World Journal of Cardiovascular Diseases
Vol.3 No.3(2013), Article ID:32532,12 pages DOI:10.4236/wjcd.2013.33044
Interventions to improve daily activity in individuals with COPD and CHF: A systematic review
![]()
Department of Physical Therapy, Grand Valley State University, Grand Rapids, USA
Email: *shoemami@gvsu.edu
Copyright © 2013 Michael J. Shoemaker et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 5 April 2013; revised 8 May 2013; accepted 16 May 2013
Keywords: Heart Failure; Chronic Obstructive Pulmonary Disease; Daily Activity
ABSTRACT
Introduction: The purpose was to systematically review the literature regarding interventions to improve daily activity in individuals with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF). Methods: Articles found by searching CINAHL Plus Full-Text, PubMed, and PsycINFO databases were included in the review if the study examined the effect of exerciseand/or psychosocial-based interventions on daily activity in individuals with COPD or CHF. Article selection, data extraction, and evaluation of methodological rigor and quality were performed by two independent reviewers. Nine articles for COPD and seven articles for CHF met the inclusion criteria and were used in this review. Results: Only four of nine studies in COPD and two of seven studies in CHF resulted in improvement in daily activity, and of those, all but one study included a psychosocial-based intervention. Improvements in daily activity did not occur concurrently with changes in other outcomes such as exercise performance, quality of life, functional status, or anxiety/depression in COPD or CHF. Conclusions: Exercise-based interventions serve a limited, if any, role in improving daily activity in individuals with COPD and CHF. Disrupting the cycle of inactivity and deconditioning requires more than just addressing the deconditioning aspect of this cycle. Psychosocial-based interventions are a promising, but under-investigated, intervention.
1. INTRODUCTION
Daily activity is associated with a variety of health outcomes in patients with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF), including life expectancy and mortality [1-7]. However, rehabilitative interventions for improving daily activity have not been well-studied. Very low levels of physical activity measured by activity questionnaires are associated with hospitalization due to exacerbation [2,3] and mortality in patients with COPD [2]. Objective measurement of daily activity with accelerometry in patients with COPD reaffirmed this finding; daily activity was an independent prognostic factor for mortality and hospitalization [4]. Similarly, in patients with CHF, low daily activity as measured by accelerometry is associated with lower survival and mortality [5-7]. It is therefore important to understand which interventions are effective for improving daily activity.
Although COPD and CHF have very distinct pathophysiologic mechanisms, both result in exercise intolerance and similar physically-limiting symptoms such as breathlessness and fatigue that lead to activity avoidance and a subsequent cycle of inactivity and deconditioning [8-12].
Chronic obstructive pulmonary disease is characterized by progressive airflow obstruction caused by a loss of elastic recoil and airway narrowing that is not fully reversible in response to bronchodilators [13]. Many individuals with COPD are inactive in daily life, most likely as a strategy to minimize dyspnea [14]. The mechanisms for dyspnea and exercise intolerance are multifactorial and include increased resistance to airflow (especially during expiration) [15], impaired gas exchange resulting in hypoxemia and hypercapnia [15], dynamic hyperinflation [16], inspiratory muscle weakness, and skeletal muscle dysfunction [17].
Chronic heart failure is a syndrome of cardiac dysfunction characterized by insufficient cardiac output [15], resulting in limited exercise performance, abnormal ventilatory response, impaired ventilatory muscle strength, and low perfusion of skeletal muscles with subsequent skeletal muscle dysfunction [8]. The symptoms that result from these various mechanisms of exercise intolerance lead to activity restriction and further functional deterioration [9].
Although the exertional symptoms associated with limited exercise tolerance may appear to account for inactivity in individuals with COPD and CHF, psychosocial factors such as depression and self-efficacy have also been demonstrated to be significant determinants of participation in daily activities [18,19]. On average, individuals with chronic diseases are 1.5 - 2.0 times more likely to be depressed [20]. Prevalence of major depresssion in individuals with COPD and CHF have been reported to be as high as 42% and 20%, respectively [21,22]. Although decreased activity is most pronounced in older patients with significant depression, individuals with less severe depression are also at risk for decline in daily activity [23]. The Longitudinal Aging Study Amsterdam found that respondents with emerging depresssion were more likely to transition to a sedentary lifestyle [24].
