Open Journal of Medical Microbiology
Vol. 3 No. 2 (2013) , Article ID: 33296 , 20 pages DOI:10.4236/ojmm.2013.32017
Global Rates and Prevalence of Urogenital Mycoplasmosis: Assembly of a Dataset from Peer-Reviewed Literature*
Department of Biological Sciences, Fisher College of Science and Mathematics, Towson University, Towson, USA
Email: jjones39@students.towson.edu, nchaba1@students.towson.edu, †mmay@towson.edu
Copyright © 2013 Jennifer A. Jones et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 3, 2013; revised March 4, 2013; accepted March 11, 2013
Keywords: Mycoplasma genitalium; Mycoplasma hominis; Ureaplasma; Urogenital Mycoplasma
ABSTRACT
Rates of urogenital mycoplasmosis associated with Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum have been reported numerous times, and frequently show a wide range of findings. Differing diagnostic techniques, population targeting, temporal and spatial data collection, and coincident infections make the conclusions from these analyses difficult to compare. We generated a single data set including the infection rate, geographic location, year, study population, diagnostic method, and clinical signs for these organisms by performing literature searches with the species names and compiling the findings. Studies focusing on basic research or reporting clinical surveys where these criteria were not reported were excluded. A statistical analysis of the dataset parameters found that: diagnostic method does not significantly correlate with positive specimen rate but does correlate with the year of publication, and the number of publications correlated significantly with year, indicating that this topic is of growing interest. Further analysis indicated that Ureaplasma species infection rate is significantly higher in pregnant women across all studies. Associations with distinct clinical presentation could not be made on datasets assembled across studies due to the number of confounding variables presented in each. The generated data set represents a large amount of temporal, geographic, and clinical data that can be utilized in future communications.
1. Introduction
Members of the genera Mycoplasma and Ureaplasma are small, wall-less bacteria that parasitize vertebrate hosts in an obligate manner. Infections with these species exhibit a spectrum of clinical manifestations ranging from asymptomatic states to the classical manifestation of a chronic inflammatory illness that is not typically fatal. The primary sites of mycoplasmal infections are mucosal surfaces, and in humans infection is typically seen in the respiratory or urogenital tract. Urogenital mycoplasmosis of humans is typically associated with Mycoplasma genitalium (MG), Mycoplasma hominis (MH), Ureaplasma urealyticum (UU), Ureaplasma parvum (UP), or a complex infection of more than one of these species. Mycoplasma penetrans and Mycoplasma pirum are not commonly associated with clinical signs [1]. All of these species can be detected in clinically normal patients, but their association with urogenital disease has been the subject of extensive exploration.
Numerous studies have reported rates of patient infections in the context of their urogenital health with ambiguous results. The clinical states associated with Ureaplasma species (Usp) and MH appear to be strainor patient-specific, and reportedly include nongonococcal urethritis (NGU) (Usp), bacterial vaginosis (MH), spontaneous abortion, or preterm labor (MH, Usp) [2]. In contrast, the causal role of MG in NGU, pelvic inflammatory disease, spontaneous abortion, and infertility is generally accepted, and as such it is now considered an emerging urogenital pathogen [3]. Infection of pregnant women with Usp has been associated with preterm labor and premature rupture of membranes, and the perinatal infants can develop Usp infections of the respiratory tract or central nervous system [4-6].
Diagnosis of urogenital mycoplasmosis was originally based on direct culture of MH or Usp from the urogenital tract, urine, semen, or cervical mucus, and often requires the services of a specialized laboratory [1]. Recovery of MG from affected tissues has historically been, and remains, particularly challenging. Due to the difficulties associated with culture, several other diagnostic methods have been developed and employed in recent years including polymerase chain reaction (PCR)-based nucleic acid detection strategies, serological diagnosis, and commercial assays based on detection of ammonia following the hydrolysis of urea (Usp) or catabolism of arginine (MH). Between the increased sensitivity of PCR and the technical limitations of culturing the organism, nucleic acid detection is used almost exclusively to diagnose MG infection.
Because the volume of clinical surveys investigating urogenital mycoplasmosis is large and the geographic location, study populations, clinical features, compounding factors, diagnostic methods, and clinical correlation with MG, MH, and Usp infection are disparate, we sought to compile a single data set describing the currently published clinical data on this topic and identify statistical trends across studies.
