World Journal of Cardiovascular Diseases
Vol.4 No.1(2014), Article ID:42118,9 pages DOI:10.4236/wjcd.2014.41004
Involvement of TLR2 and TLR4, Chlamydophila pneumoniae and Mycoplasma pneumoniae in adventitial inflammation of aortic atherosclerotic aneurysm
1Faculty of Pharmaceutical Sciences, University of São Paulo, São Paulo, SP, Brazil
2Heart Institute of Clinical Hospital, Medical School, University of São Paulo, São Paulo, SP, Brazil
Email:*anplourdes@incor.usp.br
Copyright © 2014 Renata Melo de Assis et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Renata Melo de Assis et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 1 December 2013; revised 29 December 2013; accepted 16 January 2014
ABSTRACT
Aortic atherosclerotic aneurysm (AAA) is associated with adventitial inflammation where infection is suggested to have a role. Co-infection with Chlamydophila pneumoniae (Cp) and Mycoplasma pneumoniae (Mp) was linked with coronary plaque rupture, in association with vessel dilatation and adventitial inflammation. Pathogens are recognized by Toll-like receptors (TLRs) development of the inflammatory process. Objective: Here, we studied whether co-infection by Cp and Mp was involved in the increased inflammation present in AAA and if it could be associated with deficient expression of TLRs. We compared human samples of AAA with non-dilated human aortic atherosclerotic lesions, regarding the amount of Cp and Mp antigens, and expression of TLR2 and TLR4. Methods: Two groups of aorta fragments were analyzed: G1 (n = 13) moderate atherosclerosis and G2 (n = 14) AAA samples, through immunohistochemistry and in situ hybridization methods. Results: Mp and Cp antigens in intima/medial layer were greater in G2 than G1, with no difference in adventitia. TLR2 and TLR4 were higher in G2 than G1 adventitia fat. There was a correlation between Mp versus TLR2 and of TLR4 in intima/medial layer and in adventitia of G1, but there was a lack of correlation in G2. In Cp adventitia, the correlation in G1 was high with TLR2 but not with TLR4, and in G2 the correlation was positive for both TLRs. Conclusion: This study favors the concept that symbiotic co-infection by Cp and Mp participates in the pathogenesis of AAA. It also emphasizes that adventitial fat is the initial site for colonization of these bacteria that probably reach the tissue through vasa vasorum. An exacerbated immune reaction is not efficient to control the infection that reaches and proliferates in high levels at the medial and intimal layer, contributing to the development of vessel dilatation.
KEYWORDS
Aortic Atherosclerotic Aneurysm; Inflammation; Co-Infection; Chlamydophila pneumoniae; Mycoplasma pneumoniae; Toll-Like Receptors
1. INTRODUCTION
Aortic atherosclerotic aneurysm is a complication of the atherosclerotic process, presenting an increased adventitial inflammation associated with metaloproteases in the media [1]. Inflammation participates in atherosclerosis at the onset of its development and is increased when the atheroma plaque ruptures, with thrombotic complications. The risk factors for atherosclerosis (e.g., a diet that is high in saturated fat, smoking, hypertension, hyperglycemia, obesity and insulin resistance) may trigger the expression of adhesion molecules in endothelial cells [2], but the chronic inflammation, mainly located at the outer aortic wall has been suggested to be linked to the presence of infectious agents [3]. Microbes might initiate the atherosclerotic process through the injury of vascular endothelium either directly by invasion of the vessel or indirectly through the release of lipopolysaccharides (LPS); moreover, microbes can accelerate atherosclerosis through an increased recruitment of inflammatory cells (e.g., macrophages and T lymphocytes) to pre-existing lesions, thereby stimulating the expression of adhesion molecules and the production of proinflammatory cytokines [4].
Chlamydophila pneumoniae (Cp) is the most widely studied microbe in atherosclerosis and can pass from the lung to the atheroma via circulating monocytes [5]. Cp DNA, but not Cp mRNA, has been found in aortic aneurysm samples [6]. Chlamydial heat shock protein 60 (CHSP-60) was found in human plaques in association with cytokines and metaloproteases [7], and intracellular oxidation of LDL [4] and was therefore considered a primary mediator of atherogenesis. The lack of success in antibiotic clinical trials has weakened the infectious theory of the development of atherosclerosis and its complications. The possibility that a co-infection between Cp and Mycoplasma pneumoniae (Mp) in progression for vulnerable plaques has been proposed, thus perhaps explaining the resistance to antibiotics [8]. The vulnerable plaques are associated with dilatation of the segment, which has high adventitial inflammation [9]. Also clinical studies have shown increased seropositivity to both Mp and Cp [10,11]. Inoculation of Cp and Mp in ApoE KO mice fed with cholesterol enriched diet caused aggravation of the size atherosclerotic plaques, inflammation or vessel remodeling [12]. In a recent work, we found higher amounts of both Cp and Mp in the adventitia of AAA than in severe aortic non-dilated atherosclerosis [13]. These data favor the concept that co-infection by Cp and Mp co-infection may aggravate aortic atherosclerosis leading to the development of AAA.
In the present work we have evaluated if the presence of AAA is related with different co-proliferation profiles of both bacteria (Cp and Mp) and the probability of lack in the expression of toll-like-receptors (TLRs). TLRs, a family of trans-membrane receptor proteins, can have the ability to activate antigen-presenting cells (APCs) as a sensor to eradicate pathogens through innate immunity [14]. TLRs can lead to the activation of nuclear factor kappa B (NF-kB) and to the production of proinflammatory cytokines and the expression of costimulatory molecules, thus resulting in the induction of acquired immunity. Elevated expression of both TLR4, a receptor for gram-negative bacterial LPS, and TLR2 was observed in Cp-infected foam macrophages in human atherosclerotic plaques [15]. TLR2 and TLR4 can promote the formation of foam cells, which can serve as a marker of early atherosclerosis in the presence of LDL [16]. TLR2 is also activated by dipalmitoylated lipoproteins from Mp [17], and palmitic acid is linked to the development of atherosclerosis [18].
