkaline pH) and relative compound C (acidic pH) in 24 hr.
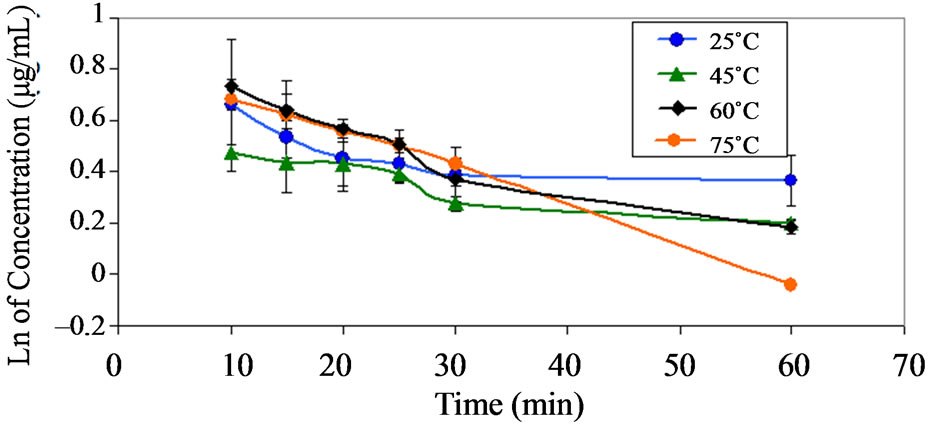
Figure 4. Pseudo first order plots showing the degradation of dantrolene at pH 1.2 and at various temperatures.
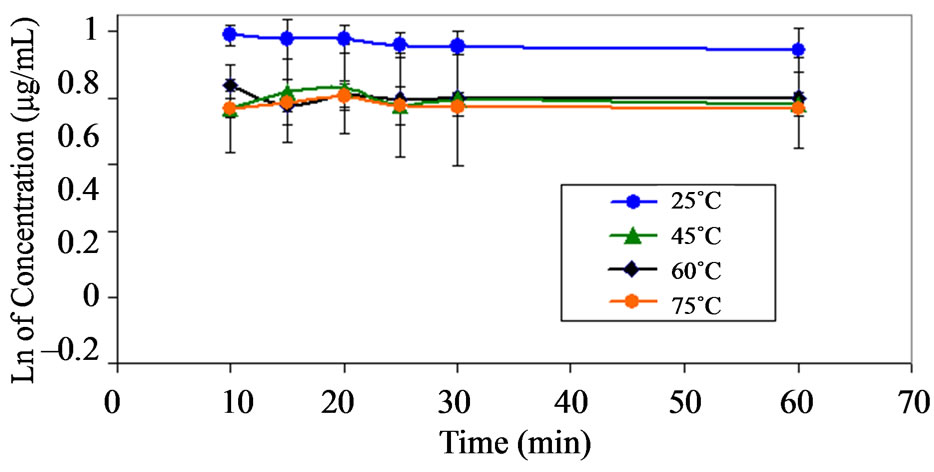
Figure 5. Pseudo first order plots showing the degradation of dantrolene at pH 7.4 and at various temperatures.
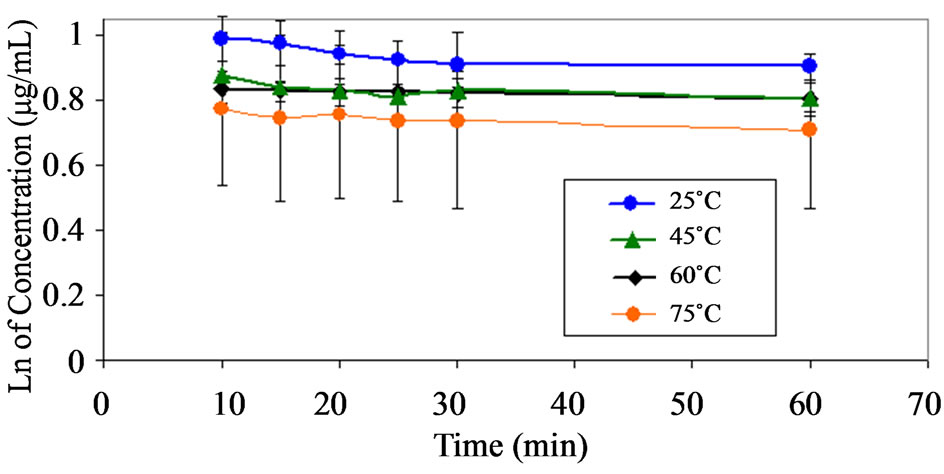
Figure 6. Pseudo first order plots showing the degradation of dantrolene at pH 9.5 and at various temperatures.
3.2. Effects of Temperature on the Stability of Dantrolene
The effect of temperature on the degradation rate of dantrolene is shown in Figure 8. It should be noted that there are small increases in the degradation of dantrolene in low pH conditions (pH 1.2 - 2.2) and at higher temperatures. Degradation of dantrolene occurred in acidic condition associated with a decrease in pH. One major decomposition product was evident by UPLC, less polar then the parent molecule. Decomposition of dantrolene in acidic condition was not statistically significant (p > 0.05, Turkey-Kramer multiple comparison test) on storage for 24 hr at 5˚C, 25˚C, 37˚C and no degradation products were detected by UPLC. This suggests that acidic condition and high temperature does facilitate the known mechanism of azomethine cleavage.