2. Materials and Methods
2.1. Literature Analysis
An analysis of primary literature was performed using the National Center for Biotechnology Information and the National Library of Medicine database PubMed. The search terms utilized were Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma. Studies describing basic research on the biology and/or pathogenicity of the bacteria were immediately excluded. The remaining medically-oriented studies were excluded if minimal clinical information was described, rates of infection were not provided, or differentiation between MG, MH, or Usp was not performed. The distinction was not drawn for U. urealyticum and U. parvum because many studies predate the description of the two distinct species. Studies were included if: 1) rates of definitive positive diagnosis of urogenital mycoplasmosis were reported, and 2) descriptions of associated clinical signs, the geographic origin of the samples, the diagnostic method, and the study population were reported. The dataset reflect studies published up to and including May 2011. Numbers of patients were sorted by clinical presentations and detected species. Asymptomatic control patients from each study were included in the dataset.
2.2. Statistical Analysis
Pearson correlation analysis between year and citations was performed. Spearman rank correlations between incidence in clinically affected individuals for each species and year, and incidence and diagnostic method were performed. Individual types of the categorical variable “diagnostic method” were assigned numeric values based on the sensitivity of the method (nucleic acid detection = 1; commercial diagnostic kits = 2, non-commercial ELISA = 3, laboratory culture = 4). The significance of incidence in pregnant vs. nonpregnant women was determined by χ2 analysis. All statistical procedures were performed using Origin 8.6 (OriginLab Corporation, Northampton, MA), and a P value of less than 0.05 was considered significant.
3. Results
3.1. Description of Dataset
A total 172 studies including data from 53 countries (in addition to Scotland and Palestine) on all populated continents were examined (Table S1). The studies were published between 1975 and 2011 in 85 different journals (Table 1). A total of 96,361 patients (27,952 for MG, 23,928 for MH, and 44,481 for Usp) were tested by one of four diagnostic methods. Different prevalence rates for each species were observed overall and for each clinical presentation (Table 2). Patient totals from individual studies are presented in Tables S2-S4 for MG, MH, and Usp, respectively. Rates of infection in patients with urethritis, adverse pregnancy outcomes, infertility, and inflammatory conditions of the genital tract are presented in Tables S5-S8, respectively.
3.2. Statistical Analysis
Significant correlations were found between the diagnostic method utilized and the year of publication (inverse, P < 0.0001), and the number of citations and year (direct, P < 0.001). The correlation between diagnostic method ranked by sensitivity and positive percent of the population tested was not significant (Table 3). Pregnant women were significantly (P < 0.005) more likely to be infected with Usp than nonpregnant women regardless of the presence or absence of symptoms. Pregnant women were significantly (P < 0.05) less likely to be infected with MG. Infection with MH had no significant association with pregnancy.
4. Discussion
An analysis of primary literature was performed, and a single dataset describing rates of urogenital mycoplasmosis was described. Significant correlations were found between the diagnostic method utilized and the year of publication (inverse, P < 0.0001), and the number of citations and year (direct, P < 0.001), indicating that increasing numbers of studies reporting incidence of urogenital mycoplasmosis as determined by more sensitive methods such as nucleic acid detection and commercial detection kits are being published. The country with the greatest number of distinct populations studied in publi-

Table 1. Parameter totals of included studies.
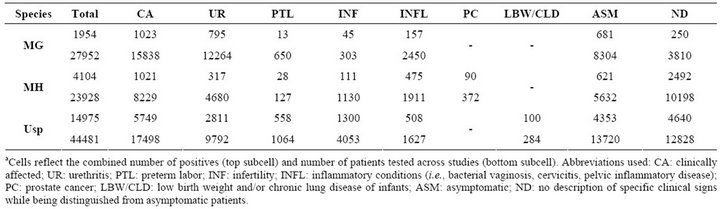
Table 2. Patient totalsa.

Table 3. Study parameters.
cations meeting our criteria was the United States (41), followed by Japan (26) and China (18). All populated continents were represented in our analysis.