In an attempt to clarify whether co-infection by Cp and Mp is involved in the increased inflammation present in AAA and if it could be associated with deficient expression of TLRs, we compare human samples of AAA with non-dilated human aortic atherosclerotic lesions, regarding the amount of Cp and Mp antigens, and expression of TLR2 and TLR4.
2. MATERIALS AND METHODS
2.1. Casuistic
Two groups of Atherosclerotic tissue were obtained from patients submitted to surgery: Group 1 (G1): tissue from coronary artery bypass surgery: 13 fragments of ascending aortas with mild/ moderate atherosclerosis and Group 2 (G2): 14 fragments from atherosclerotic aneurysms of thoracic or abdominal aortas that were removed during surgery to repair the aneurysm. This study was approved by the Ethics Committee in Human Research of the Faculty of Pharmaceutical Sciences of University of São Paulo (FCF/USP) and by the Scientific and Ethics Committee of InCor/FMUSP (Heart Institute of the Faculty of Medicine in the University of São Paulo).
2.2. Tissues and Biological Samples
After sampling, the fragments were placed into sterile tubes and divided into two equal halves. One portion was stored in a freezer at −70˚C, and the other portion was fixed in 10% buffered formalin and embedded in paraffin. micrometer serial frozen and paraffin sections were obtained for immunohistochemistry and in situ hybridization as described previously and briefly described below [8].
2.3. Immunohistochemistry (IHC)
The immunoperoxidase reaction used the following reagents: 1) a monoclonal anti-human antibody that was produced in mice against TLR2 (Abcam, Massachusetts, USA); Cp (Dako, California, USA) and Mp (Fitzgerald Industries International, Massachusetts, USA); 2) a polyclonal anti-human antibody that was produced in rabbits against TLR4 (Abcam, Massachusetts, USA). aAntigen retrieval was performed in Pascal (Dako, California, USA) with Tris-EDTA (pH 9.0) and Tween-20 (0.1%). The Envision System Labeled Polymer-HRP (Dako, California, USA) was used to stain the antigens C. pneumoniae and M. pneumoniae and Kit LSAB System-HRP (Dako, Califórnia, EUA) for another antibody. Diaminobenzidine (DAKO, California, USA) was used for detection. Tonsil fragments were used as a positive control for the TLRs. Lung and aorta fragments were used as positive controls for M. pneumoniae and C. pneumoniae respectively. As a negative control, the primary antibody was omitted.
2.4. In Situ Hybridization (ISH)
The probes sequences were based in GenBank database (www.ncbi.nlm.nih.gov) for the respective genes TLR2 (NM_003264.3), TLR4 (NM_138554.2). The initial concentration of 200 ng/ml was used as a stock solution (Bioneer Corporation, Daejeon, South Korea). The slides were incubated with their respective probes at 60˚C overnight. The Genpoint Kit (Dako, California, USA) was used for signal amplification, and the reaction was revealed with diaminobenzidine (DAKO, Califórnia, EUA). The mRNA positive control was a sequence antisense mRNA, whereas the sense mRNA sequence served as the negative control.
2.5. Analysis of IHC and ISH
The IHC and ISH immunostained sections had the images captured using the Scanscope CS System (Aperio Technologies, Inc., USA), in an objective with 20×/0.75. The positivity was identified as brown spots. Quantification of C. pneumoniae and M. pneumoniae antigens was made by the % area positive for each antigen, using the Aperio Image Scope Analysis System. TLR2 and TLR4 were quantified with the Aperio’s program for detection of positive nuclei/mm2. As AAA samples do not have well defined intima and medial layers, the comparisons were made between two regions: intima/medial versus adventitial layers.
2.6. Statistical Analysis
All of the data were expressed as the mean values and standard deviations. The variables within the groups were compared using Student’s t-test or Mann-Whitney Rank Sum Test to determine possible differences between groups and Paired t-test or Wilcoxon Signed Rank test were used to compare between Mp, Cp, TLR2 and TLR4 in fat adventitia layer versus intima/medial layer. Pearson correlation was used to verify between possible correlations between infectious agents versus TLRs.
The Statistical analysis was obtained using the Sigma Stat software, version 3.11 (Sigma Stat for Windows, Stat Software Inc, San Jose, CA). Differences were significant when the P values were < 0.05.
3. RESULTS
All 27 samples (G1 + G2) were positive for Cp and Mp antigens. The adventitial inflammation in aneurysm group was located mainly in the fat tissue. Then, this was the region analyzed at the adventitia. Comparison between G1 and G2 is demonstrated in Table 1.
The mean numbers of cells positive for TLR2 and TLR4/mm2 and the percentage area positive for the infectious antigens Mp and Cp, in G1 (mild atherosclerosis) and G2 (aneurysm) at the intima/media layer and fat adventitia are shown in Table 1.
3.1. Comparing G1 vs G2
Mp and Cp values in intima/medial layer were greater in G2 than G1 (respectively P = 0.007 and P = 0.012) with no difference in adventitia. (P = 0.6 and P = 0.98). There was no difference in intima/medial layer between G1 and G2 regarding TLR2 and TLR4 values (P = 0.07 and P = 0.27 respectively), but TLR2 and TLR4 were higher in G2 than G1 in fat adventitia layer (P < 0.001 and 0.01, respectively) (see Figures 1 and 2).