3.3. pH-Rate Profile of Dantrolene
Based on the pH of the solution dantrolene can exists in three different forms, cation DH+, free base/neutral species DH and anion D–. A V-shaped pH-rate profile of dantrolene over the pH range 1.2 - 9.5 at 25˚C was observed with maximum solution stability at pH around 7.4 (Figure 9) and the stability at this pH can be associated to the presence of dantrolene mostly in the free base/ neutral form.
In acidic solution, hydrolysis involves an initial equi-
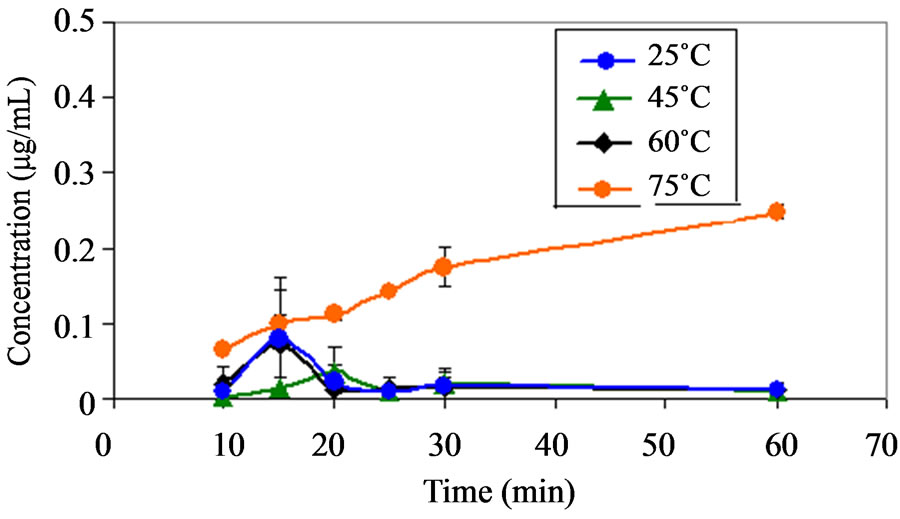 (a)
(a)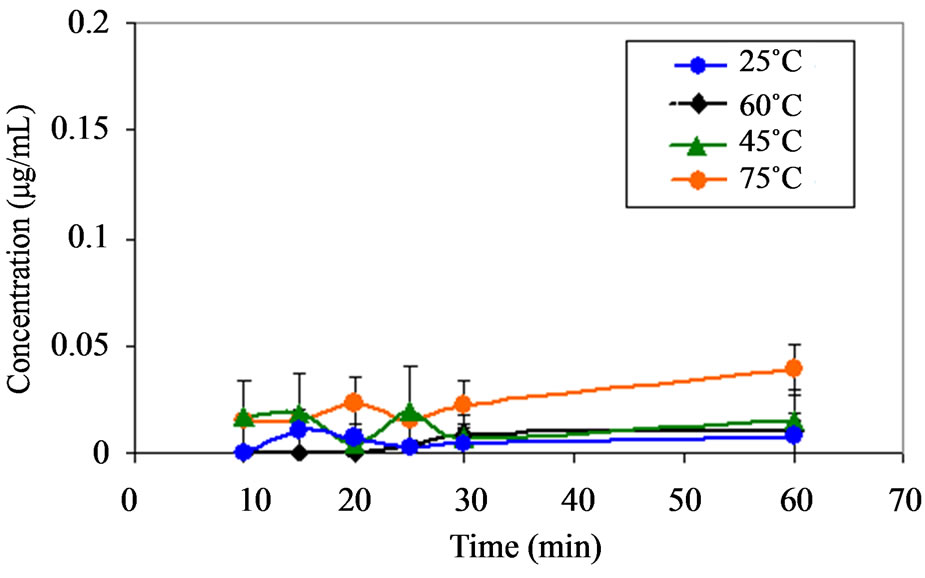 (b)
(b)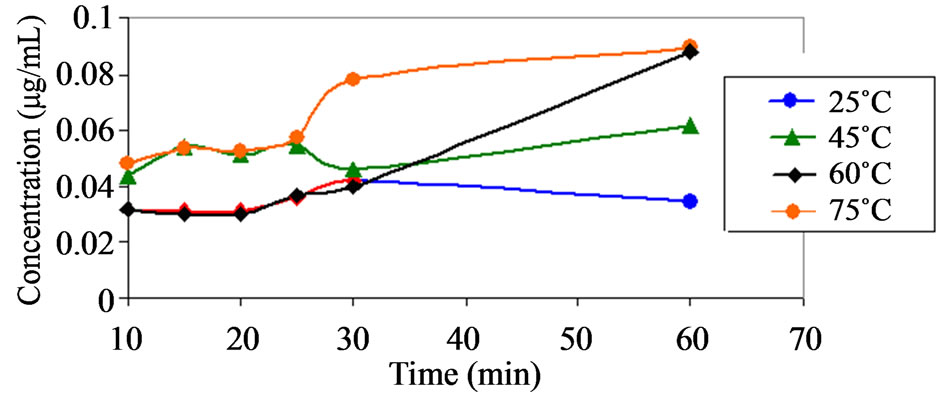 (c)
(c)
Figure 7. (a) Plot showing the appearance of compound C (a)) as a function of time at pH 1.2 at various temperatures. Note (a): No significance amount of compound C was found at pH range from 4.6 to 9.5 at temperature range of 25˚C to 75˚C; (b) Plot showing the appearance of compound C (b)) as a function of time at pH 2.2 at various temperatures. Note: No significance amount of compound C was found at pH range from 4.6 to 9.5 at temperature range of 25˚C to 75˚C; (c) Plot showing the appearance of compound B as a function of time at pH 9.5 at various temperatures. Note: No significance amount of compound B was found at pH range from 1.2 to 8.0 at temperature range of 25˚C to 75˚C.
