Open Journal of Rheumatology and Autoimmune Diseases
Vol. 2 No. 2 (2012) , Article ID: 18964 , 5 pages DOI:10.4236/ojra.2012.22005
Arterial Imaging in Digital Gangrenes Associated with Scleroderma-Spectrum Disorders*
![]()
1Department of Dermatology, Osaka University Graduate School of Medicine, Osaka, Japan; 2Department of Radiology, Osaka University Graduate School of Medicine, Osaka, Japan.
Email: #tanemura@derma.med.osaka-u.ac.jp
Received January 10th, 2012; revised February 13th, 2012; accepted February 20th, 2012
Keywords: Arterial Imaging Analysis; Digital Gangrene; Scleroderma-Spectrum Disorders
ABSTRACT
Objectives: Digital refractory gangrene is rarely found in collagen diseases, including systemic sclerosis and is possibly caused by similar underlying vascular damage in peripheral arterial disease (PAD) such as arteriosclerosis obliterans (ASO) and/or thromboangiitis obliterans (TAO) by unclarified mechanisms other than vasculitis and thrombosis. This study evaluated the radiological imaging in patients with digital gangrene associated with collagen disease and compared the images with those of PAD based on the results of laboratory and histopathological examinations. Methods: Angiography, MR angiography and/or CT angiography were performed on 6 patients with refractory gangrene or extensive ulcers accompanied by scleroderma-spectrum disorders; 3 with diffuse systemic sclerosis, 1 with limited systemic sclerosis, 1 with overlap syndrome and 1 with Sjögren’s syndrome. Results: Although the vascular alterations in collagen diseases were similar to those in PAD, the abnormal image findings (occlusion or stenosis of the arteries with smooth vessel walls) found in collagen diseases did not include atheromatous plaque, which are worm-like vessels that are characteristic of those observed in PAD. Conclusions: Some cases of digital gangrene seen in collagen diseases show similar vascular imaging patterns to those of PAD and comprehensive examinations including arterial imaging can be useful for the diagnosis of these unrecognized vascular changes other than vasculitis or digital thrombosis.
1. Introduction
Digital gangrene or digital ulcers related to Raynaud’s phenomenon or cutaneous vasculitis are the most refractory clinical manifestations in collagen vascular diseases, and are occasionally accompanied by impaired quality of life and/or life-threatening systemic infections. Digital gangrene is rarely found in collagen diseases, including systemic sclerosis and it is considered to show similar underlying vascular damage to that seen in PAD; however, it is often misdiagnosed as aggravated-digital ulcers related to Raynaud’s phenomenon, especially in case of scleroderma-spectrum disorders [1]. Treating these gangrenous conditions with medications intended for Raynaud’s phenomenon usually leads to unsatisfactory results and increasing the dosage of systemic steroids sometimes exacerbates gangrene due to the possible induction of hyperviscosity of plasma flow and platelet aggregation [2]. Therefore, the immediate and accurate evaluation of arterial circulation is required to determine the most suitable and less invasive treatment protocol for these conditions.
Recently, nonor less invasive vascular imaging tools such as MR angiography and CT angiography have been introduced in screening digital gangrene seen in collagen vascular diseases and evaluating the morphological changes of damaged blood vessels. This study used angiography, MR angiography and/or CT angiography on 6 patients with refractory gangrene or extensive ulcers of their extremities in scleroderma-spectrum disorders. In addition, the radiological imaging findings obtained in this study were compared with those of PAD.
2. Patients and Methods
We provide the clinical information of representative 2 cases.
Case 1: A 71-year-old female, with diffuse cutaneous systemic sclerosis (dcSSc) and anti-phospholipid syndrome.
The patient had developed Raynaud’s phenomenon with several clinical manifestations in 1996 and been diagnosed as dcSSc. CT angiography detected severe stenosis on the left renal artery in 2006. Digital debridement or amputations had been performed as needed because repeated digital ulcers and gangrene became more evident in winter season. She was admitted for the treatment of digital gangrene on the left 5th toe in July 2009. Laboratory findings on admission were: ANA; ×1280-(SP)↑, Anti-Scl-70 antibodies; ×160.0↑, Anti-centromere antibodies; not detected, Anti-cardiolipin antibody; 13 U/ml↑, Lupus anticoagulant; not detected, ACL-β2GPI; not detected, Anti-Sm antibodies; 61.8 U/ml↑, D-dimer; 1.13 μg/ml↑, β-TG; 52 ng/ml↑, PF-4; 11 ng/ml.