Usp have consistently been reported at higher rates in pregnant women as compared to nonpregnant women, but associations with clinical signs have been inconsistent. The generation of pathologic lesions during mycoplasmosis can be both strain variable and dependent on host factors; however, the presence or absence of MG, MH, or Usp during pregnancy as an unambiguous condition allows for concrete analysis. Across studies, we found that Usp were detected in pregnant women significantly more often than nonpregnant women. The association of Usp with low birth weight and chronic lung disease in preterm infants has been established by multiple studies [4,5], illustrating the clinical importance of this association. We also found that pregnant women were significantly less likely to be infected with MG; however, this likely represents an anomaly stemming from published study designs investigating MG and infertility. Women of infertile couples are often, but not always, significantly more likely to have MG infection than controls. The focus on infertility indicates the ideal asymptomatic control patients for such studies are pregnant women. It is far more probable that MG infection leads to infertility than pregnancy as a condition is somehow protective against MG infection.
The association of MG, MH and Usp with specific urogential health concerns remains somewhat difficult to define because of the equivocal findings of a large number of clinical studies. Confounding factors such as coinfection with additional sexually transmitted pathogens, the narrow focus on nonrepresentative populations such as infertile couples or STI clinic patients, and the differing age of patients makes the combination of data for each pathogen across studies inadvisable. Previously described differences between strains of MG, MH, and Usp include antibiotic susceptibility patterns [7-11] and the expression or variation of certain virulence factors including adhesins [12,13], biofilm formation [14], and the multiple-banded antigen of Usp [15]. In addition, comparative analysis of the complete genomes of nineteen Ureaplasma strains (representing the fourteen serovars) revealed a greater predilection for UU to undergo horizontal gene transfer, making it more subject to genome plasticity than UP [16]. UU has been associated with more severe clinical presentations than UP [16], which is potentially explained by the acquisition of novel genes. In addition, the failure to speciate between the apparently more pathogenic UU and the apparently less pathogenic UP may contribute to the ambiguity of Usp in association with clinical presentations. Heterogeneity of all three pathogens undoubtedly contributes to the spectrum of clinical presentations ranging from asymptomatic carriage to chronic inflammatory disease. Furthermore, the immune and nutritional status of the patient likely impacts the presentation of disease.
Infection with MG has been associated with increased shedding of human immunodeficiency virus (HIV) particles in two distinct studies, indicating that MG may facilitate HIV transmission [17]. Additionally, the presence of genital tract white blood cells due to urogenital inflammation regardless of cause is association with increased HIV shedding [18]. Because of the public health implications of increased HIV transmission, the prevalence of agents capable of causing urogenital inflammation should thus be monitored. Compiling the findings of studies describing rates of urogenital mycoplasmosis in clinically affected and asymptomatic patients represents a step toward illustrating the presence of these pro-inflammatory organisms.
5. Conclusion
The value of the compiled dataset is the illustration of the numbers of patients being evaluated for MG, MH, and Usp. The parameters of the dataset illustrate increasing clinical interest in the incidence of urogenital mycoplasmosis, and that increasingly molecular methods are being used to detect the organisms. Confounding variables make the analysis of infection rates across all clinically affected versus all asymptomatically infected patients inadvisable; however, the generated dataset represents a large amount of temporal, geographic, and clinical data that can be utilized in future exchanges.
REFERENCES
- D. R. Brown, M. May, J. M. Bradbury, M. F. Balish, M. J. Calcutt, J. I. Glass, S. Tasker, J. B. Messick, K.-E. Johansson and H. Neimark, “Genus I. Mycoplasma.,” In: N. R. Krieg, W. Ludwig, W. B. Whitman, B. P. Hedlund, B. J. Paster, J. T. Staley, N. Ward, D. R. Brown and A. Parte, Eds., Bergey’s Manual of Systematic Bacteriology, Springer, Inc., New York, 2010 pp. 575-644.
- K. B. Waites and D. Talkington, “New Developments in Human Disease Due to Mycoplasmas,” In: A. Blanchard and G. F. Browning, Eds., Mycoplasmas: Molecular Biology, Pathogenicity, and Strategies for Control, Horizon Bioscience, Norfolk, 2005, pp. 289-354.
- C. L. McGowin and C. Anderson-Smits, “Mycoplasma genitalium: An Emerging Cause of Sexually Transmitted Disease in Women,” PLoS Pathogens, Vol. 7, No. 5, 2011, Article ID: e1001324.