librium between dantrolene (D) and hydrogen ion followed by rate determining reaction with H2O (W):
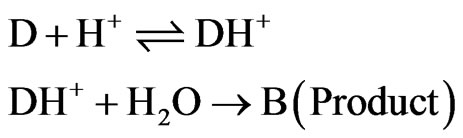
The rate law for product B formation can be obtained by the steady state approximation as follows:
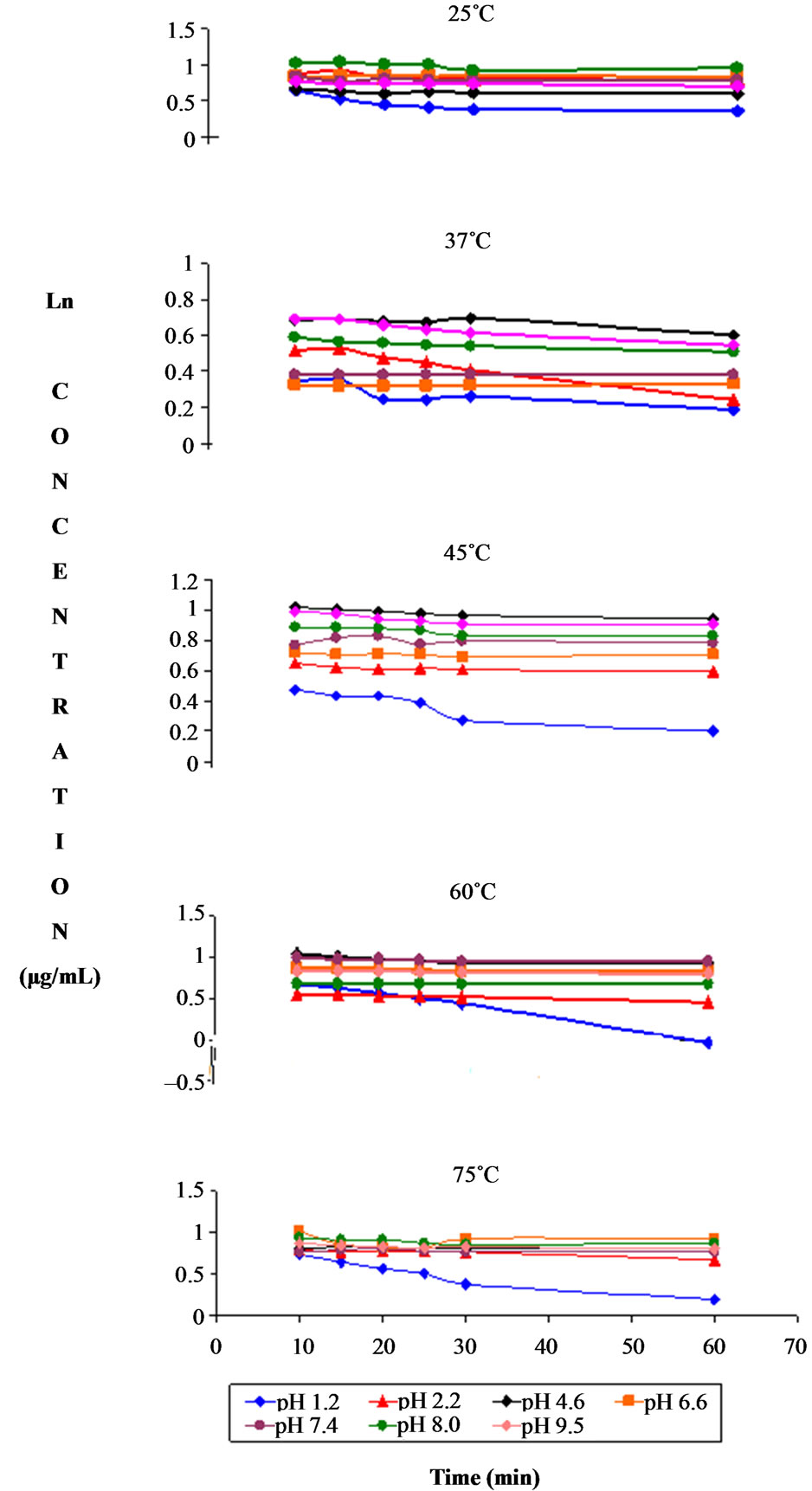
Figure 8. Pseudo first order plots showing the degradation of dantrolene at various temperatures and pH.