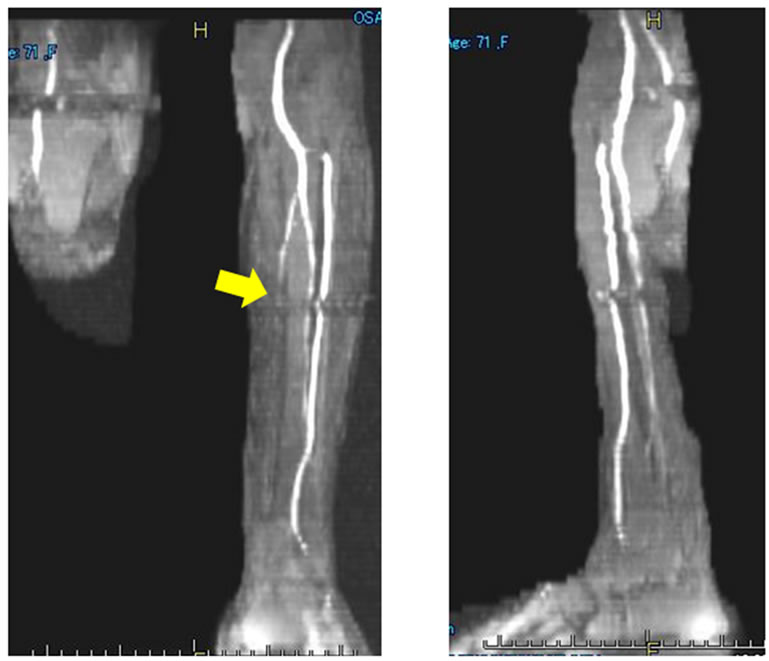
Clinial features of the digital gangrenes and plain MRA findings are shown in Figures 1 and 2, respecttively. There were ulcers or gangrene on multiple fingers, exposed bone on the left index finger (Figure 1), occlusion of the left posterior tibial arteries (Figure 2(a)) and stenosis of all digital arteries on the finger (Figure 2(b)). All of those findings were inconsistent with those of PADs.
Case 2: A 65-year-old female, limited cutaneous systemic sclerosis (lcSSc).
Raynaud’s phenomenon had developed in 2002. Digital ulcers on the right middle finger and the left index toe had been refractory to any medication since July 2009 and rapidly enlarged in multiple fingers within 3 months. The patient noticed sclerodactyly on all fingers and a histological examination showed robust collagen bundles and lack of skin apparatus on the dermis without vasculitis. Therefore, she was diagnosed with lcSSc with digital gangrene. Laboratory findings on admission were: ANA; ×5120 (HO + CE)↑, Anti-Scl-70 antibodies; not detected, ACA; 112.0↑, Anti DNA antibodies; 12 U/ml↑, D-dimer; 0.41 μg/ml, TAT; 1.0 μg/ml, β-TG; 28 ng/ml, PF-4; 8 ng/ml.