Self-efficacy, defined as an individual’s beliefs about his or her ability to exercise control over events that affect his or her life [25], is another potentially significant determinant of daily activity. Oka et al. [26] found that self-efficacy is a stronger predictor of daily physical activity in individuals with CHF than physical fitness measures or RPE. In individuals with COPD, Arnold et al. [27] found greater self-efficacy in those reporting better physical functioning. Specifically with regard to walking, lower walking self-efficacy as measured by the Self-Efficacy Questionnaire-Walking is associated with a decrease in physical activity in individuals with CHF [28], and self-efficacy is associated with objective physical performance measures such as the six-minute walk test (6MWT) in individuals with COPD [29].
Rehabilitative interventions for improving daily activity have not been well-studied. Ng et al. [30] systematiccally reviewed the effect of exercise training on daily activity in individuals with COPD. They found a small effect of exercise training on daily activity based on two randomized trials and five cohort studies. Unfortunately, their review only examined exercise interventions and did not account for other confounding determinants of daily activity. No prior systematic reviews have been conducted to examine interventions for improving daily activity of individuals with CHF, but three studies have failed to demonstrate improvement in daily activity despite improvements in exercise tolerance following exercise training [31-33]. Given that exercise-based intervenetions may not result in substantial changes in daily activity, other interventions that address impairments in psychosocial domains may be more appropriate for improving daily activity [34]. No prior systematic review has addressed non-exercise based interventions for low daily activity in individuals with COPD or CHF.
In summary, COPD and CHF result in a cycle of inactivity and deconditioning due to the symptoms of fatigue and breathlessness associated with exercise intolerance. As daily activity decreases, the risk of adverse health outcomes increases. Furthermore, psychosocial factors may substantially confound this cycle of inactivity and deconditioning such that interventions for improving daily activity may need to account for these additional factors, especially given that exercise-based intervenetions may have a minimal effect. Therefore, the purpose of the present systematic review is to consider and examine a broader range of interventions that are used to improve daily activity in individuals with COPD and CHF.
2. METHODS
2.1. Literature Search
The literature search was conducted by searching within the article text using CINAHL Plus Full-Text (Ebsco), PubMed, and PscyINFO databases on July 31, 2012. The topics of “heart failure” and “COPD” were each searched individually in conjunction with the following keywords: “daily activity”, “daily activities”, “physical activity”, “physical activities”, and “energy expenditure”.
2.2. Study Selection Criteria
Studies were included if physicalor psychosocial-based interventions were used and if a change in daily activity was assessed after the intervention period had concluded. Only peer-reviewed studies were included in the review and no limitations were imposed regarding year of publication. Studies were excluded if no full text was available or if the article was not published in the English language. To limit selection bias, two authors (BK and BS) independently searched databases and individually selected studies based on inclusion and exclusion criteria. Differences in study selection were discussed with a third author and fourth author (MS and PS), and a consensus was reached by the authors if opinions varied.
2.3. Level of Evidence
Strength of evidence for each study was appraised using levels of evidence as described by Strauss et al. [35]. The levels of evidence are ranked 1 to 5, with 1 being the highest level of evidence. Each study was independently assessed by the authors (BK and BS). If level of evidence ratings varied by author, differences were discussed with a third author and fourth author (MS and PS) and a consensus was reached. Articles rated 1b (randomized trial), 2b (cohort study), 3b (individual case control study) and 4 (low quality case control study) were included in this review.
2.4. Methodological Rigor
The methodological quality of studies included was assessed using the Medlicott and Harris [36] scale for methodological rigor. Methodological rigor was rated as follows: “Strong” 71% to 100%, “Moderate” 60% to 70%, and “Weak” 59% or less. Percentages were calculated by dividing the amount of criterion “met” by the total amount of criterion possible that could be “met” for that particular article. Randomized controlled trials with “weak” methodological rigor were given a level of evidence designation “2b.” Methodological rigor was independently assessed by the authors (BK and BS). Differences in study selection were discussed with a third author and fourth author (MS and PS) and a consensus was reached by the authors if opinions varied.