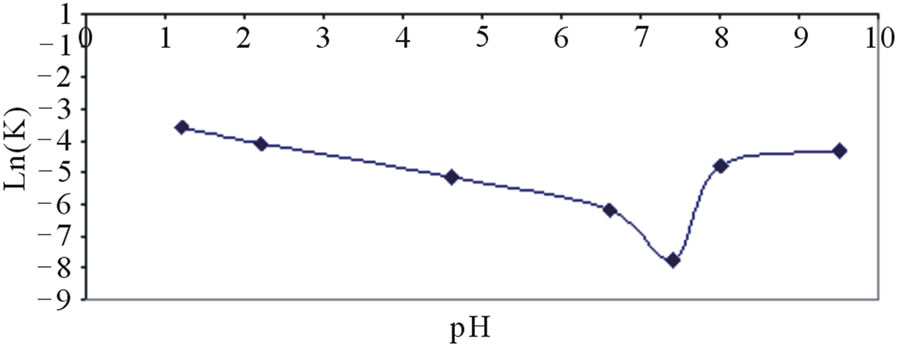
Figure 9. pH-rate (kobs, min–1) profile for dantorolene.
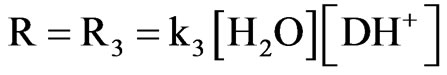 (3)
(3)
To remove the intermediate from the rate law, the Equation becomes as follows:
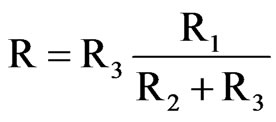 (4)
(4)
Rearranging to substitute rate equations for R1, R2 and R3, Equation (4) becomes:
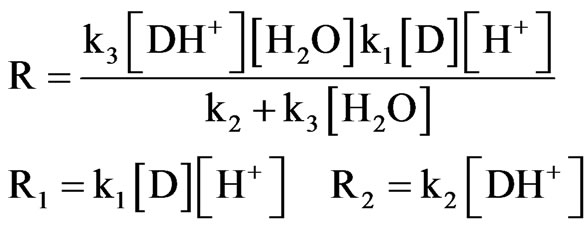 (5)
(5)
when the constant k3 >>> k2, the product B is formed and the further simplifying Equation (5), the rate law Equation becomes as follows:
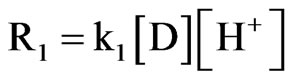 (6)
(6)
The H+ in this rate Equation (6) above signifies a specific acid catalysis. At a given pH, pseudo first order reaction is written as:
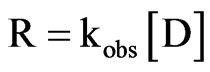 (7)
(7)
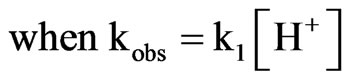 (8)
(8)
Inserting Equation (8) for kobs in Equation (7) and solving for kobs by taking natural logarithm of both sides result in the following expression:
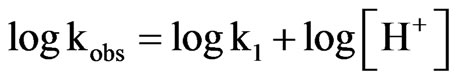 (9)
(9)
Equation (9) can be written as follows:
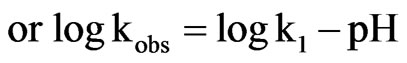 (10)
(10)
Thus Figure 9 shows that in acidic conditions from pH 1 to 7.4, the slope is negative with a value of minus 1 from pH 6 to 7.4. In the alkaline condition, the product C is formed by the scheme as shown as follows:
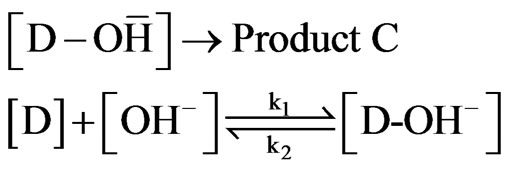
Similar to the derivation of acidic condition, the rate law for Product C can be obtained as shown in the Equation below:
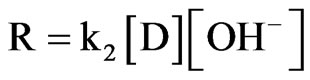 (11)
(11)
Since kw = [H+][OH–] and solving for [OH–] and inserting it into Equation (11), the expression becomes as follows:
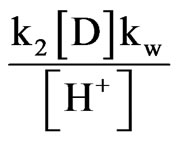 (12)
(12)
The observable rate constant, kobs can be re-written as follows:
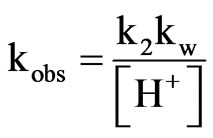 (13)
(13)
Inserting Equation (13) into Equation (12) and then solving for kobs by taking natural logarithm of both sides of the Equation results in:
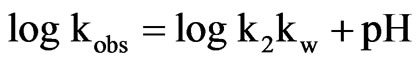 (14)
(14)
Therefore, as seen in Figure 9, the slope is positive at higher pH for this specific base catalysis. Around pH 7.4, a minimum of natural logarithm of kobs is observed suggesting a water catalytic effect.
The estimated degradation rate constants were 0.028 (min−1) (pH 1.2), 0.0004 (min–1) (pH 7.4), 0.014 (min–1) (pH 9.5) at 37˚C and the values differed significantly (p < 0.05). The slope of the logkobs versus pH profile was less than unity over pH 1.2 - 9.5; indicating the mechanism of the degradation reaction was specific acid or base-catalyzed hydrolysis.
Degradation rate profiles are, however, often complex for molecules such as dantrolene that have multiple ionization sites within the molecule. This is because the observed degradation rate at any specified pH is composed of contributions from acid and base on all existing species of the drug in the solution (Figure 9). The effect of pH on the degradation of dantrolene in aqueous solution and constant μ (0.2) at 37˚C is shown in plots of logkabs versus pH (Figure 9).