- B. Larsen and J. Hwang, “Mycoplasma, Ureaplasma, and Adverse Pregnancy Outcomes: A Fresh Look,” Infectious Diseases in Obstetrics and Gynecology, Vol. 2010, 2010, Article ID: 521921. doi:10.1155/2010/521921
- R. M. Viscardi, “Ureaplasma Species: Role in Diseases of Prematurity,” Clinics in Perinatology, Vol. 37, No. 2, 2010, pp. 393-409. doi:10.1016/j.clp.2009.12.003
- R. M. Viscardi, W. M. Manimtim, C. C. Sun, L. Duffy and G. H. Cassell, “Lung Pathology in Premature Infants with Ureaplasma urealyticum Infection,” Pediatric and Developmental Pathology Vol. 5, No. 2, 2002, pp. 141-150.
- M. A. De Francesco, S. Caracciolo, C. Bonfanti and N. Manca, “Incidence and Antibiotic Susceptibility of Mycoplasma hominis and Ureaplasma urealyticum Isolated in Brescia, Italy, over 7 Years,” Journal of Infection and Chemotherapy, 2012.
- R. Krausse and S. Schubert, “In-Vitro Activities of Tetracyclines, Macrolides, Fluoroquinolones and Clindamycin against Mycoplasma hominis and Ureaplasma ssp. Isolated in Germany over 20 Years,” Clinical Microbiology and Infection, Vol. 16, No. 11, 2010, pp. 1649-1655. doi:10.1111/j.1469-0691.2010.03155.x
- Y. Shimada, T. Deguchi, K. Nakane, T. Masue, M. Yasuda, S. Yokoi, S. Ito, M. Nakano and H. Ishiko, “Emergence of Clinical Strains of Mycoplasma genitalium Harbouring Alterations in ParC Associated with Fluoroquinolone Resistance,” International Journal of Antimicrobial Agents, Vol. 36, No. 3, 2010, pp. 255-258. doi:10.1016/j.ijantimicag.2010.05.011
- Y. Shimada, T. Deguchi, K. Nakane, M. Yasuda, S. Yokoi, S. Ito, M. Nakano and H. Ishiko, “Macrolide Resistance-Associated 23S rRNA Mutation in Mycoplasma genitalium, Japan,” Emerging Infectious Diseases, Vol. 17, No. 6, 2011, pp. 1148-1150. doi:10.3201/eid1706.101055
- Y. Shimada, T. Deguchi, Y. Yamaguchi, M. Yasuda, K. Nakane, S. Yokoi, S. Ito, M. Nakano and H. Ishiko, “gyrB and parE Mutations in Urinary Mycoplasma genitalium DNA from Men with Non-Gonococcal Urethritis,” International Journal of Antimicrobial Agents, Vol. 36, No. 5, 2010, pp. 477-478. doi:10.1016/j.ijantimicag.2010.07.013
- S. L. Iverson-Cabral, S. G. Astete, C. R. Cohen and P. A. Totten, “mgpB and mgpC Sequence Diversity in Mycoplasma Genitalium Is Generated by Segmental Reciprocal Recombination with Repetitive Chromosomal Sequences,” Molecular Microbiology, Vol. 66, No. 1, 2007, pp. 55-73. doi:10.1111/j.1365-2958.2007.05898.x
- S. L. Iverson-Cabral, S. G. Astete, C. R. Cohen, E. P. Rocha and P. A. Totten, “Intrastrain Heterogeneity of the mgpB Gene in Mycoplasma genitalium Is Extensive in Vitro and in Vivo and Suggests That Variation Is Generated via Recombination with Repetitive Chromosomal Sequences,” Infection and Immunity, Vol. 74, No. 7, 2006, pp. 3715-3726. doi:10.1128/IAI.00239-06
- K. Pandelidis, K. A. McCarthy, K. L. Chesko and R. M. Viscardi, “Role of Biofilm Formation in Ureaplasma Antibiotic Susceptibility and Development of Bronchopulmonary Dysplasia in Preterm Neonates,” Pediatric Infectious Disease Journal, Vol. 32, No. 4, 2013, pp. 394-398.
- L. J. Teng, X. Zheng, J. I. Glass, H. L. Watson, J. Tsai and G. H. Cassell, “Ureaplasma urealyticum Biovar Specificity and Diversity Are Encoded in Multiple-Banded Antigen Gene,” Journal of Clinical Microbiology, Vol. 32, No. 6, 1994, pp. 1464-1469.