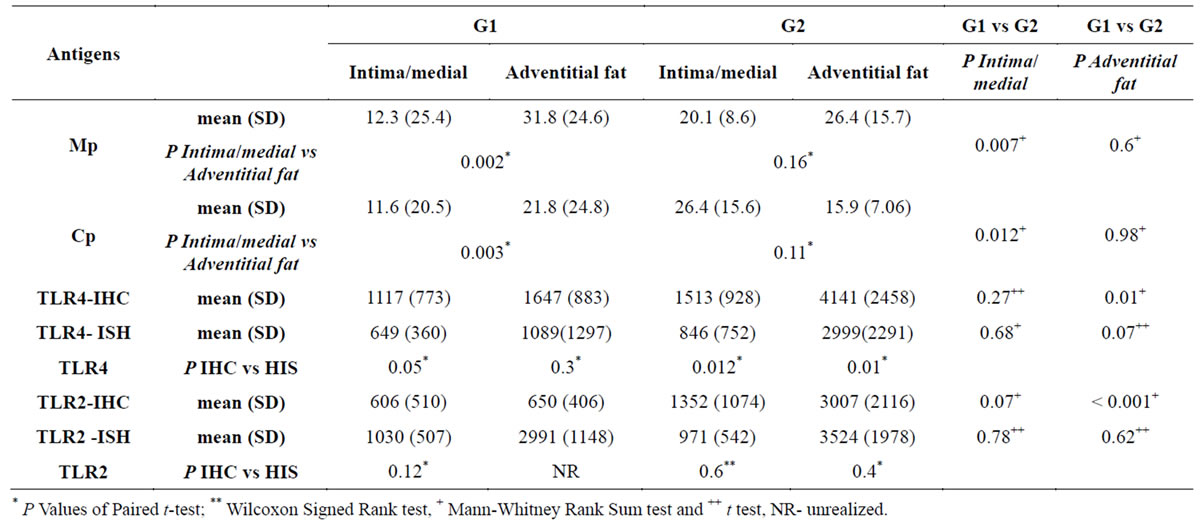
Table 1. Mean (SD) P values in the comparison of antigens and Intima/Medial and Adventitial fat by Immunohistochemistry (IHC) and in situ hybridization (ISH) and also between groups G1 vs G2.
* P Values of Paired t-test; ** Wilcoxon Signed Rank test, + Mann-Whitney Rank Sum test and ++ t test, NRunrealized.
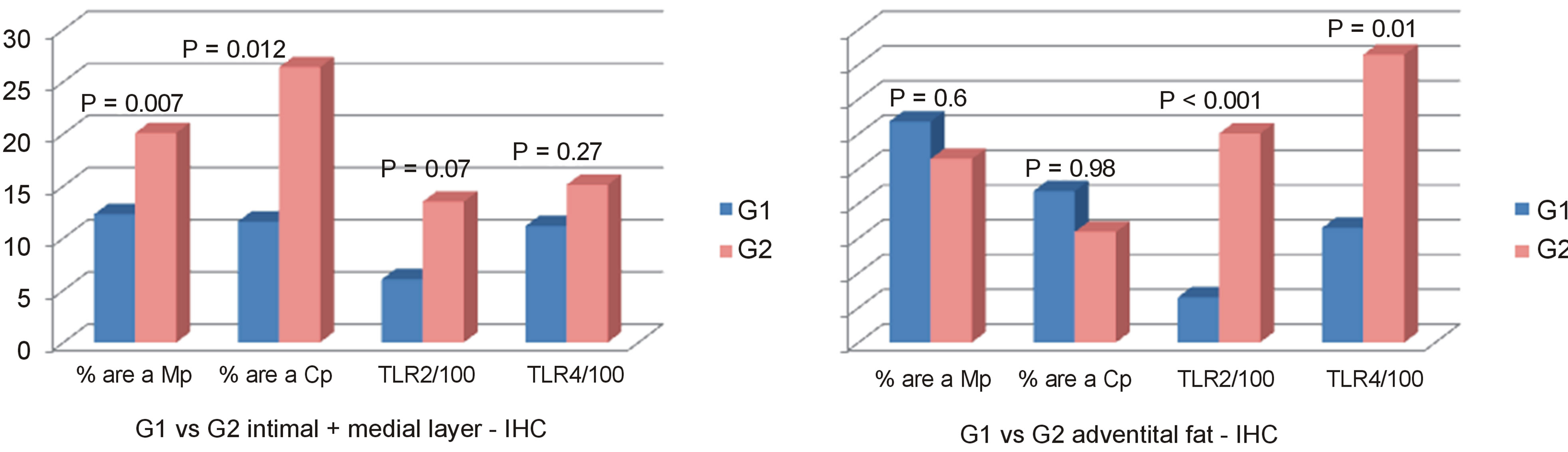
Figure 1. Mean values of % positive areas for Mp and Cp antigens, and mean numbers (/100)/mm2 of TLR2 and TLR4 antigens and DNA positive cells in the intima + medial layers and adventitial fat in groups atherosclerosis (G1) and aneurysm (G2).
