Natural Resources
Vol.4 No.8(2013), Article ID:41451,10 pages DOI:10.4236/nr.2013.48061
Prosopis L. Invasion in the South-Western Region of Botswana: The Perceptions of Rural Communities and Management Options
![]()
1Department of Geography and Environmental Science, University of Fort Hare, Alice, South Africa; 2Department of Geography and Environmental Science, North-West University, Mafikeng, South Africa; 3Department of Biological Science, University of Botswana, Gaborone, Botswana.
Email: sammosweu@gmail.com
Copyright © 2013 Samuel Mosweu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Samuel Mosweu et al. All Copyright © 2013 are guarded by law and by SCIRP as a guardian.
Received September 26th, 2013; revised November 3rd, 2013; accepted November 21st, 2013
Keywords: Rural Communities; Perceptions; Prosopis Invasion; Prosopis Management
ABSTRACT
This study was aimed to determine the extent to which Prosopis species had invaded four settlements (Bokspits, Rappelspan, Vaalhoek and Struizendam) located in the Kgalagadi Desert south west of Botswana, investigate the perceptions of the communities about the existence of the species in their environment and assess possible control options for the spread of Prosopis plants in the area. Prosopis plants were sampled in 42 quadrats of 625 m2 along a 70 km Prosopis invasion gradient from Struizendam to Rappelspan. Using the Global Positioning System (GPS), the locations of all quadrats were established. The distribution map of Prosopis plants was produced using ArcGIS 9.2 (ESRI Inc.). Questionnaire survey and focused group discussions were used to collect data on the perceptions of rural communities about the species. A total of 342 respondents comprising 139 males and 203 females were interviewed, and four focussed group discussions were conducted. The results indicated that the invasion of Prosopis species was prominent in and around settlements suggesting that anthropogenic activities had a significant role in the spread of Prosopis plants in the area. The perceptions of rural communities about Prosopis plants appeared to be moulded by the impacts of the plants on their livelihoods as well as their micro-economic status. The respondents (71.30%) expressed the view that the invasion of Prosopis species negatively affected the livelihoods of the communities in the study area. They identified eradication as the preferred method of controlling the spread of Prosopis plants. On the contrary, this study recommended the integrated environmental management paradigm as the best options for the control of the spread of Prosopis plants in the area.
1. Introduction
Prosopis Linnaeus amend. Burkart genus belongs to the family Leguminosae (Fabaceae), sub-family Mimosoideae [1]. Prosopis species are trees or shrubs of various sizes which are primarily xerophilous, aculeate, and spiny [2]. The taxonomy of Prosopis genus compiled by [2] included 44 Prosopis species and a number of varieties. The range of the genus covers arid and semi-arid regions in Africa, Asia, Central, Northern and Southern regions of America [1]. Prosopis juliflora is the most common and widely spread Prosopis species [1,3].
Prosopis species were introduced in various areas primarily to combat desertification and improve the quality and quantity of fodder resources in arid regions [1,4,5]. However, the introduction of Prosopis species in many areas resulted in undesirable ecological and socio-economic consequences. Many communities inhabiting areas where Prosopis species have been introduced initially welcomed the introduction of the species until the species developed conspicuous invasive characteristics that impacted negatively on their livelihoods [6,7].
Owing to allelophathy, Prosopis plants suppress growth of other plants and threaten plant diversity in areas where Prosopis plants grow [7,8]. Prosopis tap roots are able to reach a depth of 20 to 25 m, and some Prosopis trees whose roots reached beyond this depth have been reported globally [1]. The roots allow Prosopis plants to tap water from deep underground causing shortage of underground water by lowering the water table [1]. Most Prosopis species have large thorns [9] which are often detrimental to people and farm equipment. In addition, reports that Prosopis plants cause allergies and diseases have been documented [7,8]. To this end, Prosopis plants are often associated with the term “invasive alien species” [7,10,11].
Although Prosopis species is frequently associated with the term “invasive alien species” which more often than not implies negativity [11]; positive ecological and socio-economic impacts have been noted about the species. Positive impacts of Prosopis species are evident in areas where the species are used to stabilize sand dunes [5,12,13], used as fuel energy resources [1,6,7], used to improve soil fertility [13], used for soil moisture conservation [1,11,14], used as construction timber, shade and furniture wood [1], used as feed and forage for livestock [15,16], used as food resources for humans [1,7], used for honey production [17], used for the creation of employment [1,7], used for production of exude gums [1,18], used for production of fibres, tannins and dyes [19] and used for medicinal purposes [20].
The coexistence of positive and negative ecological and socio-economic impacts associated with Prosopis species has instigated researchers to investigate whether Prospis plants are “weed or wonder”, “pest or providence” and “friend or foe” [1,7,21,22]. Empirical research generally indicates that, despite the general perception that Prosopis species are alien invasive plants in the arid and semi-arid regions, the benefits derived by the local communities from the presence of Prosopis plants in the environment outweigh the benefits that could be drawn from the absence of the plants [1,6,7]. Additionally, the perceptions about the impacts of invasive plants by rural people are normally influenced by the impacts that the species have on their livelihoods [23].
Diversity in the perceptions of different rural communities about Prosopis species have been reported globally [1,6,7], but paucity of work in this line of research still exists particularly with reference to the rural communities inhabiting the Kgalagadi Desert which covers the central part of Botswana, eastern Namibia and north western regions of the Republic of South Africa. Therefore, the aim of this study was to determine the extent to which Prosopis species had invaded four settlements in the Kgalagadi area south west of Botswana, investigate the perceptions of the communities about the existence of the species in their environments and assess possible control options for the spread of Prosopis plants in the area.