The degradation of dantrolene is described by the catalytic effect of specific acid and water; however, the specific base catalysis becomes more predominant as the pH goes up from 7.5 - 9.5 resulting in an increase in the degradation rate, indicating degradation of the neutral species was catalyzed by OH ions. Equation 15 is a general Equation describing degradation rate of dantrolene as a function of pH:
kobs = kH [H+] + kOH [OH–] + kw(15)
where kH, kOH, kw is the first-order constant rate for degradation of this species catalyzed by protons, hydroxyl ions, and water respectively.
3.4. Mass Spectrometry and NMR Analysis of Degradation Products
UPLC analysis showed that dantrolene in buffer solutions over acidic solution of pH 1.2 - 4.6 and alkaline solution of pH 9.5 produced two degradation products with a retention time 1.1 min for related compound B and 2.1 min for related compound C. The corresponding positive ion ESI mass spectra of these compounds yielded an m/e 333 (M + H) and 218 (M + H), which is consistent with compound B and C (MW = 332 and 217) as a result of hydrolysis of the hydantoin ring (compound B) and imine linkage (compound C) (Figure 10). These degradation products were further confirmed by 1HNMR spectroscopy (Figure 11).
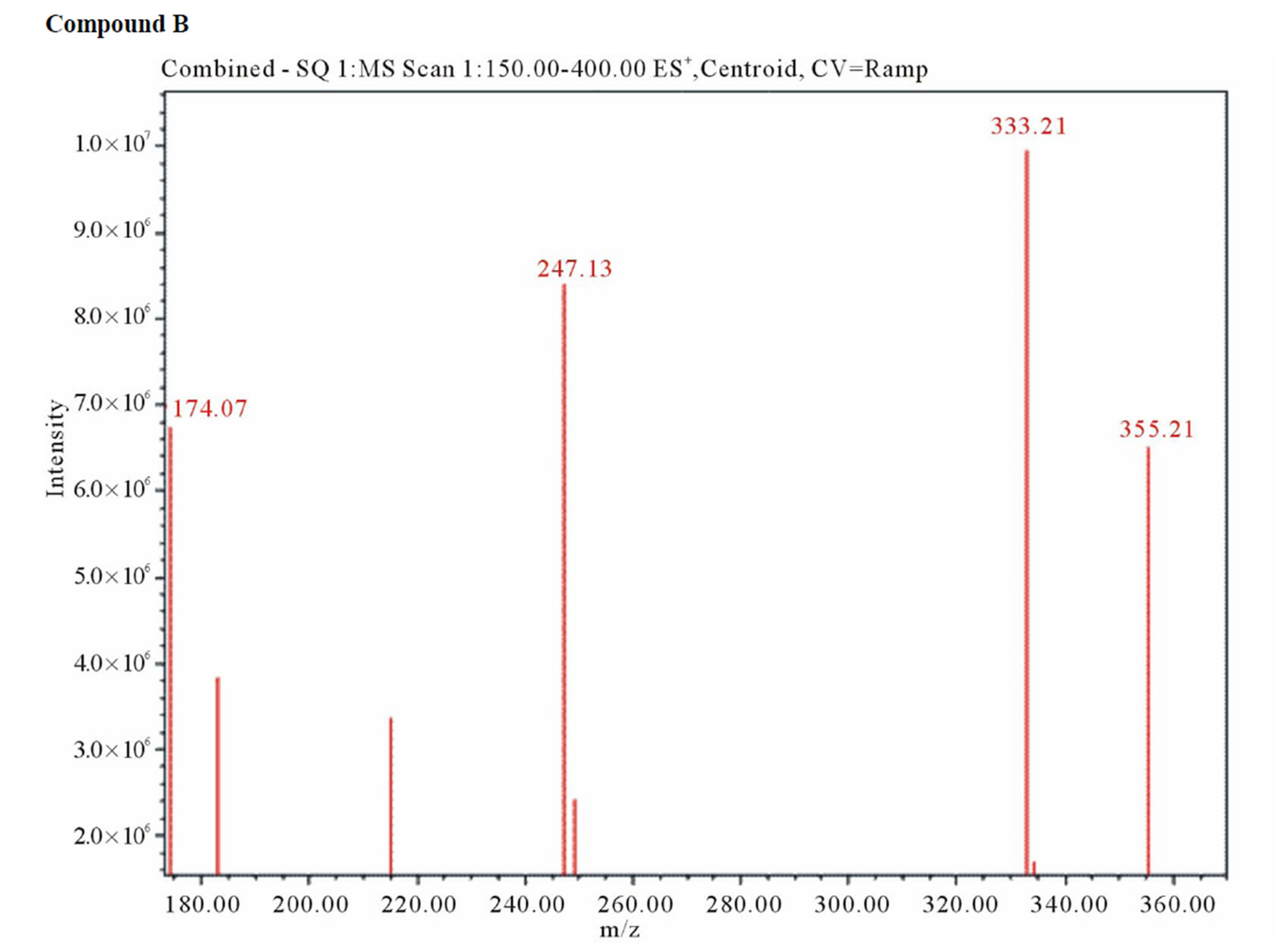
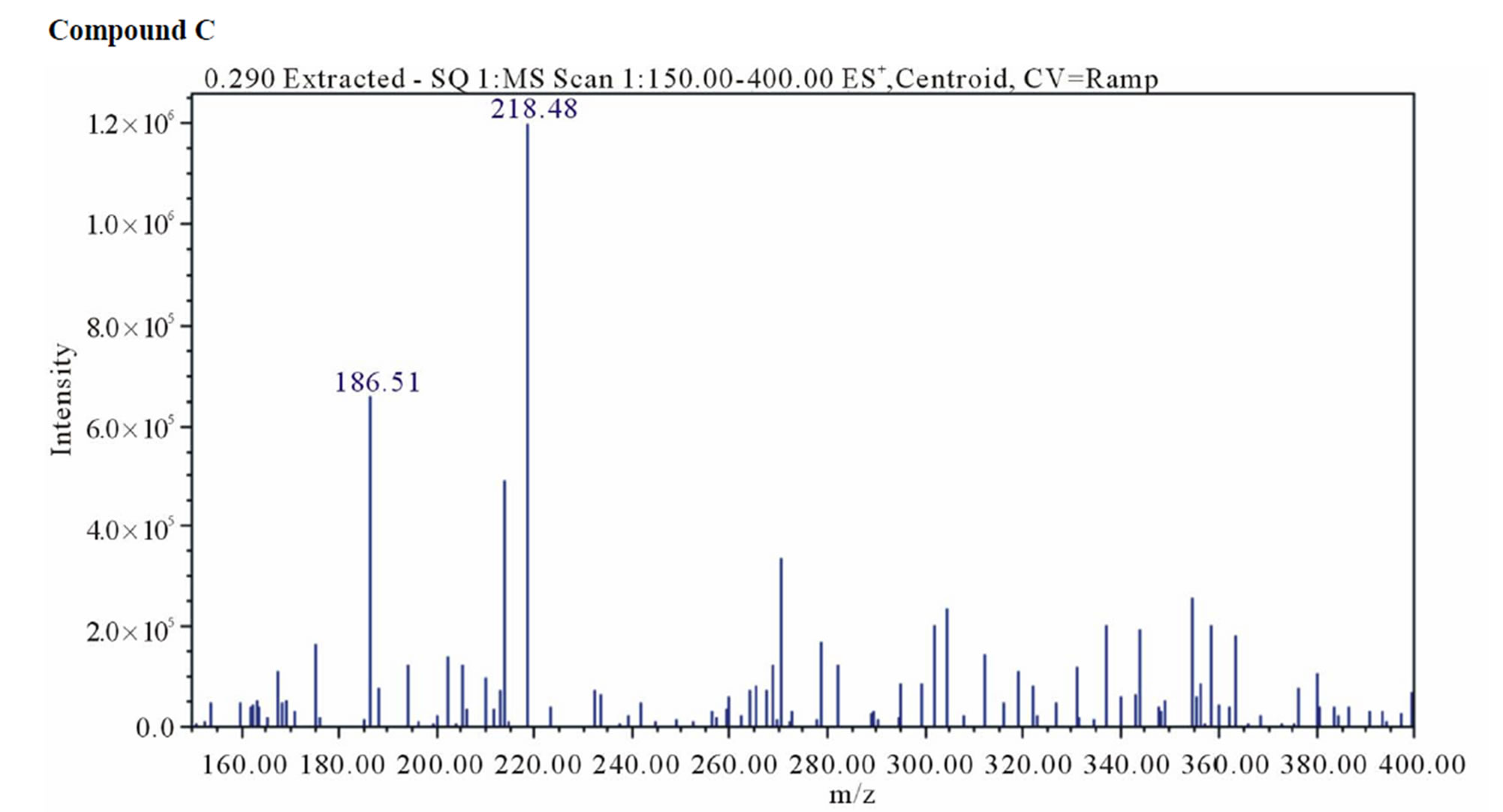
Figure 10. Positive ion electrospray ionization mass spectra for hydrolysis product: Compound B (m/z = M + H = 333) and C (m/z = M + H = 218).