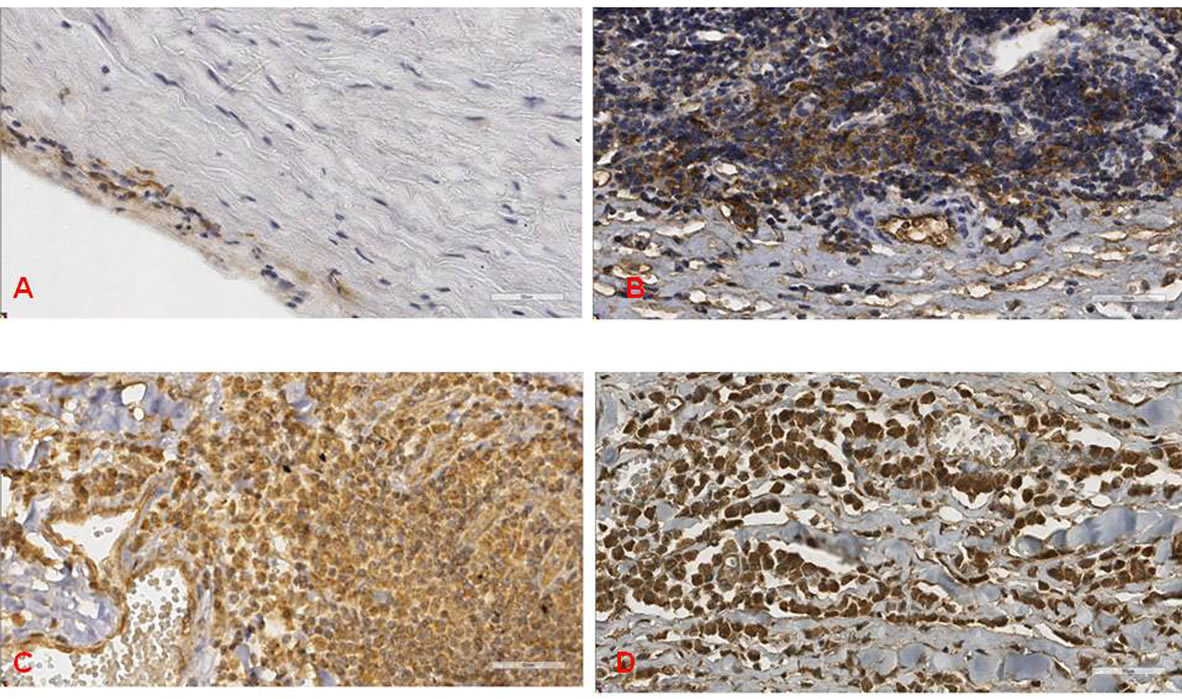
Figure 2. Shows some examples of the immunohistochemical reactions against infectious agent and TLR antigens. A) C. pneumoniae at the intima of G1 case; B) M. pneumoniae at the adventitia of G2 case; C) and D) showing a G2 case with increased numbers of TLR2 and TLR4 at the adventitia, respectively, (scale bar = 50 mm).
3.2. Comparing Adventitial with Intima/Medial Layer
The comparison between Mp in fat adventitia versus intima/medial layer showed higher values of Mp adventitial fat in G1 (P = 0.002), but not in G2 (P = 0.16). Also, there was a significant higher amount of Cp in fat adventitia than in intima/medial layers of G1 (P = 0.003) but not of G2 (P = 0.11) (Table 1).
3.3. Correlations between Infectious Agents versus TLRs
There was a positive correlation between Mp values versus mean numbers of TLR2 and of TLR4 in intima/medial layer and in adventitia of G1, but lack of correlation in G2. The correlation was also very high regarding Cp and TLR2 and TLR4 at intima/medial layer of G1 but not with TLR4 in G2. In Cp adventitia, the correlation in G1 was high with TLR2 but not with TLR4, and in G2 the correlation was positive for both TLRs. There was a positive correlation between Mp and Cp in G1, in all layers, and weaker correlation in G2 (Table 2). A strong correlation between ISH and IHC expression occurred with TLR4 mainly in adventitial layer of G2 group (P < 0.001) (Table 3).
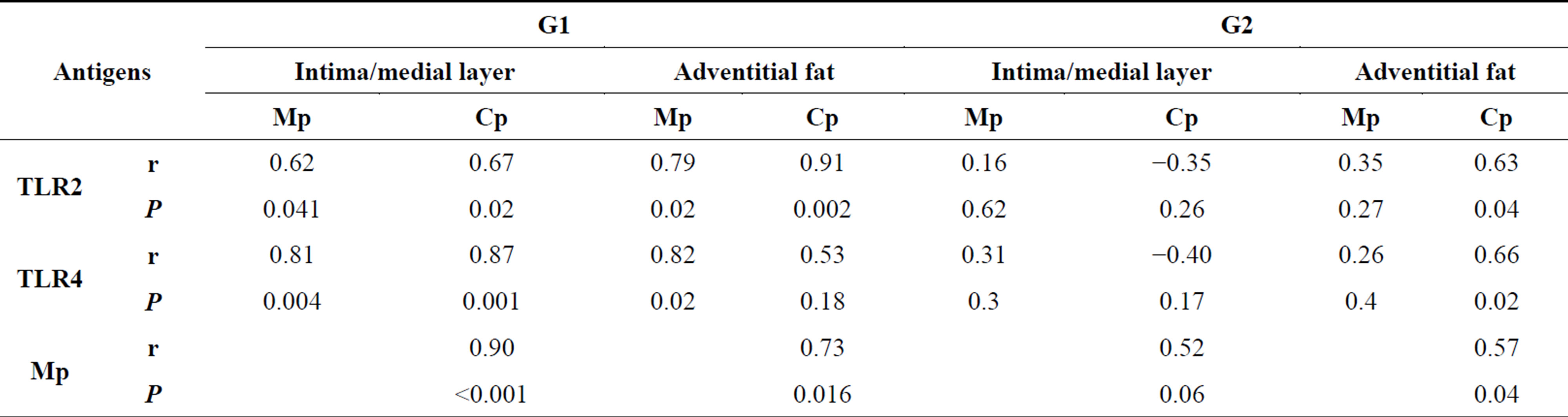
Table 2. Comparison of antigens TLR2, TLR4, Cp and Mp in intima/medial and Adventitial fat layer and between groups (r = Correlation Coefficient, P = value) of Pearson Correlation test.
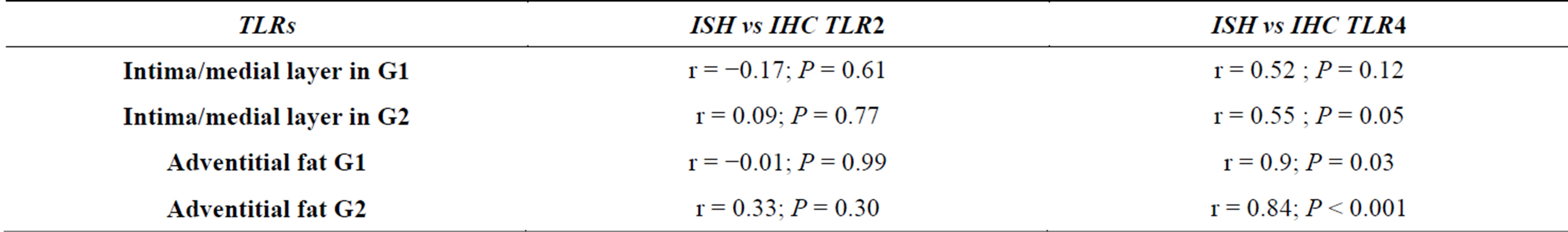
Table 3. Comparison between TLRs values of ISH versus IHC techniques in intima/media and adventitial fat layers.