The clinical features of the digital gangrene (Figure
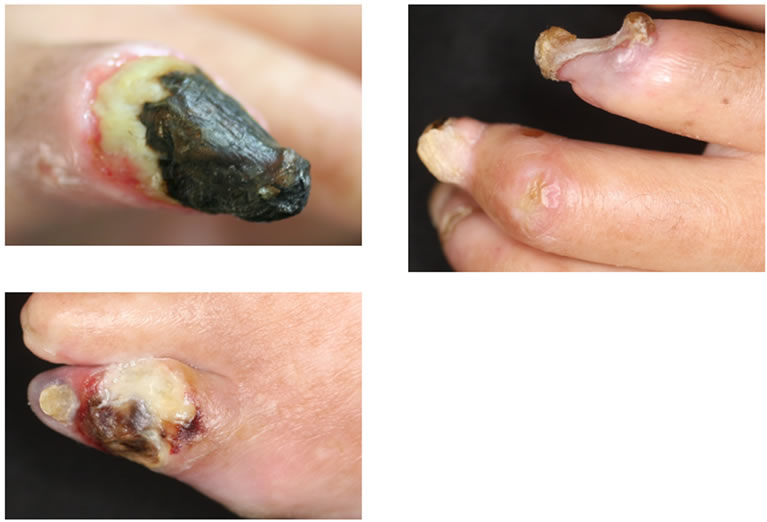
Figure 1. Clinical feature of the digital gangrene in Case 1. The necrotic ulcers or gangrene involved multiple fingers and toes. The digital bone on the Lt. index finger was exposed.
 (a)
(a) (b)
(b)
Figure 2. (a) Plain MR angiography imaging on the bilateral lower extremity in Case 1. The imaging indicates complete occlusion of the Lt. posterior tibial artery (arrow); (b) Plain MR angiography imaging on the bilateral hand in Case 1. The imaging findings suggest severe stenosis and meandering of the digital arch (circle).
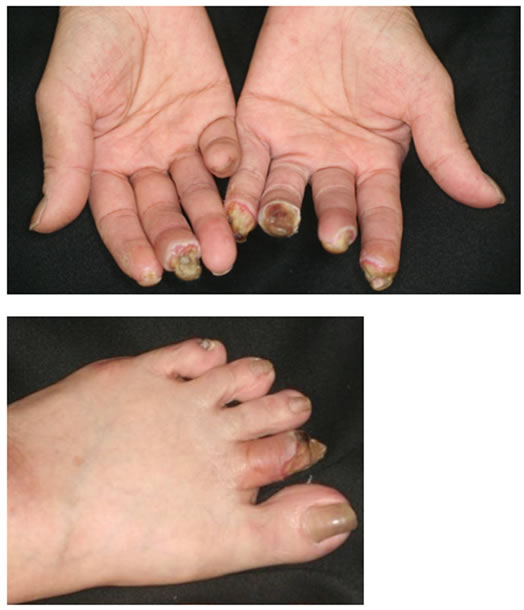
3(a)) and angiography findings are shown in Figure 3(b) and Figure 3(c). Briefly, the undermined ulcers developed rapidly in multiple fingers (Figure 3(a)) and stenosis of the common and the proper digital arteries (Figure 3(b)) and occlusion of the bilateral anterior tibial arteries and the right posterior arteries at the ankle level (Figure 3(c)) were demonstrated and both of these findings were inconsistent with those of PADs.
The detailed patients’ characteristics of all 6 patients on this study are summarized in Table 1.
3. Discussion
Microvascular involvement in SSc and related disorders are limited to the digital artery, possibly associated with cold induced—vascular contraction due to Raynaud’s
 (a)
(a) (b)
(b)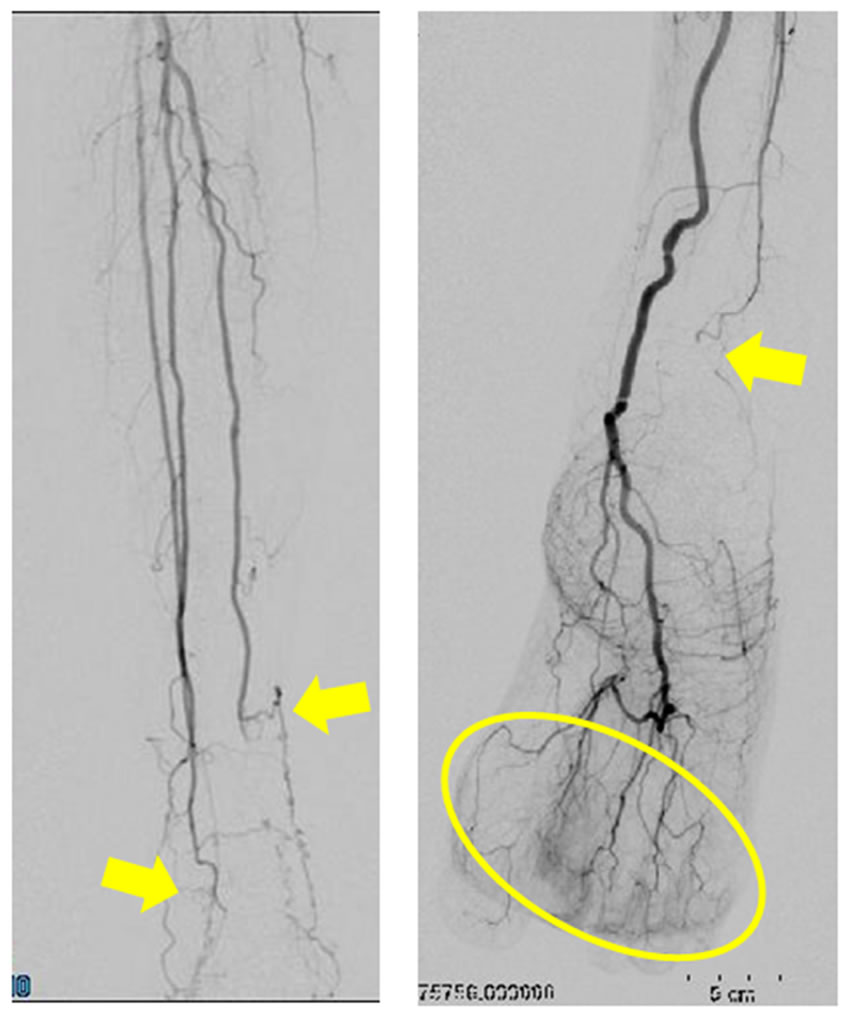 (c)
(c)