4. Conclusion
In summary, the present study shows that degradation of dantrolene follows pseudo-first-order kinetics. The pHrate profile is V-shaped and can be rationalized by the different catalytic effects of various components in dantrolene solution. Degradation pathways of dantrolene include acidic and basic hydrolysis and results provide useful information for formulation development of a stable dantrolene injectable at physiological pH. The available dantrolene sodium/commercial injection is formulated to yield a high pH of 8.8 to 11.0 when reconstituted in water for injection and is allowed to have not more
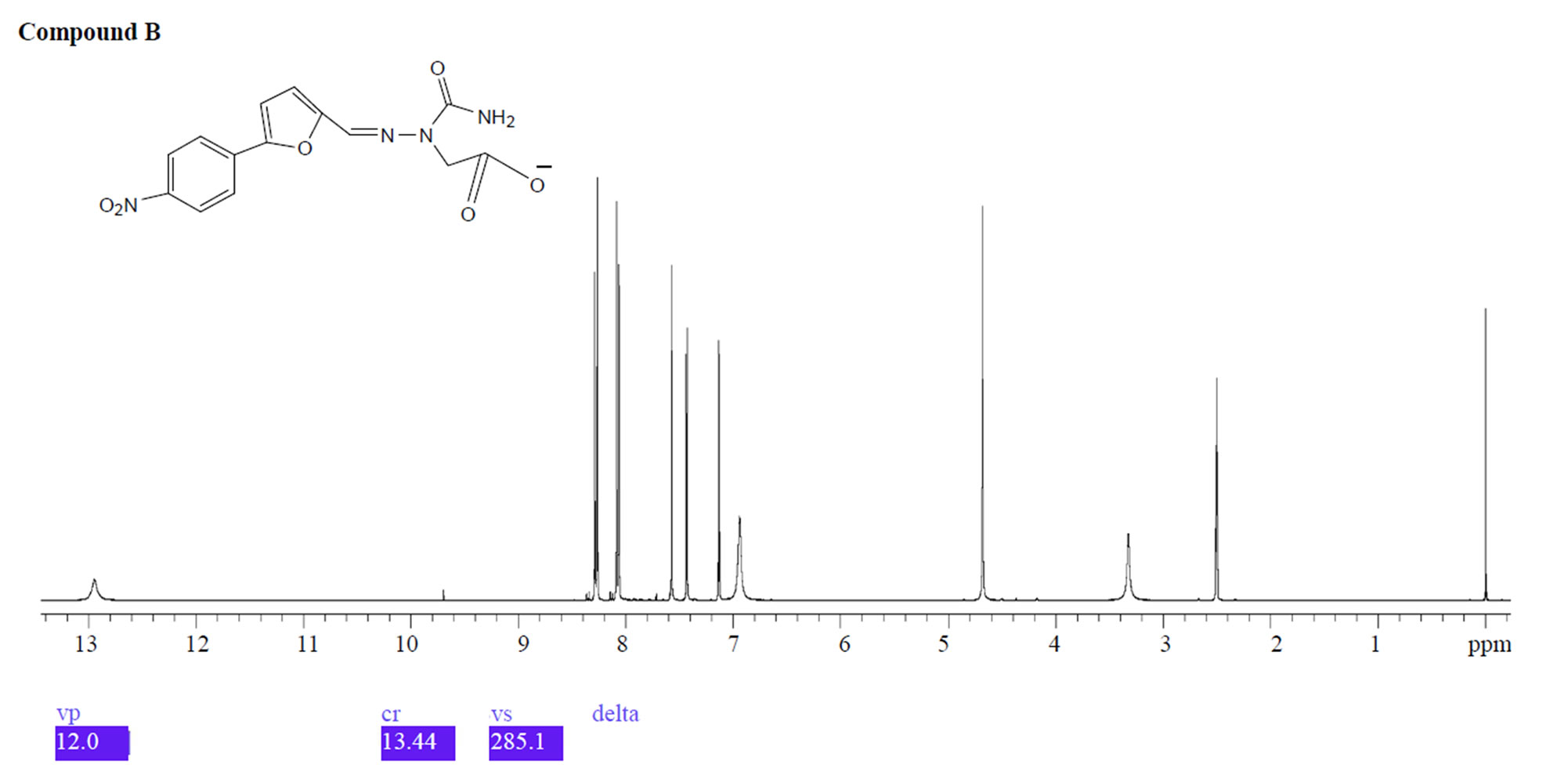
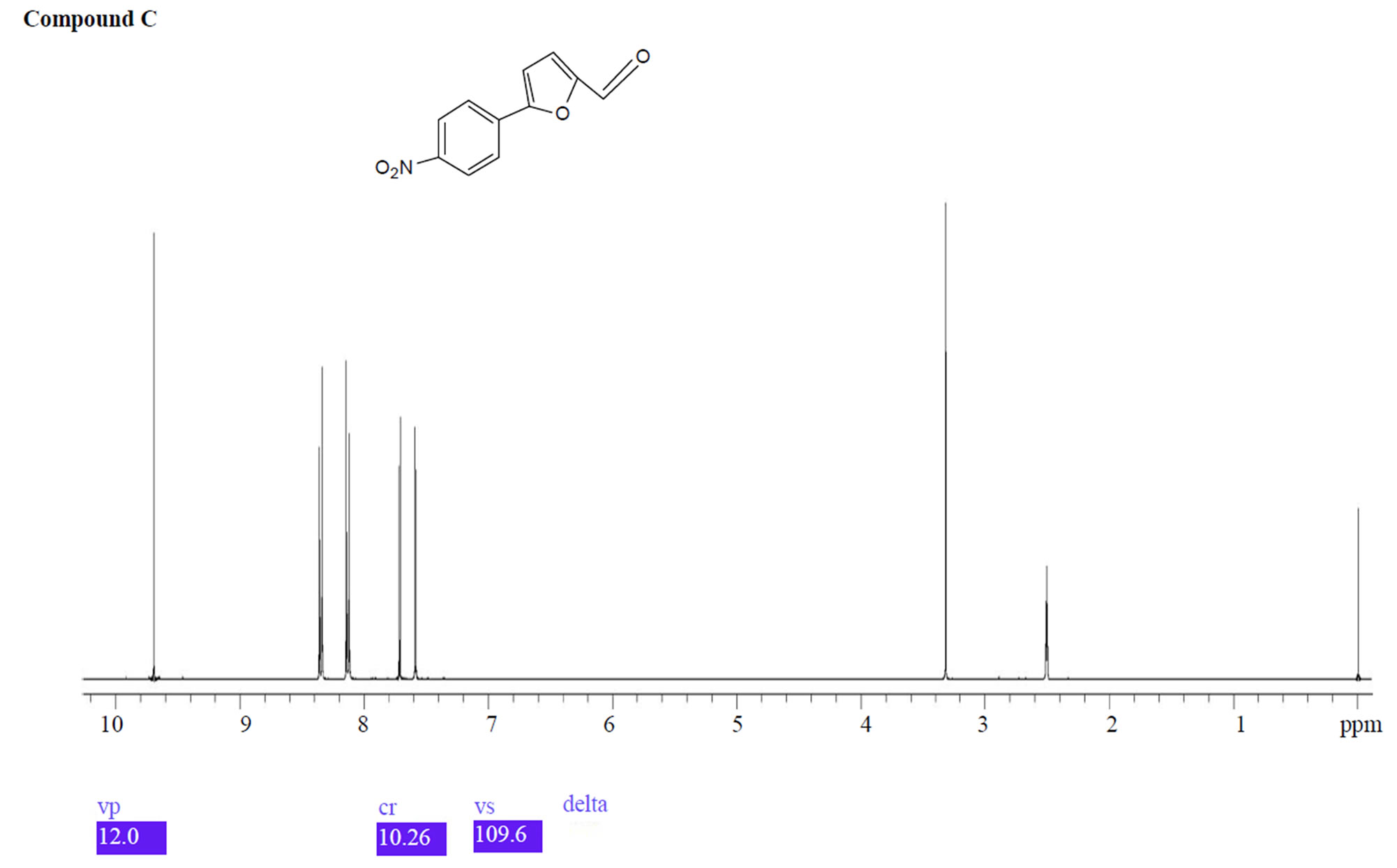
Figure 11. 1HNMR spectra of compound B and C.