2. Material and Methods
2.1. Description of the Study Area
The study focused on four villages (Bokspits, Rappelspan, Vaalhoek and Struizendam) located in the south west of Botswana in the southern Kgalagadi district (Figure 1). The study is located within a vast area covered in sand stretching between the Orange River and the Zambezi River including the western and central part of Botswana, eastern Namibia and North western regions of South Africa identified as the Kgalagadi Desert. The sandstone and quartz comprise the rocky outcrops in the study area with calcrete dominating the riparian zones along the Nossob-Molopo River valley.
The vegetation of the area is generally open tree and grass savanna with sparse cover of tussock grasses. Acacia erioloba, Acacia haematoxylon, Rhigozum trichotomum, Lycium namaquense, Monechma incanum, Prosopis chilensis, Prosopis velutina, Prosopis juliflora, Prosopis glandulosa, hybrids of P. juliflora and P. glandulosa, P. Juliflora and P. pallida, P. Chilensis and P. glandulosa, P. Glandulosa and P. pallida, and P. juliflora and Acacia karoo comprise the main trees and shrubs found in the study area [24]. Schmidtia pappophoroides and Eragrostis species are the main grass species growing in the area [4].
The study area is located in the driest part of Botswana where the mean annual rainfall is 300 mm and the rainfall season is characterized by erratic rainfall patterns [25]. The area experiences very high temperatures in summer which may reach up to over 40˚C, while the winter temperatures are normally between 2˚C to 4˚C [4].
The San are the first inhabitants of the Kgalagadi area in Botswana [26]. The Kgalagadi communities were initially nomadic hunters and gatherers and depended on sip holes for water [27] until they ceased their nomadic life by the end of the first quarter of the 20th century after the advent of pit wells and underground water extraction technologies. The main livelihood activity and land use type in the study area is pastoral farming at both commercial and subsistence levels.
2.2. Survey of the Spread of Prosopis Plants
Sampling quadrats of 25 m × 25 m (625 m2) were used to sample Prosopis plants along a 70 km Prosopis invasion gradient from Struizendam to Rappelspan area. The quadrats were spaced by a distance of 500 m. The Global Positioning System (GPS) was used to determine the coordinates of all quadrats. The density of Prosopis plants was determined in a total of 42 quadrats. ArcGIS 9.2
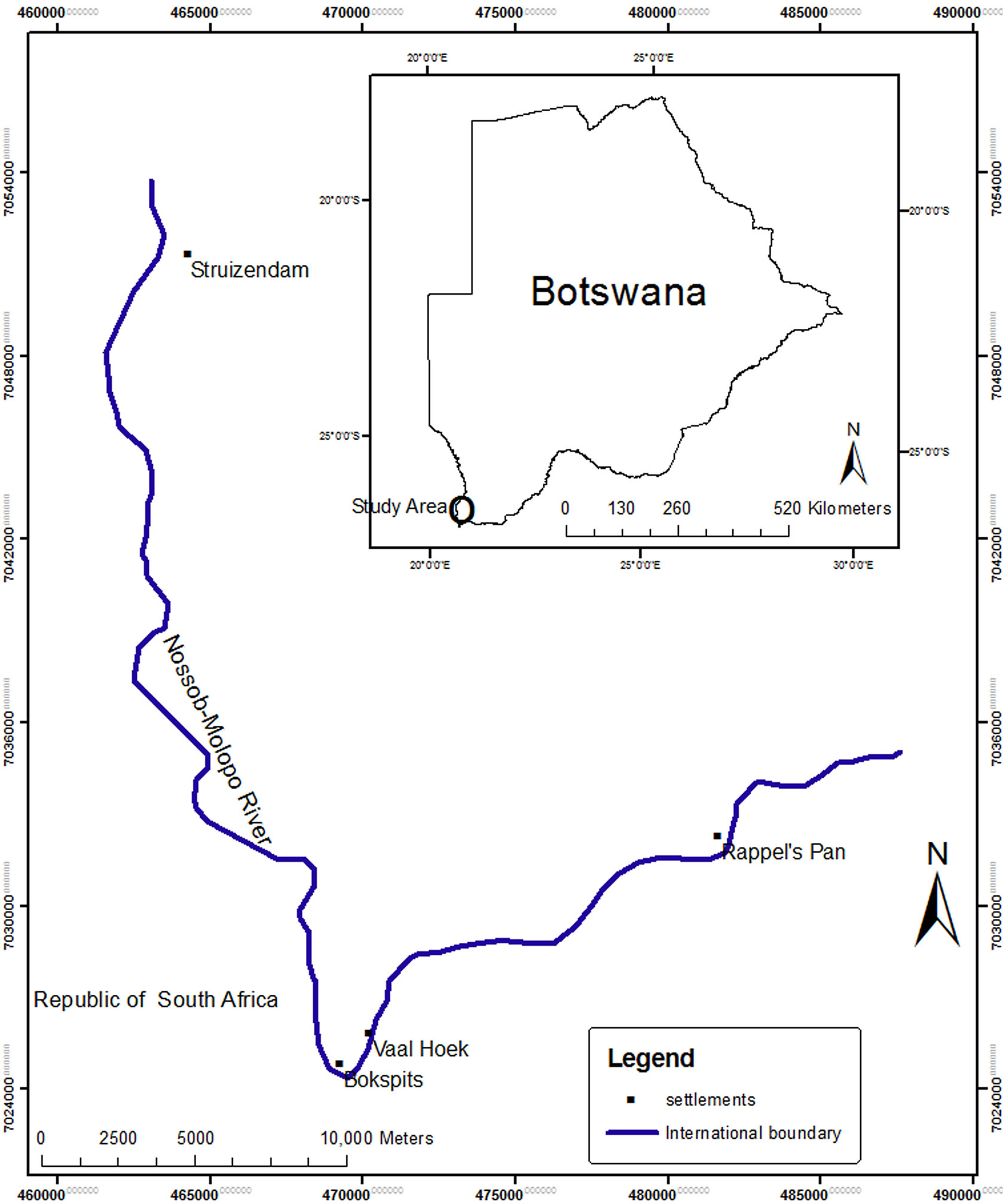
Figure 1. Location of the study site.
