Open Journal of Applied Sciences
Vol.05 No.10(2015), Article ID:60611,4 pages
10.4236/ojapps.2015.510058
Development of Multiphoton Ionization Technique for Detection of Polycyclic Aromatic Hydrocarbon (PAH) in Solution
Hoa Do Quang1*, Duong Vu1, Nghia Nguyen Trong1, Totaro Imasaka2
1Center for Quantum Electronics, Institute of Physics, Vietnam Academy of Science and Technology (VAST), Hanoi City, Vietnam
2Department of Applied Chemistry, Kyushu University, Fukuoka, Japan
Email: *hoado@iop.vast.ac.vn
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 September 2015; accepted 24 October 2015; published 27 October 2015
ABSTRACT
A simple low-cost system for detection of polycyclic aromatic hydrocarbon (PAH) in solution based on multiphoton ionization configuration is designed using a circulating ionization cell of 0.1 × 2 × 5 mm dimension with quartz optical window. Fourth harmonic emission of Nd:YAG laser (266 nm, 6 ns, 10 Hz, and 2 mJ) and second harmonic generation of distributed feedback dye laser (278 - 286 nm, 20 ps, 10 Hz, and 300 μJ) were used as the ionization source. A high voltage of 800 V was applied to separate the ions after ionization. The photocurrent includes a sharp peak and a broad tail indexed to electron and ion currents, respectively. The lowest concentration of anthrax- cene (C14H10) in order of few nano-grams per milliliter was detected by this multiphoton ioniza- tion configuration.
Keywords:
UV Laser, Multiphoton Ionization, Polycyclic Aromatic Hydrocarbon

1. Introduction
The interaction between intense laser and organic molecules is interested in spectroscopic analytical methods and applications. Such process has been investigated depending on the laser power [1] [2] , the excitation wavelength [3] , and the absorbance of analytical molecules [4] . These scientific results cause the development of gas chromatograph combining with mass spectrograph (GC/MS) method. The detection of PAH and several isomers of polychloro-p-dibenzodioxin (PCDD) and polychloro-dibenzofuran (PCDF) or polychlorinated biphenyl (PCB) using technique of multiphoton ionization-mass spectroscopy (MPI-MS) have been reported [5] .
One of the simple method, which can be employed in the analysis of PAH organics is demonstrated by S. Yamada [6] [7] and other authors. A photoionization cell was designed which constituted of a pair of transparent glasses playing the roles both of optical windows and electrodes. A concentration of 3.4 × 10−9 mol/L (860 pg/mL) of benzo[a]pyrene was detected by using a laser of 0.6 mJ, 355 nm for ionization energy source. A low DC potential (c.a. less than 100 V) applied into the pair electrodes was used to accelerate the charged carriers. Therefore, it’s difficult to separate different weight ionization molecules.
In this report, a novel cell configuration which can be used to detect the PAH (for example, anthracene C14H10) molecules is demonstrated. A nanosecond fourth harmonic generation (FGH) of a Nd:YAG laser combined with a picosecond second harmonic generation (SHG) laser of a distributed feedback dye laser (DFDL) [8] is employed as the multiphoton ionization source. A high DC voltage is applied to the electrodes in order to increase the separation efficiency, i.e. detected photocurrent.
2. Experimental Section
2.1. Samples
178.2 mg (1 × 10−3 Mol) anthracene purchased from Merk Co. Ltd. is dissolved in 100 ml of pure alcohol. After stirring throughout 30 minutes, original solution was diluted to a series of samples with various concentrations from 1 × 10−8 Mol/L to 1 × 10−5 Mol/L.
2.2. Apparatus
The schematic of the experimental setup is shown in Figure 1. The fourth harmonic generation (FGH) beam of a Nd:YAG laser (Quantel Brilliant, 266 nm, 1.5 mJ, 5 ns, 10Hz) and second harmonic generation (SGH) of a distributed feedback dye laser (DFDL) using Rh6G as the active medium (287 nm, 12 ps, 89 μJ) were temporal overlapped and focused into the cell as the excitation source. DFDL was pumped by second harmonic emission from the same Nd:YAG laser. In order to avoid any damage of the optical windows, the intensity of FHG beam is limited by a slit to the energy of 0.48 ÷ 0.64 mJ/mm2.