Figure 3. (a) Clinical features of the digital gangrene in Case 2. The undermined necrotic ulcers rapidly developed in multiple fingers and toes; (b) Digital angiography imaging on the bilateral hand in Case 2. The imaging clearly indicates severe stenosis and occasional occlusion of the common and proper digital arteries (circle); (c) Digital angiography imaging on the bilateral lower leg in Case 2. The imaging clearly indicates severe stenosis on the peripheral arteries and the complete occlusion of the Rt. posterior tibial artery at the ankle levels (arrows). The Rt. digital blood flow is mainly supplied by the anterior tibial artery (circle).
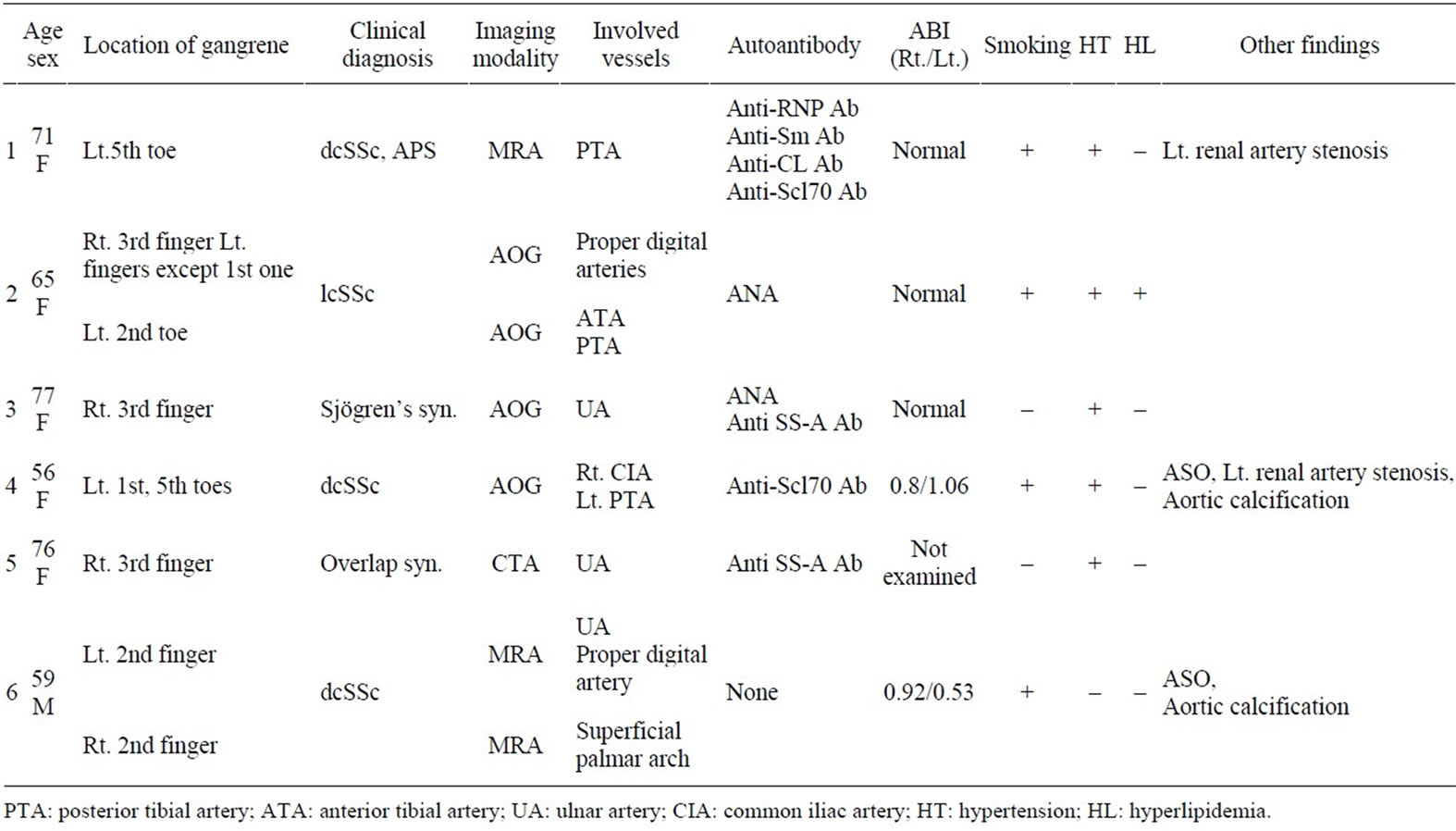
Table 1. Clinical characteristics of the enrolled patients.
phenomenon. However, recent studies suggest that middle-sized vessels distal from the elbow or knee are occasionally involved in scleroderma spectrum disorders [3- 6]. These include significant vascular alterations such as occlusion of the ulnar artery observed in one third of SSc patients [3-5]. Radial artery involvement is rare [4-6] and the primarily affected vessels are the proper digital arteries [6]. Cases with involvement in the lower extremities show occlusion and stenosis of middle-sized vessels such as the anterior and posterior tibial artery [4].
There are 2 distinct pathophysiological mechanisms associated with the microvascular and macrovascular involvement in SSc and related disorders. One is similar to PAD and related to the findings such as arteriosclerosis in the renal artery or common iliac artery, as shown in the present cases and arterial wall stiffness and thickness reported in the previous studies [7-9]. Another might be closely related to the pathogenesis of SSc without the findings characteristic of PAD, such as atheromatous plaque, and worm-like vessels. Unrecognized vascular injury closely related to Raynaud’s phenomenon and/or endothelial injury due to possible anti-endothelial antibodies or oxidative stress might play important roles in the induction of microvascular involvement [10-12]. It is difficult to determine the underlying phenomenon because these distinct etiologies are occasionally concomitant in the vascular damage in SSc and its related disorders. There seem to be other mechanisms associated with vascular damage in SSc or other collagen diseases, although, at present, the precise mechanisms have not yet been revealed by vascular imaging analyses.
In conclusion, this study described several cases of digital gangrene with more proximal vascular damages in SSc and related disorders. Although it is sometimes difficult to distinguish the vascular damages in SSc and those in PAD by new vascular imaging tools such as MR and CT angiography, imaging analysis itself is less invasive and definitely valuable for understanding the disease condition, including the vascular damage. Although there were abnormal images of arteries in the sclerodermaspectrum disorders examined in this study there were no distinct differences in the radio-imaging findings between SSc and PAD. The underlying mechanisms associated with vascular damage in SSc or other collagen diseases must be determined in subsequent studies. The development of more precise imaging tools covering more peripheral regions of circulation will hopefully be able to clarify the mechanisms underlying these conditions.