(ESRI Inc.) was used to analyse data and produce a map showing the distribution of Prosopis plants in the study area through interpolation process.
2.3. Questionnaire Survey and Focused Group Discussions
Questionnaire survey and focused group discussions were used to investigate the perceptions of the communities about Prosopis species. To seek relevant consent, the research permit issued by the Botswana Government for this study was presented and the objectives of the study were explained to local authorities and all participants. Questionnaires that had multiple answers were administered to 342 people comprising 139 males and 203 females (Table 1). The most predictable answers had been pre-stated, but were not read out to respondents to avoid influencing their opinions. It is generally believed that young members of communities normally lack knowledge on the changes that occur in their environment, hence this study used participants who were at least 20 years old.
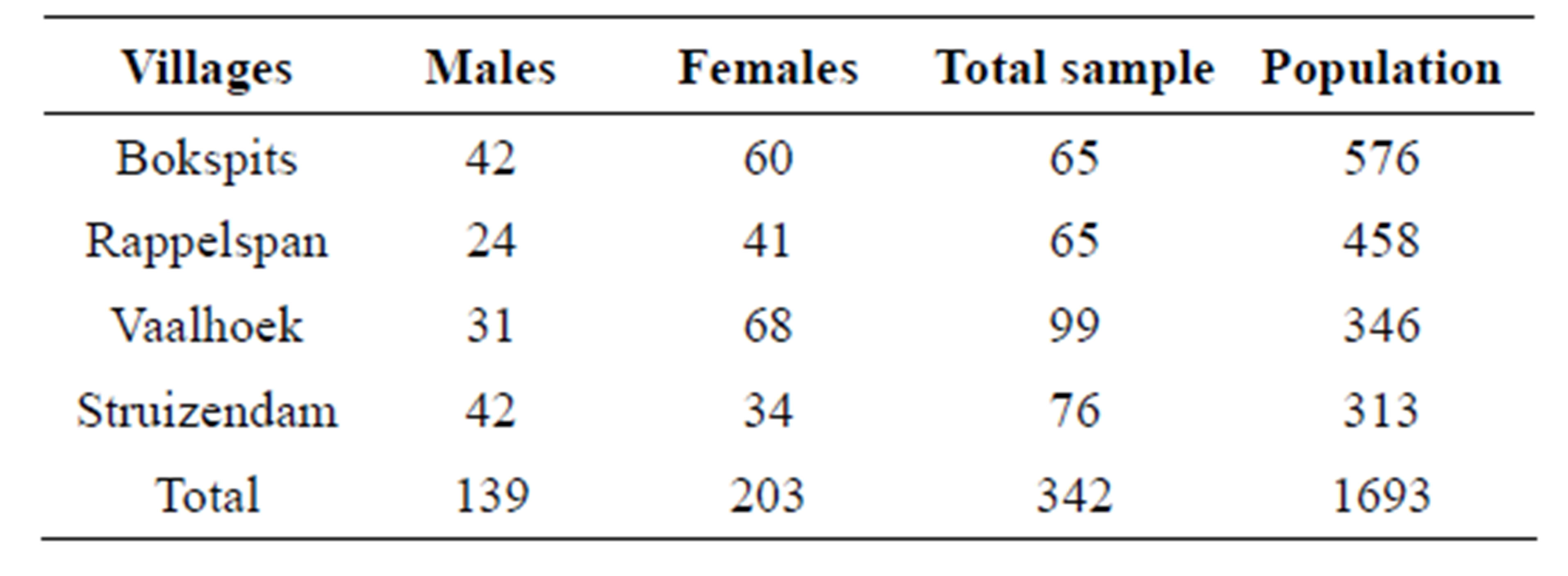
Table 1. Sampling parameters for investigating the perceptions of the communities about Prosopis plants.
Open-ended questions were used in the focused group discussions conducted in the four settlements. In Bokspits, the focused group discussion was attended by the BORAVAST (Bokspits, Rappelspan, Vaalshoek & Struizendam) Trust Community Based Natural Resource Management Committee, the Chiefs and their assistants, headmen, Village Development Committee (VDC) members and some elders of the communities from the four settlements. The Chiefs, VDC members accompanied by some elders of the communities were engaged in the focused group discussions separately held in Struizendam and Vaalshoek. In Rappelspan, the focused group discussion was conducted through a meeting attended by the Chief and his assistant, VDC members and other members of the community. All focused group discussions were facilitated by the researcher to avoid bias toward the perceptions of the most vocal participants as this could compromise data quality. The Predictive Analytics SoftWare (PASW) Statistics 19.0 was used to process data obtained from the interviews and focused group discussions.
3. Results
3.1. The Distribution of Prosopis Species
The invasion of Prosopis species was mostly noticeable in the settlements areas and their surroundings (Figure 2). High density of Prosopis trees was particularly observed around livestock water points (boreholes and wells). Heavily invaded patches that were found outside the settlements corresponded with locations of farms that were not fenced. In the farms, the density of Prosopis plants was high around livestock water points diminishing with increase in distance from water points.
3.2. Questionnaire Survey and Focused Group Discussions
The respondents (78.44%) indicated that Prosopis species were mainly introduced into the study area in the early 1980s by the defunct Department of Forestry which was under the Ministry of Agriculture. They also mentioned that before the Department of Forestry brought Prosopis plants into the area, some individuals in their communities had already began, as early as before and around the 1970s, to bring the plants into the area from Namibia and South Africa. The respondents (94.20%) observed that Prosopis plants were allellopathic.