A quartz thin plate with area of 2 × 5 mm2 having the same spectral response to the FGH, SGH is used as the optical windows. The photo-ionized ions are forwarded to the electrodes forming the photocurrent which is converted to voltage by an amplifier (Hamamatsu C-6438) and observing by an oscilloscope (100 MHz, Tektronix Co.). The laser pulse energy was calibrated by a photodiode at the same moment. Q-switch pulse was taken to trigger of data acquisition.
Figure 1. Experimental scheme of multiphoton ionization system using two UV laser beams to detect PAH in solution.
Ionization cell was formed by two inner surfaces of couple quartz plate which set on soda glass substrate. Narrow gap distance between two quartz plates is about 0.1 mm connected to two gold coating electrodes (Figure 1). The spatial separation between electrodes was about 7 mm, which allows applying a power supply from regulated high voltage equipment (Ortec526). Sample solution was injected into the cell by a small volume syringe. The injection speed is programmable by controlling the speed of the step-motor used to press the syringe.
The DFB laser wavelength, injection speed and all electrical signals (calibration, photocurrent…) are controlled by NI USB6009 DAQ unit.
3. Results and Discussion
In this configuration, the photocurrent waveform consists of a sharp peak and a long tail about twenty microseconds (Figure 2). The signal shape is almost unchanged when the voltage between two electrodes was in range of 600 V to 900 V. The sharp peak indexes to the moving of photoelectron and the other was depended on the motion of ion. At low concentration of the reagent, a weak narrow peak, and steep decay was observed. The photocurrent waveform describes several dynamic processes such as the motion of ions and electrons in liquid medium, the quenching of ion flow by the other molecules (solute, residual impurities etc.), recombination of anions and cations or charge transfer between ions and the molecules [9] [10] .
The change of photocurrent at different voltage is investigated with two anthracene concentrations (1 × 10−6 mol/L and 1 × 10−7 mol/L) as shown in Figure 3. At low voltage, the change of photocurrent is not so clear since high possibility of recombination of anions and cations. By increasing the voltage, the charge carriers are accelerated and the relaxation to initial states was decreased. A critic high level of voltage applied was set in order to avoid breaking the cell. The voltage of 800V is applied in our experiment for detection of various concentration of anthracene solution in ethanol.
As known well, the probability of generation the anion-cation pair depends on electric field, E, the temperature of medium, T, and active radius of molecule, r, which is expressed in elsewhere as follow [7] [9]
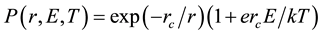 .
.
Here rc is effect radius of molecule, which is influenced by dielectric constant of solvent, k the Boltzman constant. The energy needed to ionize anthracene molecules alternatively depends on electric field intensity caused by laser beam and on temporal temperature. The ionization threshold for all PHAs is about 9 eV (c.a. 138 nm) [9] . This light source is impossible to generate at ambiance condition because of the strong absorbance of oxygen in the air. Multiphoton absorption is frequently applied in order to meet the ionization energy. In our experiment, two-color included the fluence of 0.64 mJ/mm2 at 266 nm wavelength (4.661 eV) and 0.3 mJ/mm2 of picoseconds distributed feedback dye laser at 278 nm wavelength (4.432 eV) is applied. As described in the equation above, the ionization efficiency is proportional to the temperature. Therefore, the other advantage of using an ultraviolet nanosecond laser unfocused on to sample is increasing the local temperature before temporal superimpose with the picosecond beam. Figure 4 shows the photocurrent intensity with various concentrations
Figure 2. Photocurrent of ionized antracen molecules under interaction of two color laser beams.
Figure 3. Signal changes at different voltages on two electrodes with the sample concentration of 10−6 mol/L (square) and 10-7mol/L (circle).