REFERENCES
- K. Takehara, Y. Soma and Y. Ishibashi, “Early Detection of Scleroderma Spectrum Disorders in Patients with Raynaud’s Phenomenon,” Dermatologica, Vol. 183, No. 3, 1991, pp. 164-168. doi:10.1159/000247662
- P. Youssef, T. Brama, H. Englert and J. Bertouch, “Limited Scleroderma Is Associated with Increased Prevalence of Macro Vascular Disease,” The Journal of Rheumatology, Vol. 22, No. 3, 1995, pp. 469-472.
- M. Stücker, S. Quinna, U. Memmel, A. Röchling, M. Traupe, K. Hoffmann, et al., “Macroangiopathy of the Upper Extremities in Progressive Systemic Sclerosis,” European Journal of Medical Research, Vol. 5, No. 7, 2000, pp. 295-302.
- M. Hasegawa, Y. Nagai, A. Tamura and O. Ishikawa, “Arteriographic Evaluation of Vascular Changes of the Extremities in Patients with Systemic Sclerosis,” British Journal of Dermatology, Vol. 155, No. 6, 2006, pp. 1159- 1164. doi:10.1111/j.1365-2133.2006.07475.x
- L. Dabich, J. J. Bookstein, A. Zweifler and C. J. Zarafonetis, “Digital Arteries in Patients with Scleroderma: Arteriographic and Plethysmographic Study,” Archives of Internal Medicine, Vol. 130, No. 5, 1972, pp. 708-714. doi:10.1001/archinte.1972.03650050036006
- B. Janevski, “Arteries of the Hand in Patients with Sclero- derma,” Diagnostic Imaging in Clinical Medicine, Vol. 55, No. 4-5, 1986, pp. 262-265.
- G. N. Anderson, L. Mincheva-Nilsson, E. Kazzam, G. Nyberg, N. Klintland, A. S. Petersson, et al., “Assessment of Vascular Function in Systemic Sclerosis: Indications of the Development of Nitrate Tolerance as a Result of Enhanced Endothelial Nitric Oxide Production,” Arthritis and Rheumatism, Vol. 46, No. 5, 2002, pp. 1324-1332. doi:10.1002/art.10191
- A. Cypiene, A. Laucevicius, A. Venalis, J. Dadoniene, L. Ryliskyte, Z. Petrulioniene, et al., “The Impact of Systemic Sclerosis on Arterial Wall Stiffness Parameters and Endothelial Function,” Clinical Rheumatology, Vol. 27, No. 12, 2008, pp. 1517-1522. doi:10.1007/s10067-008-0958-1
- M. Ho, D. Veale, C. Eastmond, G. Nuki and J. Belch, “Macrovascular Disease and Systemic Sclerosis,” Annals of Rheumatic Diseases, Vol. 59, No. 1, 2000, pp. 39-43. doi:10.1136/ard.59.1.39
- M. B. Hill, J. L. Phipps, R. J. Cartwright, et al., “Antibodies to Membranes of Endothelial Cells and Fibroblasts in Scleroderma,” Clinical and Experimental Immunology, Vol. 106, No. 3, 1996, pp. 491-497. doi:10.1046/j.1365-2249.1996.d01-867.x
- D. Abraham, O. Distler, et al., “How Does Endothelial Cell Injury Start? The Role of Endothelin in Systemic Sclerosis,” Arthritis Research and Therapy, Vol. 9, Suppl. 2, 2007, p. S2. doi:10.1186/ar2186
- S. Guiducci, O. Distler, et al., “Mechanisms of Vascular Damage in SSc—Implications for Vascular Treatment Strategies,” Rheumatology, Vol. 47, 2008, pp. 18-20. doi:10.1093/rheumatology/ken267
Abbreviations
PAD: Peripheral arterial disease;
ASO: Arteriosclerosis obliterans;
NOTES
*Conflict of interest: none.
#Corresponding author.