The introduction of Prosopis plants was initially embraced and the communities interacted with the plants harmonious until the early 1990s when the spread of Prosopis plants reached an alarming rate in the study area. However, the impacts of Prosopis invasion on the livelihoods of the communities became an issue of concern to the respondents (59.9%) around the year 2000. Upon realizing the seriousness of the impacts of the invasion of Prosopis species on their livelihoods, some attempts were made by the communities to eradicate or at least control the spread of Prosopis plants. The respondents (69.07%) indicated that the efforts that were made to arrest the invasion of Prosopis species mainly included pruning and uprooting mature Prosopis plant. Notwithstanding this, the rate at which Prosopis plants invaded the area continued unabated over the years.
The respondents (76.83%) believed that the dispersal of Prosopis seeds by livestock which feed on Prosopis seed pods exacerbated Prosopis invasion. Consequently, 71.30% of the respondents asserted that the invasion of Prosopis species resulted in a decline in the livelihoods of the communities, and 80% of the respondents viewed Prosopis plants as environmental nuisance. Although the respondents generally viewed Prosopis plants as foe, they mentioned that there were some socio-economic benefits such as availability of firewood, timber for fencing and seed pods for livestock feed associated with the spread of Prosopis plants in the area. The views that the respondents expressed against Prosopis species mirrored the notion of other rural communities which inhabit areas affected by Prosopis invasion elsewhere [e.g. 7,10]. The views indicated that feeding of livestock on Prosopis seed pods caused death to livestock, Prosopis plants killed other plant species growing in the study area, depleted underground water resources and have large thorns that caused tyre deflation and injury to people.
The respondents (72.30%) mentioned that efforts to solicit external help concerning the control of the spread of Prosopis plants were made in several occasions by the communities. They further pointed out that the Government incorporated clearing of Prosopis plants in one of the poverty alleviation programmes as a reaction to their appeal for assistance. Prosopis management options identified by the respondents included eradication of Prosopis plants by uprooting all Prosopis plants growing in the area, cutting or pruning of all mature Prosopis plants, selective uprooting of Prosopis plants (uprooting applied to Prosopis trees which grow where they may cause impediment) and the use of a combination of uprooting and
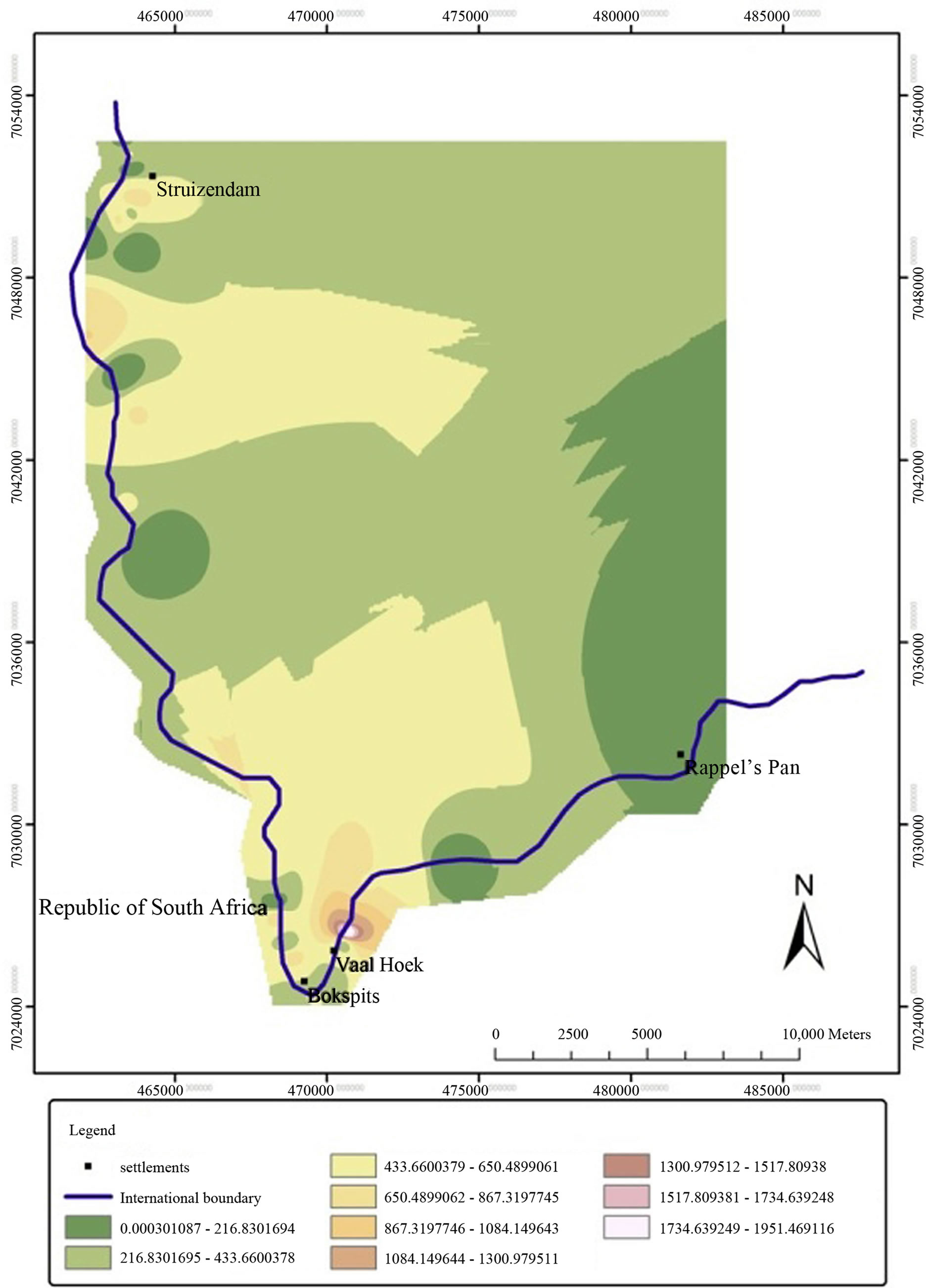
Figure 2. Interpolated distribution of Prosopis species in the study area (Prosopis trees/Ha).
chemical treatment to eliminate regeneration.