4. DISCUSSION
Aortic atherosclerotic aneurysm (AAA) is a life threatening condition associated with significant morbidity and mortality. The half-marks in the pathogenesis of aortic aneurysms are destruction of elastin and collagen combined with adventitial inflammation, however their etiology are complex and multifactorial [19]. Infections with bacteria such as C. pneumoniae are proposed to trigger the secretion of inflammatory cytokines that leads to atherogenesis with activation of TLRs- [20-22]. However, different techniques have yielded different rates of infection in atherosclerotic lesions. Atherosclerotic arteries were negative for Cp in PCR studies, and IHC positivity was interpreted by the authors as an artifact [23]. In aorta, Cp antigens were found in 100% in stenotic aortic valves [24], 33% - 100% in atherosclerotic aortas [25,26]. Cp DNA was detected in ascending atherosclerotic aortas in 30% and 50% of cases using real-time PCR and nested PCR, respectively [27]. However, in 148 thoracic and abdominal aortic dissections, real-time PCR (using the Taqman system) was negative for Cp in all cases [28]. Cp and Mp antigens were detected in 100% of calcified aortic valves [29] and in mild atherosclerosis of aorta [30]. Higher amount of Cp and Mp was detected in adventitia of AAA [13].
The present study searched for antigens of Cp and Mp, in aortic atherosclerotic samples from two groups: G1- AAA and G2-mild/ moderate atherosclerosis, and the TLR2 and TLR4 expression, looking for different TLRs immune response that might explain abnormal growth of these pathogens in aorta of AAA.
We found Cp antigens in 100% of G1 and G2 samples, but increased amount of both antigens in intima/medial layers of G2 versus G1 (P = 0.007) with no difference in fat adventitia layer (P = 0.6). On the other hand, there was higher number of positive cells of TLR2 and TLR4 in the adventitial fat of G2, compared with G1 (P < 0.001 and 0.01, respectively). Correlations that were found in G1 using ISH in the adventitia layer suggest an activation of TLR4 by Cp. In G2, we found a correlation of TLR4 with Cp in the adventitial and intimal layers. Co-infection with Mp and Cp was described in atheroma plaques for the first time by Higuchi et al. [31]. This group also found higher numbers and a high correlation of Cp antigens and Mp DNA, and archaea-like microparticles in intimal association with Mp and Cp in vulnerable plaques, which might explain a local increased vessel inflammation and dilatation [32]. A similar atherosclerotic complication might be occurring in AAA lesions. It means that, the response was exacerbated in G2, in front of a same level of infectious antigens of G1 but possibly having other factors acting in G2 such as microparticles. This might be reflecting a more efficient immune response in the group G1 as there was a significant positive correlation between the amount of Cp and Mp versus expression of TLR 2 and TLR4 in this group, at intima/medial layer and also in advential fat unless the TLR4 against Cp in adventitial fat. This efficient immune response also would explain the lower level of Cp and Mp in intima/medial layer of G1 compared with G2.
The expression in fat adventitia of TLR4 values by ISH versus IHC was highly correlated (Tables 1 and 3), in both G1 and G2, supporting the hypothesis that the infectious load in the blood may reach the aorta by the vasa vasorum, explaining the increased iInflammatory infiltrate at the adventitia layer. According to some authors, as lipoproteins constitute part of innate immune system response by binding and inactivating microorganisms and their toxic products through formation of circulating complexes, such aggregates may obstruct arterial vasa vasorum producing ischemia and cell death within the arterial wall leading to the creation of a vulnerable plaque [33,34].
Exacerbated immune response in adventitia fat in G2 is also re-enforced by the fact of increased numbers of TLRs in in adventitia of G2 than in G1, with lack of correlation between amount of Cp and Mp versus TLR2 and TLR4. A correlation between TLR4 antigen and RNA expressions in fat adventitia of G2 indicates an active production of such receptor in these AAA lesions, possibly against Cp antigens. However, this exacerbated immune response is apparently non-effective as those bacteria reach medial/intimal layer in much higher level in AAA group. In G2, the increased correlation between Cp and TLR2 and TLR4, and Cp versus Mp suggests that both bacteria are in symbiosis that creates more difficult in defense against co-infections in adventitia of aneurysm atherosclerotic lesions.
Functionally, the TLRs alert the immune system to the presence of foreign microorganisms in the host organism. In conjunction with its ligand (which can be either endogenous or exogenous), TLRs lead to the production of inflammatory cytokines, chemokines and effector molecules (adapters), depending on the type of TLR that is activated [35,36]. In the present study, immune-staining identified the antigens of TLR2 and TLR4 in 100% of G1 and G2. There were high correlation between Mp and Cp each other and versus TLR2 antigen expression at intima/medial layer in G1, but not in G2.
TLRs modulate the innate immune system and activate the acquired immune system to generate a response to a pathogen [37-39]. In G2, many correlations between infectious agents, TLR2 and TLR4 indicate a cooperative action of these receptors as found by others. Laflamme et al. showed synergism between TLR2 and TLR4 in the mouse brain [40]. Whereas TLR4 is absolutely necessary to initiate the innate immune response, TLR2 participates in the regulation of the TNF-a and IL-12 genes and is therefore decisive regarding the change of the innate immune system to the adaptive system [38]. It was observed by some authors that lipoprotein triacylglycerol originated from MP activate NF-κB through TLR1 and TLR2 but not TLR6 [17,41]. However, the interruption of TLR2 signaling that was induced by MP infection by the release of IL-4 and IL-13 (allergic cytokines) decreased the clearance of MP from the lungs of mice, leaving the tissue more susceptible to bacterial diseases [42]. Using ISH, we observed correlations between Cp and TLR2 in the aneurysm group. In spite of CP is a gram-negative bacterium, we found hyperactivation of TLR2, which is a receptor for gram-positive bacteria. Other studies found similar results [43-46]. It was shown that human cells exposed to microbial products of Cp in the early stages of atherogenesis expressed TLR4 and TLR2 [47-49].