Figure 4. Changes of photocurrent signal of anthracene concentration in single laser beam of 266 nm (square) and double beams (circle).
of the anthracene in ethanol. A linear dependence of the photocurrent versus concentration of anthracene was found in the concentration range of 10−8 - 10−6 M for both of two excitation condition. The black-square data is taken while only nanosecond beam light is introduced to the cell. In the case of two UV beams (UV nanosecond pulse and UV picosecond beam), red-circle data points, a higher sensitivity of detection of anthracene sample was observed.
4. Conclusion
This report shows the simple equipment based on strong interaction between two-color laser beam and PAH molecules. This device could be applied to the qualitative or semi-quantitative analysis of PAH substances in solution. Especially, the sensitivity of the method is increased by the overlap of two UV laser pulses with different wavelength and pulsewidth.
Acknowledgements
This work is supported by the cooperation between Vietnam Academy of Science and Technology (VAST) and Japanese Society of Promotion Science (JSPS), and National Foundation of Science and Technology under grant number of 103.03-2013.10.
Cite this paper
Hoa DoQuang,DuongVu,Nghia NguyenTrong,TotaroImasaka, (2015) Development of Multiphoton Ionization Technique for Detection of Polycyclic Aromatic Hydrocarbon (PAH) in Solution. Open Journal of Applied Sciences,05,595-599. doi: 10.4236/ojapps.2015.510058
References
- 1. Oser, H., Copic, K., Coggiola, M.J., Faris, G.W. and Crosly, D.R. (2001) Congener-Specific Detection of Dioxins Using Jet-REMPI. Chromophere, 43, 469.
- 2. Lacorte, S. and Fernandez-Alba, A.R. (2006) Time of Flight Mass Spectrometry Applied to the Liquid Chromatographic Analysis of Pesticides in Water and Food. Mass Spectrometry Reviews, 25, 866-880. http://dx.doi.org/10.1002/mas.20094
- 3. Wei, J., Zhang, B., Fang, L., Zhang, L. and Cai, J. (1998) REMPI Time-of-Flight Mass Spectra of C2H7N Isomers. Optics Communications, 156, 331-336. http://dx.doi.org/10.1016/S0030-4018(98)00457-X
- 4. Schwarz, J., Rambo, P. and Diels, J.C. (2001) Measurements of Multiphoton Ionization Coefficients with Ultrashort Ultraviolet Laser Pulses. Applied Physics B, 72, 343. http://dx.doi.org/10.1007/s003400100496
- 5. Matsui, T., Fukazawa, K., Fujimoto, M. and Imasaka, T. (2012) Analysis of Persistent Organic Pollutants at Sub- Femtogram Levels Using a High-Power Picosecond Laser for Multiphoton Ionization in Conjunction with Gas Chromatography/Time-of-Flight Mass Spectrometry. Analytical Science, 28, 445.
- 6. Yamada. S. (1991) A Submicroliter Transparent Cell for Laser Multiphoton Ionization Detection in Solution. Analytical Chemistry, 63, 1894-1897. http://dx.doi.org/10.1021/ac00017a041
- 7. Yamada. S. (2009) Application of Strong Interactions between Photons and Molecules to Analytical Sciences. Analytical Science, 29, 1059.
- 8. Hoa, D.Q., Duong, V., Long, P. and Nhung, T.H. (2008) Generation Short-Pulse Laser by Using a Quenched Distributed Feedback Dye Laser. Journal of the Korean Physical Society, 53, 3823.
- 9. Van den Bron, A.J., Kapelios, M., et al. (2005) Photodissociation and Photoionization of Pyrrole Following the Multiphoton Excitation at 243 and 364 nm. Physical Chemistry Chemical Physics, 5, 892.
- 10. Kjellberg, M., Bulgakov, A.V., Goto, M., Johansson, O. and Kassen, K. (2010) Femtosecond Electron Spectroscopy of Coronene, Benzo[GHI]perylene, and Anthracene. The Journal of Chemical Physics, 133, Article ID: 074308. http://dx.doi.org/10.1063/1.3466925
NOTES
*Corresponding author.