The respondents perceived lack of market (77.10%) and low prices for Prosopis derived products as the main challenges constraining the exploitation of the spread of Prosopis plants in the study area. In spite of the challenges faced by the communities in exploiting the spread of Prosopis plants, some respondents (48.98%) mentioned that there were some ways in which the communities could generate income from Prosopis derived products. The respondents who expressed this view identified fire wood harvesting and fodder production as feasible options of community development activities. However, the respondents also indicated that in the absence of external support, the identified and other potential community development options would be negated by limitations such as lack of resources, lack of market and low prices for Prosopis derived products. The respondents (82.16%) expressed their willingness to embrace innovative ideas that could assist them to harness the spread of Prosopis plants.
4. Discussion
4.1. Prosopis Species Distribution
The invasion of Prosopis species mostly affected settlements, farms and livestock water points. This suggested that anthropogenic activities, particularly livestock rearing, significantly influenced the spread of Prosopis plants in the study area. The observation also showed that livestock had a major contribution in the dispersal of Prosopis seeds. Lack of surface water sources and low rainfall in the area appears to be the cause of the concentration of livestock around water points (boreholes and wells) which promoted high rates of Prosopis seeds dispersal and the invasion of Prosopis plants around settlements and farms.
4.2. Socio-Economic Aspects and Perceptions
The respondents expressed the view that the negative socio-economic impacts of Prosopis species outweighed the benefits derived from the species. The explanation of this view was premised upon two theories. The first theory states that the perceptions of people about invasive species are shaped by the economic impacts of the species on their livelihoods [1,23]. The second theory is founded upon micro-economic theory of consumer preferences [22]. It indicates that preferences over commodities are dictated by the characteristics of households, including occupation, proximity to forests, user of the invasive plants, as well as the characteristics of the invasive plants. The communities in the study area were predominantly poor and the improvements that they preferred in their livelihoods were those that could mitigate their poverty such as creation of employment opportunities. Although some respondents mentioned some benefits enjoyed by the communities from Prosopis plants, the invasion of Prosopis species had not addressed the basic needs of the communities in the area. Therefore, the perception that the negative socio-economic impacts of Prosopis species outweighed the benefits derived from the species was considered to be influenced by the belief that Prosopis plants did not produce preferred positive socio-economic impacts on the livelihoods of the communities.
The potential for generation of funds from Prosopis plants was not realised by more than half (51.02%) of the respondents due to their view that the plants had insignificant positive socio-economic benefits. However, it is worth noting that during focused group discussions, reference to some socio-economic benefits derived from Prosopis plants elsewhere stimulated interest among the respondents. Additionally, the willingness of the respondents to accept new ideas of exploiting the spread of Prosopis plants for the improvement of their livelihoods was noted. This suggested that the communities in the study area may change their perceptions about Prosopis plants if the nature of goods and services derived from the plants changes. Even so, lack of essential resources in the communities implied that without external support, the goods and services potentially attainable from Prosopis plants remained impracticable.
The most notable socio-economic benefit of Prosopis according to the respondents was the improvement of the quality and quantity of fodder resources in the study area. In line with this, research has shown that Prosopis seed pods are very nutritious fodder resources which are high in soluble sugars, and contain low concentrations of tannins and other unpleasant chemicals, with moderate to high digestibility [1]. To this end, external support could assist the rural communities to maximize the utilization of Prosopis plants in the production of fodder resources as part of a sustainable means of managing the spread of the species in the area.
4.3. Ecological Aspects and Perceptions
The respondents indicated that the invasion of Prosopis species had reduced plant diversity in the study area. Similar to other areas around the world where there is Prosopis invasion [e.g.1,7,10,11,23], this observation was alluded to the ability of the species to become established over a large area from a few scattered trees and the strong survival characteristics of the species. Although the respondents acknowledged the importance of Prosopis plants as part of fodder resources in the study area, they indicated that they had experienced incidences of death of livestock, particularly donkeys and horses,
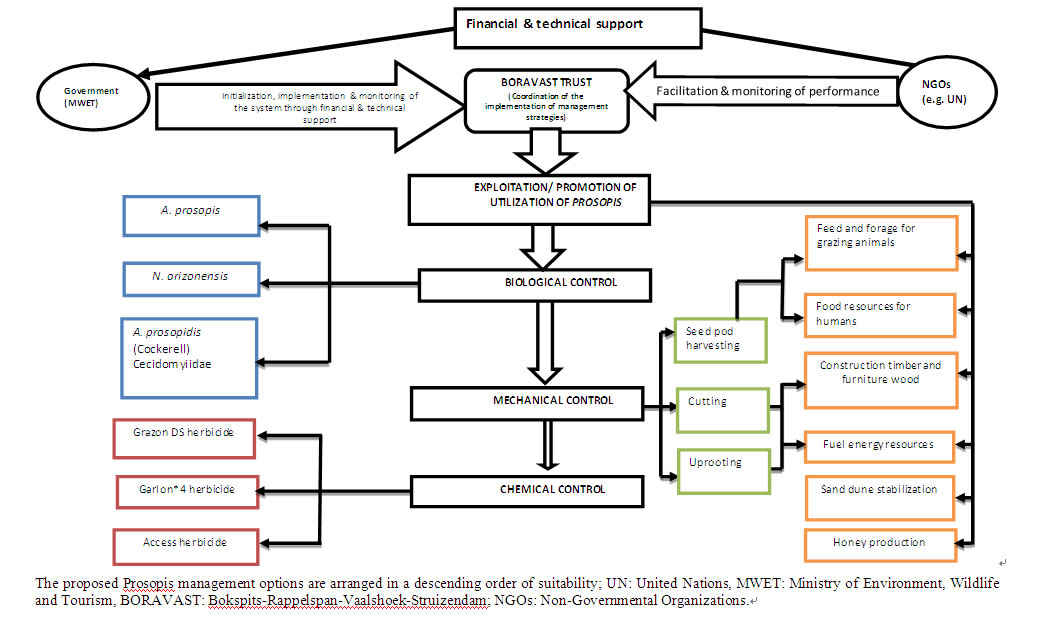
Figure 3. The Integrated Management System for Prosopis species recommended for the study area.