5. CONCLUSION
The present study favors the concept that symbiotic coinfection by Cp and Mp participates in the pathogenesis of AAA. Also this study emphasizes that adventitial fat is the initial site for colonization of these bacteria that probably reach the tissue through vasa vasorum. An exacerbated immune reaction is not efficient to control the infection that reaches and proliferates in high levels at the medial and intimal layer, contributing to the development of vessel dilatation.
ACKNOWLEDGMENT
This work was supported by CNPQ (National Counsel of Technological and Scientific Development of Federal Government grant 132905/ 2006-0.) and FAPESP (Foundation that supports research in the State of São Paulo, grant 562444/2007).
REFERENCES
- Guo, D.C., Papke, C.L., He, R. and Milewicz, D.M. (2006) Pathogenesis of thoracic and abdominal aortic aneurysm. Annals of the New York Academy of Science, 1085, 339- 352. http://dx.doi.org/10.1196/annals.1383.013
- Libby, P. (2006) Inflammation and cardiovascular disease mechanisms. The American Journal of Clinical Nutrition, 83, 456S-460S. http://ajcn.nutrition.org/content/83/2/456S.full.pdf+html
- Lindholt, J.S. and Shl, G.P. (2006) Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. European Journal of Vascular Surgery, 31, 453- 463. http://dx.doi.org/10.1016/j.ejvs.2005.10.030
- Fong, I.W. (2003) Chlamydia pneumoniae and the cardiovascular system. In: Infections and the Cardiovascular System: New Perspectives, Kluwer Academic/Plenum Publishers, New York, 121-156. http://dx.doi.org/10.1007/0-306-47926-5_5
- Haranaga, S., Yamaguchi, H., Friedman, H., Izumi, S. and Yamamoto, Y. (2001) Chlamydia pneumoniae infects and multiplies in lymphocytes in vitro. Infection and Immunity, 69, 7753-7759. http://dx.doi.org/10.1128/IAI.69.12.7753-7759.2001
- Edvinsson, M., Thelin, S., Hjelm, E., Friman, G. and Nystrom-Rosander, C. (2010) Persistent chlamydophila pneumoniae infection in thoracic aortic aneurysm and aortic dissection? Upsala Journal of Medical Sciences, 115, 181-186. http://dx.doi.org/10.3109/03009731003778719
- Kol, A., Sukhova, G.K., Lichtmanm, A.H. and Libby, P. (1998) Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation, 28, 300-307. http://dx.doi.org/10.1161/01.CIR.98.4.300
- Higuchi, M.L., Reis, M.M., Sambiase, N.V., Palomino, S.A.P. Castelli, J.B., Gutierrez, P.S., Aiello, V.D. and Ramires, J.A.F. (2003) Co-infection with Mycoplasma pneumoniae and Chlamydia pneumoniae in ruptured plaques associated with acute myocardial infarction. Arquivos Brasileiros de Cardiologia, 81, 12-22. http://dx.doi.org/10.1590/S0066-782X2003000900001
- Higuchi, M.L., Gutierrez, P.S., Bezerra, H.G., Palomino, S.A., Aiello, V.D., Silvestre, J.M, Libby, P. and Ramires, J.A. (2002) Comparison between adventitial and intimal inflammation of ruptured and nonruptured atherosclerotic plaques in human coronary arteries. Arquivos Brasileiros de Cardiologia, 79, 20-24. http://dx.doi.org/10.1590/S0066-782X2002001000003
- Momiyama, Y., Ohmori, R., Taniguchi, H., Nakamura, H. and Ohsuzu, F. (2004) Association of Mycoplasma pneumoniae infection with coronary artery disease and its interaction with chlamydial infection. Atherosclerosis, 176, 139-144. http://dx.doi.org/10.1016/j.atherosclerosis.2004.04.01
- Maia, I.L., Nicolau, J.C., Machado, M. de N., Maia, L.N., Takakura, I.T., Rocha, P.R., Cordeiro, J.A. and Ramires, J.A.F. (2009) Prevalence of Chlamydia pneumoniae and Mycoplasma pneumoniae in different forms of coronary disease. Arquivos Brasileiros de Cardiologia, 92, 405- 411. http://dx.doi.org/10.1590/S0066-782X2009000600005
- Damy, S.B., Higuchi, M.L., Timenetsky, J., Reis, M.M., Palomino, S.A., Ikegami, R.N., Santos, F.P., Osaka, J.T. and Figueiredo, L.P. (2009) Mycoplasma pneumoniae and/ or Chlamydophila pneumoniae inoculation causing different aggravations in cholesterol-induced atherosclerosis in apoE KO male mice. BMC Microbiology, 9,194. http://dx.doi.org/10.1186/1471-2180-9-194
- Roggério, A., Sambiase, N.V., Palomino, S..A.P., de Castro, M.A., da Silva, E.S., Stolf, N.G. and de Lourdes Higuchi, M. (2013) Correlation of bacterial coinfection versus matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 expression in aortic aneurysm and atherosclerosis. Annals of Vascular Surgery, 27, 964-971. http://dx.doi.org/10.1016/j.avsg.2013.02.012
- Kaisho, T. and Akira, S. (2006) Toll-like receptor function and signaling. Journal of Allergy and Clinical Immunology, 117, 979-987. http://dx.doi.org/10.1016/j.jaci.2006.02.023
- Cao, F., Castrillo, A., Tontonoz, P., Re, F. and Byrne, G.I. (2007) Chlamydia pneumoniae-induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infection and Immunity, 75, 753-759. http://dx.doi.org/10.1128/IAI.01386-06
- Ashida, K., Miyazaki, K., Takayama, E., Tsujimoto, H., Ayaori, M., Yakushiji, T., Iwamoto, N., Yonemura, A., Isoda, K., Mochizuki, H., Hiraide, H., Kusuhara, M. and Ohsuzu, F. (2005) Characterization of the expression of TLR2 (toll-like receptor 2) and TLR4 on circulating monocytes in coronary artery disease. Journal of Atherosclerosis and Thrombosis, 12, 53-60. http://dx.doi.org/10.5551/jat.12.53