- V. Paralanov, J. Lu, L. B. Duffy, D. M. Crabb, S. Shrivastava, B. A. Methé, J. Inman, S. Yooseph, L. Xiao, G. H. Cassell, K. B. Waites and J. I. Glass, “Comparative Genome Analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum Strains,” BMC Microbiology, Vol. 12, 2012, p. 88.
- L. E. Manhart, S. B. Mostad, J. M. Baeten, S. G. Astete, K. Mandaliya and P. A. Totten, “High Mycoplasma Genitalium Organism Burden Is Associated with Shedding of HIV-1 DNA from the Cervix,” Journal of Infectious Diseases, Vol. 197, No. 5, 2008, pp. 733-736. doi:10.1086/526501
- C. L. McGowin, R. S. Annan, A. J. Quayle, S. J. Greene, L. Ma, M. M. Mancuso, D. Adegboye and D. H. Martin, “Persistent Mycoplasma genitalium Infection of Human Endocervical Epithelial Cells Elicits Chronic Inflammatory Cytokine Secretion,” Infection and Immunity, Vol. 80, No. 11, 2012, pp. 3842-3849. doi:10.1128/IAI.00819-12
Supplement











Table S1. Peer-reviewed studies represented in this dataset.
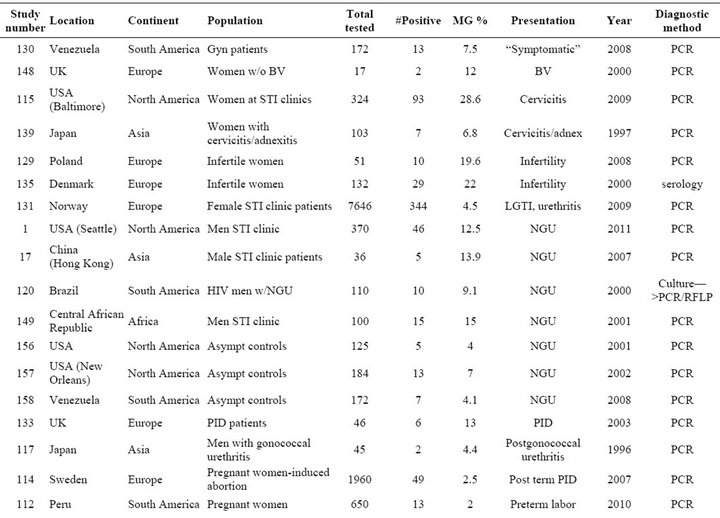
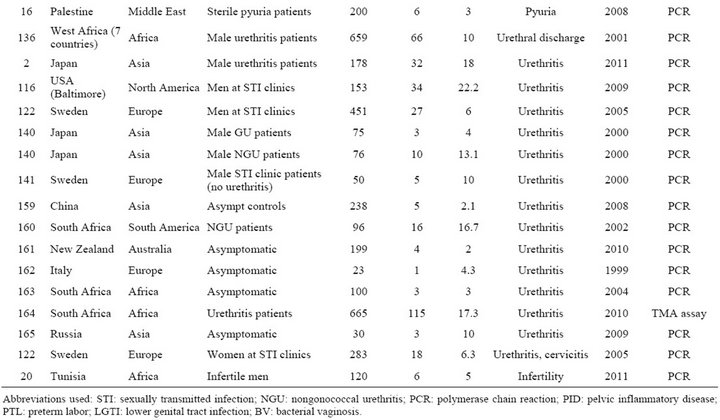
Table S2. Rates and clinical presentations of Mycoplasma genitalium.