which fed on Prosopis seed pods. The respondents mentioned that the digestive systems of donkeys and horses could not effectively digest the seed pods. Accounts of incidences of death of livestock which fed on Prosopis seed pods have also been recorded elsewhere [1,10,11]. However, [16] observed that the cause of illness and death among livestock that were on an exclusively Prosopis-based diet was due to ruminal impaction caused by Prosopis seeds which had been insufficiently digested. Contrary to the perceptions of the respondents, no correlation between the ability of an animal to digest Prosopis seeds and the resilience of the animal towards ruminal impaction was found in other studies [e.g. 16,28-30]. The quantity of Prosopis seeds consumed by an animal was found to be a crucial factor that determined the effects of the seeds on the animal’s health [16, 28-30]. Free range farming was the most common pastoral farming system practiced in the study area. As a result, farmers were not in control of the amount of Prosopis seed pods consumed by livestock within a given period of time. Therefore, the deaths of livestock that fed on Prosopis seed pods reported in the study area may possibly be linked to excessive and uncontrolled consumption of Prosopis seed pods.
Respondents considered donkeys and horses as the main animals that contributed significantly in the dispersal of Prosopis seeds as they observed that the seeds normally traversed the digestive systems of donkeys and horses without damage. Elsewhere, it was observed that 82% of Prosopis seed germinated after passing through horses, 69% through cattle and 25% through sheep [31]. However, this study recommended crushing of Prosopis seed pods before their use for livestock feed to minimise seed dispersal.
4.4. Perceptions and Management Options
Various control approaches have been recommended as solutions to Prosopis invasion from a range of quarters around the world. The approaches are basically dichotomous in nature. One category focuses on eradication, while the other centres on management of the species. Eradication approaches entail systematic control of Prosopis plant population that leads to the elimination of Prosopis species from a particular area [32]. Time is a critical factor in this method. Otherwise, eradication approaches may tend to be management approaches if conducted indefinitely. Management approaches involve systematic and sustainable containment of Prosopis plant population [10,33]. Containment reduces the rate of the spread of the plant species [33]. It is on this background that the perceptions of the respondents were considered in relation to the management options of the spread of Prosopis plants in the study area.
The respondents identified eradication approaches as their preferred option of controlling the spread of Prosopis plants. However, the implementation of eradication approaches on Prosopis plants have failed in other areas around the world. For example, campaigns to implement eradication methods on Prosopis plants were initiated in 1995 in Sudan following a declaration for eradication of the species by the Sudanese President [10,11]. The eradication process was undertaken at the expense of millions of US Dollars [10,11]. Notwithstanding the high costs of the eradication project, the rate of success was relatively insignificant even when Prosopis plants were uprooted [10,11]. Furthermore, experiences from America, Asia, Australia and South Africa indicated that eradication of Prosopis plants was costly, difficult and often impracticable [34,35]. Eradication of Prosopis plants is also complicated by the long dormancy period (up to 10 years) of their seeds in the soil seed bank which normally germinate immensely under environmental disturbance [34]. Therefore, eradication approaches were considered unsuitable option for the control of the spread of Prosopis plants in the study area. At best, eradication methods could be applied as supplementary method to other potential management approaches (see Section 4.5).
4.5. Potential Prosopis Management Options
Different studies have shown that the war against the invasion of Prosopis would not be won by engaging a single management approach [10,34]. Therefore, the establishment of a sustainable management system for Prosopis species in the study area requires consideration of various management approaches that have been implemented with success to address the invasion of Prosopis in other countries like Argentina, Kenya, India and the Republic of South Africa [e.g. 1,7,36-38]. It is on this basis that the integrated management model (Figure 3) in which possible management options are implemented on a case based approach to control the spread of Prosopis plants was recommended for the study area. Not only this, but the model was also considered applicable in other areas where the invasion of Prosopis specis was a cause for concern in the Kgalagadi region. The model emphasizes the essence of external support to the rural communities toward sustainable natural resources management.
It is important to note that in cases where the need to apply biological control methods arises, the selection of insects to be used should be confined to those destroying seeds only, as seeds are considered key attribute of Prosopis invasiveness [35]. In addition, host specificity of insects applicable to Prosopis species growing in the study area is another critical aspect in biological control methods that warrants consideration. The focus on host specific seed-feeding insects aims to address issues related to unplanned destruction of the useful non-seed Prosopis properties and also promote sustainability. Worth noting is the observation that the costs of herbicides, potential soil pollution, possible poisoning of livestock and other undesirable consequences associated with chemicals [34] rendered chemical control methods as the least recommended method of controlling the spread of Prosopis plants in the study area.