- Shimizu, T., Kida, Y. and Kuwano, K. (2005) A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappa B through TLR1, TLR2, and TLR6 Journal of Immunology, 175, 4641-4646. http://www.jimmunol.org/content/175/7/4641
- Ishiyama, J., Taguchi, R., Yamamoto, A. and Murakami, K. (2010) Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis, 209, 118-124. http://dx.doi.org/10.1016/j.atherosclerosis.2009.09.004
- Wassef, M., Baxter, B.T., Chisholm, R.L., Dalman, R.L., Fillinger, M.F., Heinecke, J., Humphrey, J.D., Kuivaniemi, H., Parks, W.C., Pearce, W.H., Platsoucas, C.D., Sukhova, G.K., Thompson, R.W., Tilson, M.D. and Zarins, C.K. (2001) Pathogenesis of abdominal aortic aneurysms: A multidisciplinary research program supported by the National Heart, Lung, and Blood Institute. Journal of Vascular Surgery, 34, 730738 http://dx.doi.org/10.1067/mva.2001.116966
- Triantafilou, M., Gamper, F.G., Lepper, P.M. Mouratis, M.A., Schumann, C., Harokopakis, E., Schifferle, R.E., Hajishengallis, G. and Triantafilou, K. (2007) Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cellular Microbiology, 9, 2030-2039. http://dx.doi.org/10.1111/j.1462-5822.2007.00935.x
- Wang, S.S., Tondella, M.L., Bajpai, A., Mathew, A.G., Mehranpour, P., Li, W., Kacharava, A.G., Fields, B.S., Austin, H. and Zafari, A.M. (2007) Circulating Chlamydia pneumoniae DNA and advanced coronary artery disease. International Journal of Cardiology, 118, 215-219. http://dx.doi.org/10.1016/j.ijcard.2006.07.013
- Shi, Y. and Tokunaga, O. (2002) Chlamydia pneumoniae and multiple infections in the aorta contribute to atherosclerosis. Pathology International, 52, 755-763. http://dx.doi.org/10.1046/j.1440-1827.2002.01422.x
- Hoymans, V.Y., Bosmans, J.M,, Ursi, D., Martinet, W., Wuyts, F.L., Van Marck, E., Altwegg, M., Vrints, C.J. and Ieven, M.M. (2004) Immunohistostaining assays for detection of Chlamydia pneumoniae in atherosclerotic arteries indicate cross-reactions with nonchlamydial plaque constituents. Journal of Clinical Microbiology, 42, 3219-3224. http://dx.doi.org/10.1046/j.1440-1827.2002.01422.x
- Pierri, H., Higuchi-dos-Santos, M.H., Higuchi, M. de L, Palomino, S., Sambiase, N.V., Demarchi, L.M., Rodrigues, G.H., Nussbacher, A., Ramires, J.A. and Wajngarten, M. (2006) Density of Chlamydia pneumoniae is increased in fibrotic and calcified areas of degenerative aortic stenosis. International Journal of Cardiology, 108, 43-47. http://dx.doi.org/10.1016/j.ijcard.2005.04.022
- Kuo, C.C., Gown, A.M., Benditt, E.P. and Grayston, J.T. (1993) Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. . Arteriosclerosis, Thrombosis, and Vascular Biology, 13, 1501-1504 http://dx.doi.org/10.1161/01.ATV.13.10.1501
- Juvonen, T., Laurila, A., Alakärppä, H., Lounatmaa, K., Surcel, H.M., Leinonen, M., Kairaluoma, M.I. and Saikku, P. (1997) Demonstration of Chlamydia pneumoniae in the walls of abdominal aortic aneurysms. Journal Vascular Surgery, 25, 499-505. http://dx.doi.org/10.1161/01.ATV.13.10.1501
- Nyström-Rosander, C., Edvinsson, M., Thelin, S., Hjelm, E. and Friman, G. (2006) Chlamydophila pneumonia: Specific mRNA in aorta ascendens in patients undergoing coronary artery by-pass grafting. Scandinavian Journal of Infectious Diseases, 38, 758-763. http://dx.doi.org/10.1080/00365540600617058
- Sodeck, G., Domanovits, H., Khanakah, G., Schillinger, M., Thalmann, M., Bayegan, K., Schoder, M., Grabenwoeger, M., Hoelzenbein, T., Boehmig, G., Laggner, A.N. and Stanek, G. (2004) The role of Chlamydia pneumoniae in human aortic disease-a hypothesis revisited. European Journal of Vascular and Endovascular Surgery, 28, 547-552. http://dx.doi.org/10.1016/j.ejvs.2004.07.019
- Higuchi-Dos-Santos, M.H., Pierri, H., Higuchi, M. de L., Nussbacher, A., Palomino, S., Sambiase, N.V., Ramires, J.A. and Wajngarten, M. (2005) Chlamydia pneumoniae and Mycoplasma pneumoniae in calcified nodes of stenosed aortic valves. Arquivos Brasileiros de Cardiologia, 84, 443-448. http://dx.doi.org/10.1590/S0066-782X2005000600002
- Gois, J.M., Higuchi, M.L., Reis, M.M., Diament, J., Sousa, J.M., Ramires, J.A.F. and Oliveira, S.A. (2006) Infectious agentes, inflammation and growth factors. How do they interact in the progression or stabilization of mild human atherosclerotic lessions? Annals of Vascular Surgery, 20, 638-645. http://dx.doi.org/10.1007/S10016-006-9076-1
- Higuchi, M.L., Sambiase, N.V., Palomino, S., Gutierrez, P., Demarchi, L.M., Aiello, V.D. and Ramires, J.A.F. (2000) Detection of Mycoplasma pneumoniae and Chlamydia pneumoniae in ruptured atherosclerotic plaques. Brazilian Journal of Medical and Biological Research, 33, 1023-1026. http://dx.doi.org/10.1590/S0100-879X2000000900005
- Higuchi, M.L., Santos, M.H., Roggério, A., Kawakami, J.T., Bezerra, H.G. and Canzian, M. (2006) A role for archaeal organisms in development of atherosclerotic vulnerable plaques and myxoid matrices. Clinics, 61, 473-478. http://dx.doi.org/10.1590/S1807-59322006000500016
- Ravnskov, U. and McCully, K. (2009) Review and Hypothesis: Vulnerable plaque formation from obstruction of Vasa vasorum by homocysteinylated and oxidized lipoprotein aggregates complexed with microbial remnants and LDL autoantibodies. Annals of Clinical Laboratory Science, 39, 3-16.