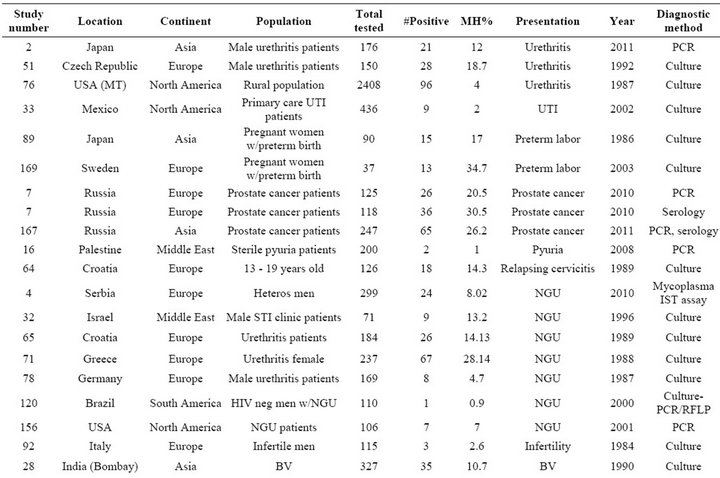
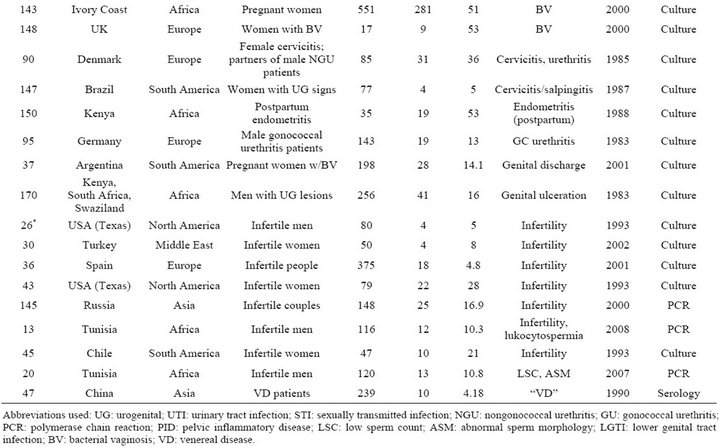
Table S3. Rates and clinical presentations of Mycoplasma hominis.
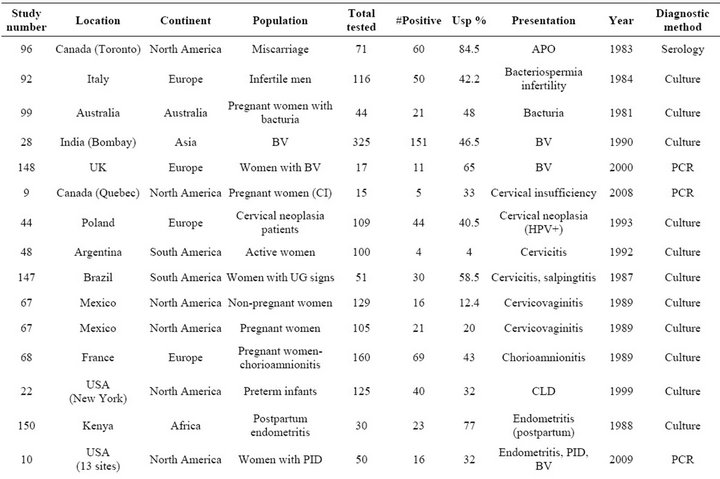
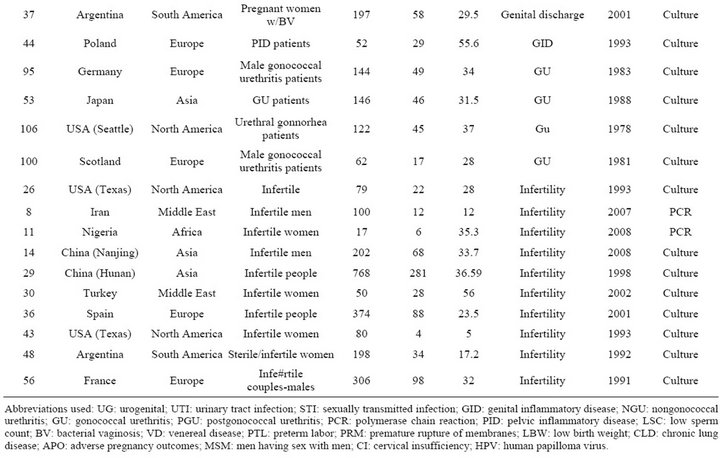
Table S4. Rates and clinical presentations of Ureaplasma species.