5. Conclusion
The purpose of this study was to determine the extent to which Prosopis species had invaded four settlements in the Kgalagadi area south west of Botswana, investigate the perceptions of the communities about the existence of the species in their environments and assess possible control options for the spread of Prosopis plants in the area. The study has shown that the invasion of Prosopis was prominent in settlements areas and their surroundings. Thus, it was concluded that anthropogenic activities significantly influenced the spread and spatial distribution of Prosopis plants in the study area. Additionally, it was observed that the perceptions of the communities about Prosopis plants were moulded by the impacts of the species on their livelihoods as well as their microeconomic background. As a result, rural communities viewed Prosopis plants as environmental nuisance because the plants lacked desired influence over their livelihoods. This study highlighted the need for external support to facilitate systematic and sustainable control of Prosopis species in the study area. Eradication as a single method of controlling the spread of Prosopis plants in the study area was considered impracticable. Instead, this study recommended an integrated management approach in which potential methods of controlling the spread of Prosopis plants are implemented on a case-based system. The study also recommended that in the implementation of the recommended model, socio-economic benefits associated with Prosopis plants should be promoted to foster sustainability in community development and natural resources management.
REFERENCES
- N. M. Pasiecznik, “The Prosopis juliflora-Prosopis pallida Complex: A Monograph,” HDRA, Coventry, 2001.
- A. Burkart, “A Monograph of the Genus Prosopis (Leguminosae sub-fam. Mimosoideae). (Part 1 and 2). Catalogue of the Recognized Species of Prosopis,” Journal of the Arnold Arboretum, Vol. 57, 1976, pp. 219-249.
- N. M. Pasiecznik, “Prosopis: Pest or Providence, Weed or Wonder Tree?” European Tropical Forest Research Network Newsletter, Vol. 28, 1999, pp. 12-14.
- J. Timberlake, “Handbook of Botswana Acacias,” Ministry of Agriculture, Gaborone, 1980.
- M. A. El Fadl and O. Luukkanen, “Effect of Pruning on Prosopis juliflora: Considerations for Tropical Dryland Agroforerstry,” Journal of Arid Environments, Vol. 53, No. 4, 2003, pp. 441- 455. http://dx.doi.org/10.1006/jare.2002.1069
- Varshney, “Overview of the Use of Prosopis juliflora for Livestock Feed, Gum, Honey, and Charcoal, As Well As Incombating Drought and Desertification: A Regional Case Studyfrom Gujarat, India,” In: P. Felker and J. Moss, Eds., Prosopis: Semiarid Fuelwood and Forage Tree; Building Consensus for the Disenfranchised, Center for Semi-Arid Forest Resources, Kingsville, 1996, pp. 6.35- 6.4
- E. Mwangi and B. Swallow, “Invasion of Prosopis juliflora and Local Livelihoods: Case Study from the Lake Baringo Area of Kenya,” ICRAF Working Paper No. 3, World Agroforestry Centre, Nairobi, 2005.
- Centre for Applied Research, “Review of Institutional and Legal Arrangements for Community-Based Management of Rangelands in Botswana,” IVPBOT04/020, Botswana, 2004.
- A. Burkart and B. B. Simpson, “The Genus Prosopis and Annoted Key to the Species of the World,” In: B. B. Simpson, Ed., Mesquite: Its Biology in Two Desert Ecosystems, Hutchinson and Ross, Dowden, 1977, pp. 201- 215.
- H. Bokrezion, “The Ecological and Socio-Economic Role of Prosopis juliflora in Eritrea; An Analytical Assessment within the Context of Rural Development in the Horn of Africa,” Ph.D. Thesis, University of Mainz, Mainz, 2008.
- J. Laxén, “Is Prosopis a Curse or a Blessing?—An Ecological-Economic Analysis of an Invasive Alien Tree Species in Sudan,” Viiki Tropical Resources Institute, Finland, 2005.
- T. Mutsambiwa, G. E. Ali and I. H. ElTahir, “Community Forestry Project,” UNEP Evaluation Mission Report, Sudan, 1998.
- M. A. El Fadl, “Management of Prosopis juliflora for Use in Agroforestry Systems in the Sudan,” Tropical Forestry Reports 16, University of Helsinki, Helsinki, 1997.
- V. Muthaiya and P. Felker, “Influence of Phosphorus and Silviculture Treatments on Leaf and Soil Nitrogen and Phosphorus Concentrations in a Mature Prosopis glandulosa (Mesquite) Stand,” Journal of Arid Environments, Vol. 35, No. 3,1997, pp. 487-498. http://dx.doi.org/10.1006/jare.1995.0152
- P. Felker and J. Moss, Eds., “Prosopis: Semiarid Fuelwood and Forage Tree; Building Consensus for the Disenfranchised,” Center for Semi-Arid Forest Resources, Kingsville, 1996.
- A. I. Abdel Gaabar, “1. Proximate Composition of Mesquite Prosopis chilensis (Mollina) Stuntz Pods, Seeds and Leaves and 2. Digestibility Trials,” Pamphlet No. 2, Prosopis Project, Sudan, Forestry Research Centre, 1986, p. 10.
- P. Felker, “Economic, Environmental and Social Advantages of Intensively Managed Short Rotation Mesquite (Prosopis spp.) Biomass Energy Farms,” Biomass, Vol. 5, No. 1, 1984, pp. 65-77. http://dx.doi.org/10.1016/0144-4565(84)90070-2
- O. P. Vimal and P. D. Tyagi, “Prosopis juliflora: Chemistry and Utilization,” In: V. J. Patel, Ed., The Role of Prosopis in Wasteland Development, Agroforestry Center, Gujarat, 1986, pp. OVP1-OVP8.