than 8% of dantrolene related compound B. The high pH of the intravenous formulation results in high level of degradation products that could potentially increase the risk of tissue necrosis. To address this concern a precautionary care note is included in the labeling to inform the practitioners to prevent extravasation of dantrolene sodium solution into the surrounding tissues. Using the degradation rate reported, it appears that a solution formulated at physiological pH 7.4 would have sufficient solution solubility and can overcome the problem of tissue necrosis associated with high pH of the intravenous formulation. Although the focus of this paper was on the mechanism of hydrolysis of dantrolene, the poor aqueous solubility of dantrolene, the developed understanding can be utilized to improve the quality of the formulation and the risk associated with the extravasation of dantrolene sodium solution in its current form.
5. Acknowledgements
This scientific contribution is intended to support regulatory policy development. The views presented in this article have not been adopted as regulatory policies by the Food and Drug Administration at this time.
6. Declaration of Interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- T. Krause, M. U. Gerbershagen, M. Fiege, R. Weißhorn and F. Wappler, “Dantrolene—A Review of Its Pharmacology, Therapeutic Use and New Developments,” Anaesthesia, Vol. 59, No. 4, 2004, pp. 364-373. doi:10.1111/j.1365-2044.2004.03658.x
- H. R. Snyder, C. S. Davis, R. K. Bickerton and R. P. Halliday, “1-[5Arylfurfurylidene)amino] Hydantoins: A New Class of Muscle Relaxants,” Journal of Medicinal Chemistry, Vol. 10, No. 5, 1967, pp. 807-810. doi:10.1021/jm00317a011
- K. O. Ellis, A. W. Castellion, L. J. Honkomp, F. L. Wessels, J. F. Carpenter and R. P. Halliday, “Dantrolene, a Direct Acting Skeletal Muscle Relaxant,” Journal of Pharmaceutical Science, Vol. 62, No. 6, 1973, pp. 948- 951. doi:10.1002/jps.2600620619
- M. Nakano, N. Inotsume, N. Kohri and T. Arita, “Reversible Ring-Opening Reaction of Diazepam in Acid Media Around Body Temperature,” International Journal of Pharmaceutics, Vol. 3, No. 4-5, 1979, pp. 195-204. doi:10.1016/0378-5173(79)90003-6
- K. A. Connors, V. J. Amidon and V. J. Stella, “Chemical Stability of Pharmaceuticals,” A Handbook for Pharmacists, John Wiley & Sons, New York, 1986.
- N. Inotsume and M. Nakano, “Hydrolytic Behavior of Dantrolene in Acidic Media at Body Temperature,” International Journal of Pharmaceutics, Vol. 17, No. 2-3, 1983, pp. 357-360. doi:10.1016/0378-5173(83)90047-9
- P. L. Cox, J. P. Heotis, D. Polin and G. M. Rose, “Quantitative Determination of Dantrolene Sodium and Its Metabolites by Differential pulse Polarography,” Journal of Pharmaceutical Science, Vol. 58, No. 8, 1969, pp. 987-989. doi:10.1002/jps.2600580818
- J. D. Conklin and R. J. Sobers, “Qualitative Method for Dantrolene and a Related Metabolite in Urine,” Journal of Pharmaceutical Sciences, Vol. 62, No. 6, 1973, pp. 1024-1025. doi:10.1002/jps.2600620641
- R. D. Hollifield and J. D. Conklin, “Determination of Dantrolene in Biological Specimens Containing DrugRelated Metabolites,” Journal of Pharmaceutical Science, Vol. 62, No. 2, 1973, pp. 271-274. doi:10.1002/jps.2600620219
- M. A. Tawakkul, P. J. Faustino, V. A. Sayeed, M. A. Khan and S. R. Khan, “Development and Application of a Validated Stability-Indicating Ultra-Performance Liquid Chromatography (UPLC) Method for the Determination of Dantrolene and Its Related Impurities,” Clinical Research and Regulatory Affairs, Vol. 27, No. 1, 2010, pp. 21-29. doi:10.3109/10601331003623309
NOTES
*The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
#Corresponding author.