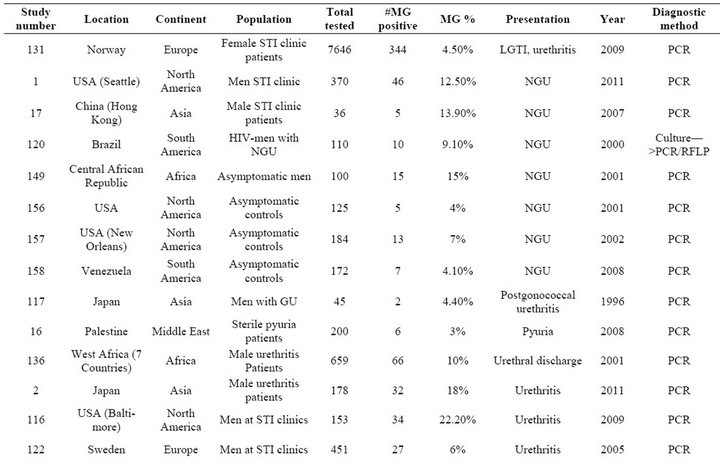

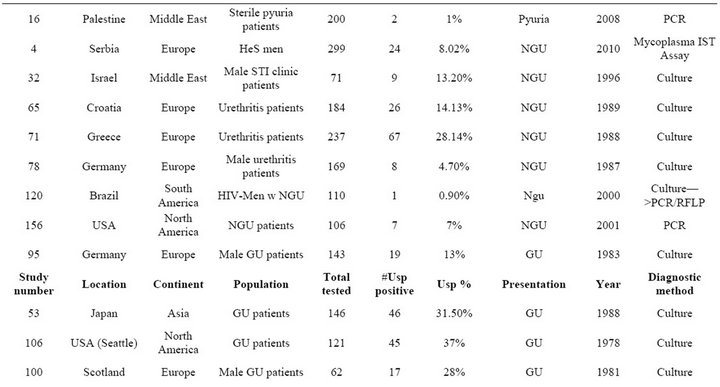


Table S5. Rates of all species in cases of urethritis.

Table S6. Rates of all species in cases of preterm labor and adverse pregnancy outcomes.


Table S7. Rates of all species in cases of infertility.
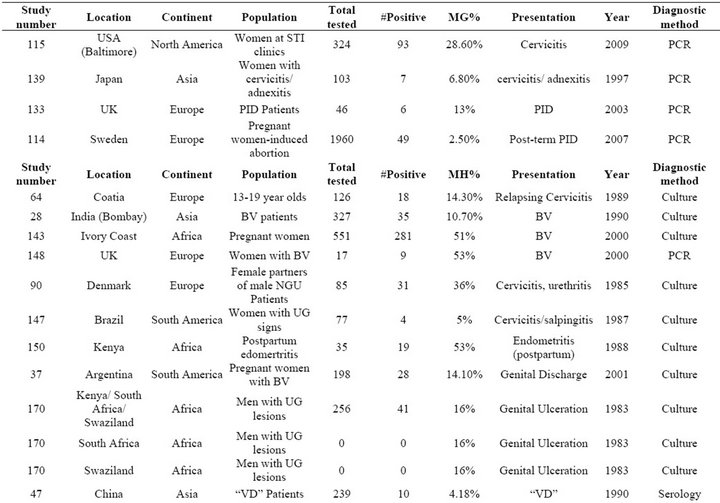
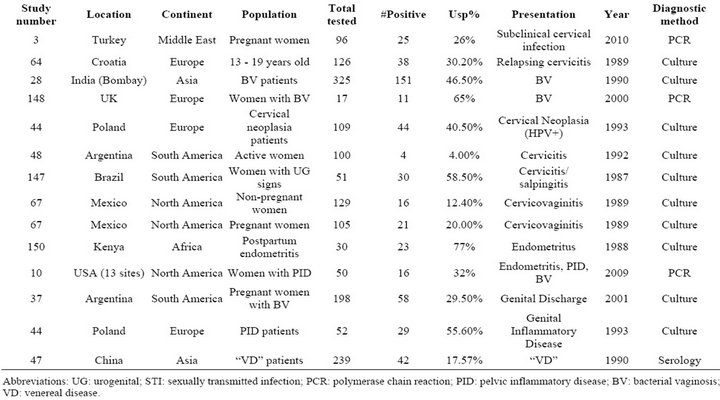
Table S8. Rates of all species in cases of genital inflammation including bacterial vaginosis, cervicitis, pelvic inflammatory disease, and nonspecific inflammatory conditions.
NOTES
*This work was supported by funds from the Robert M. Fisher Memorial Foundation (MM).
#These authors contributed equally to this work.
†Corresponding author.