- J. Doat, “Tannins in Tropical Woods,” Bois et Forêts des Tropiques, Vol. 182, 1978, pp. 37-54.
- H. D. Neuwinger, “African Ethnobotany: Poisons and Drugs,” Chapman and Hall, London, 1996.
- J. Foster and L. A. Sandberg, “Friend or Foe? Invasive Species and Public Green Space in Toronto,” Geographical Review, Vol. 94, No. 2, 2004, pp. 178-198. http://dx.doi.org/10.1111/j.1931-0846.2004.tb00166.x
- K. R. Rai, H. Scarborough, N. Subedi and B. Lamichhane, “Invasive Plants: Do They Devastate or Diversify Rural Livelihoods? Rural Farmers’ Perceptions of Three Invasive Plants in Nepal,” Journal for Nature Conservation, Vol. 20, No. 3, 2012, pp. 170-176. http://dx.doi.org/10.1016/j.jnc.2012.01.003
- P. Binggeli, “The Human Dimension of Invasive Woody Plants,” In: J. A. McNeely, Ed., The Great ReshufflingHuman Dimensions of Invasive Alien Species, IUCN, Gland, 2001, pp.145-159.
- M. Muzila, M. P. Setshogo, B. Moseki and R. Morapedi, “An Assessment of Prosopis L. in the Bokspits Area, South-Western Botswana, Based on Morphology,” The African Journal of Plant Science and Biotechnology, Vol. 5, 2011, pp. 75-80.
- Y. P. R. Bhalotra, “Rainfall Maps of Botswana,” Department of Meteorological Services, Gaborone, 1985.
- R. B. Lee and I. Devore, “Kalahari Hunter-Gatherers: Studies of the Kung San and their Neighbours,” Harvard University Press, Cambridge, 1976.
- P. Devitt, “Man and His Environment in Western Kalahari or a Little Technology Is Dangerous Thing,” Botswana Notes and Records, Vol. 3, 1971, pp. 50-56.
- I. M. Tabosa, J. C. Souza, D. L. Graca, J. M. Barbosa Filho, R. N. Almeida and F. Riet-Correa, “Neuronal Vacuolation of the Trigeminal Nuclei in Goats Caused by Ingestion of Prosopis juliflora Pods,” Veterinary and Humum Toxicology, Vol. 42, 2000, pp. 155-158.
- M. Mahgoub, T. Isam, I. T. Kadim, N. E. Forsberg, D. S. Al-Ajmi, N. M. Al-Saqry, A. S. Al-Abri and K. Annamalai, “Evaluation of Meskit (Prosopis juliflora) Pods as a Feed for Goats,” Animal Feed Science and Technology, Vol. 121, No. 3, 2005, pp. 319-327. http://dx.doi.org/10.1016/j.anifeedsci.2005.01.016
- O. Mahgoub, I. T. Kadim, E. H. Johnson, A. Srikandakumar, N. M. Al-saqri, A. S. Al-abri and A. Ritchie, “The Use of a Concentrate Containing Meskit (Prosopis juliflora) Pods and Date Palm By-Products to Replacecommercial Concentrate in Diets of Omani Sheep,” Animal Feed Science and Technology, Vol. 120, No. 1, 2005, pp. 33-41. http://dx.doi.org/10.1016/j.anifeedsci.2005.01.011
- C. E. Fisher, C. H. Meadors, R. Behrens, E. D. Robinson, P. T. Marion and H. L. Morton, “Control of Mesquite on Grazing Lands,” Texas Agricultural Experiment Bulletin, Vol. 935, 1959, pp. 1-24.
- R. H. Groves and F. D. Panetta, “Some General Principles for Weed Eradication Programs,” In: H. Spafford Jacob, J. Dodd and J. H. Moore, Eds., 13th Australian Weeds Conference: Papers and Proceedings, Plant Protection Society of WA Inc., Perth, 2002, pp. 307-310.
- A. A. Sharov and A. M. Liebhold, “Bioeconomics of the Managing the Spread of Exotic Pest Species with Barrier Zones,” Ecological Application, Vol. 8, No. 3, 1998, pp. 833-845.
- S. Csurhes, “Mesquite (Prosopis spp.) in Queensland – Pest Status Review Series,” Department of Natural resources and Mines, Queensland, 1996.
- H. G. Zimmermann and N. M. Pasiecznik, “Realistic Approaches to the Management of Prosopis Species in South Africa,” HDRA, Coventry, 2005.
- J. H. Hoffmann, F. A. C. Impson and V. C. Moran, “Competitive Interactions between Two Bruchid Species (Algarobius spp.) Introduced into South Africa for Biological Control of Mesquite Weeds (Prosopis spp.),” Biological Control, Vol. 3, No. 3, 1993, pp 215-220. http://dx.doi.org/10.1006/bcon.1993.1030
- J. H. Hoffmann, F. A. C. Impson and V. C. Moran, “Biological Control of Mesquite Weeds in South Africa Using a Seed-Feeding Bruchid, Algarobius prosopis: Initial Levels of Interference by Native Parasitoids,” Biological Control, Vol. 3, No. 1, 1993, pp. 17-21. http://dx.doi.org/10.1006/bcon.1993.1003
- W. Coetzer and J. H. Hoffmann, “Establishment of Neltumius arizonensis (Coleoptera: Bruchidae) on Mesquite (Prosopis Species: Mimosaceae) in South Africa,” Biological Control, Vol. 10, No. 3, 1997, pp. 187-192. http://dx.doi.org/10.1006/bcon.1997.0558
NOTES
*Corresponding author.