- Ravnskov, U. and McCully, K.S. (2012) Infections may be causal in the pathogenesis of atherosclerosis. The American Journal of the Medical Sciences, 344, 391-394. http://dx.doi.org/10.1097/MAJ.0b013e31824ba6e0
- Galkina, E. and Ley, K. (2009) Immune and inflammatory mechanisms of atherosclerosis. Annual Review of Immunolog, 27,165-197. http://dx.doi.org/10.1146/annurev.immunol.021908.132620
- Armant, M.A. and Fenton, M.J. (2002) Toll-like receptors: A family of pattern-recognition receptors in mammals. Genome Biology, 3. http://genomebiology.com/2002/3/8/reviews/3011
- O’Neill, L.A. (2006) How toll-like receptors signal: What we know and what we don’t know. Current Opinion in Immunology, 18, 3-9. http://dx.doi.org/10.1016/j.coi.2005.11.012
- O’Neill, L.A., Bryant, C.E. and Doyle, S.L. (2009) Therapeutic targeting of toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacological Reviews, 61, 177-197. http://dx.doi.org/10.1124/pr.109.001073
- O’Neill, L.A. (2006) Targeting signal transduction as a strategy to treat inflammatory diseases. Nature Reviews Drug Discovery, 5, 549-563. http://dx.doi.org/10.1038/nrd2070
- Laflamme, N., Echchannaoui, H., Landmann, R. and Rivest, S. (2003) Cooperation between toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from gram-negative and gram-positive bacteria. European Journal of Immunology, 33, 1127- 1138. http://dx.doi.org/10.1002/eji.200323821
- Shimizu, T., Kida, Y. and Kuwano, K. (2007) Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-κB through toll-like receptors 1 and 2. Immunology, 121, 473-483. http://dx.doi.org/10.1111/j.1365-2567.2007.02594.x
- Wu, Q., Martin, R.J., LaFasto, S., Efaw, B.J., Rino, J.G., Harbeck, R.J. and Chu, H.W. (2008) Toll-like receptor 2 down-regulation in established mouse allergic lungs contributes to decreased mycoplasma clearance. American Journal of Respiratory and Critical Care Medicine, 177, 720-729. http://dx.doi.org/10.1164/rccm.200709-1387OC
- Moon, S.K., Woo, J.I., Lee, H.Y., Park, R., Shimada, J., Pan, H., Gellibolian, R. and Lim, D.J. (2007) Toll-like receptor 2-dependent NF-κB activation is involved in nontypeable Haemophilus influenzae-induced monocyte chemotactic protein 1 up-regulation in the spiral ligament fibrocytes of the inner ear. Infection and Immunity, 75, 3361-3372. http://dx.doi.org/10.1128/IAI.01886-06
- Prebeck, S., Kirschning, C., Dürr, S., da Costa, C., Donath, B., Brand, K., Redecke, V., Wagner, H. and Miethke, T. (2001) Predominant role of toll-like receptor 2 versus 4 in Chlamydia pneumoniae-induced activation of dendritic cells. Journal of Immunology, 167, 3316-3323. http://www.jimmunol.org/content/167/6/3316
- Mueller, M., Postius, S., Thimm, J.G., Gueinzius, K., Muehldorfer, I. and Hermann, C. (2004) Toll-like receptors 2 and 4 do not contribute to clearance of Chlamydophila pneumoniae in mice, but are necessary for the release of monokines. Immunobiology, 209, 599-608. http://dx.doi.org/10.1016/j.imbio.2004.08.003
- Ikeda, H., Sasaki, M., Ishikawa, A., Sato, Y., Harada, K., Zen, Y., Kazumori, H. and Nakanuma, Y. (2007) Interaction of toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Laboratory Investigation, 87, 559-571. http://dx.doi.org/10.1038/labinvest.3700556
- Yang, X., Coriolan, D., Schultz, K., Golenbock, D.T. and Beasley, D. (2005) Toll-like receptor 2 mediates persistent chemokine release by Chlamydia pneumoniae-infected vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 2308-2314. http://dx.doi.org/10.1161/01.ATV.0000187468.00675.a3
- de Graaf, R., Kloppenburg, G., Kitslaar, P.J., Bruggeman, C.A. and Stassen, F. (2006) Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through toll-like receptors 2 and 4. Microbes and Infection, 8, 1859-1865. http://dx.doi.org/10.1016/j.micinf.2006.02.024
- Doherty, T.M., Fisher, E.A. and Arditi, M. (2006) TLR signaling and trapped vascular dendritic cells in the development of atherosclerosis. Trends in Immunology, 27, 222-227. http://dx.doi.org/10.1016/j.it.2006.03.006
NOTES
*Corresponding author.