3. RESULTS
3.1. Heart Failure
3.1.1. Literature Search
Three hundred and sixteen unique articles were found using the search strategy previously outlined. The most frequent reasons for exclusion were heterogeneous populations, no measure of daily activity, or a lack of a physical or psychosocial intervention. Ultimately, seven studies [37-43] met the inclusion criteria for this review. The results of the search strategy are outlined in Figure 1. Included studies were then analyzed for their methods, outcome measures, level of evidence, and methodological rigor. Tables 1 and 2 summarize the methodological rigor and extracted data of the included studies.
3.1.2. Levels of Evidence
All seven studies [37-43] were Level 1b based on being randomized in design and having a methodological rigor score greater than or equal to 60%.
3.1.3. Methodological Rigor
Methodological rigor scores ranged from 70% to 90% and are summarized in Table 1. Five articles [38,40-43] were rated as “strong” (“yes” percentage from 75 - 100), two [37,39] were judged to be “moderate” (“yes” percentage from 50 - 74), and none were determined to be “weak” (“yes” percentage from 0 - 49). The most notable deficiencies in rigor were related to randomization, blinding, and long term follow-up.
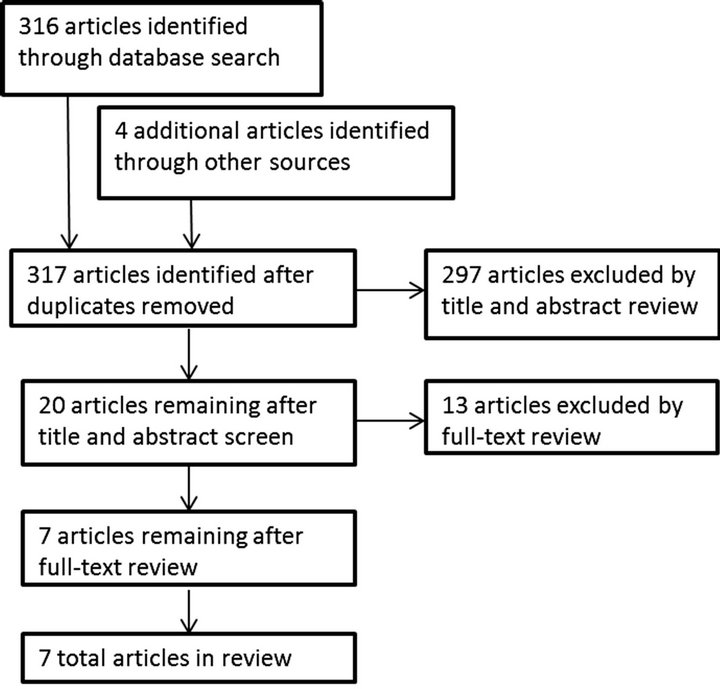
Figure 1. Search results for chronic heart failure.
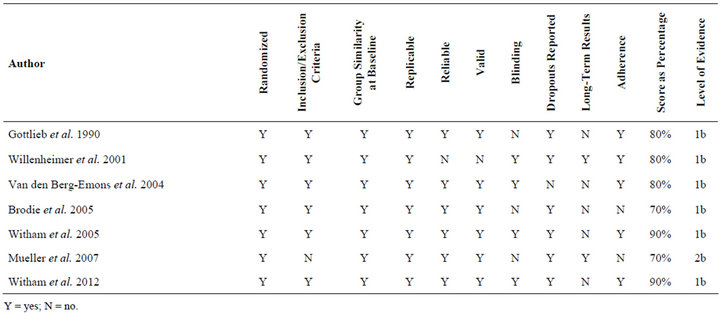
Table 1. Methodological rigor of included studies in individuals with chronic heart failure.
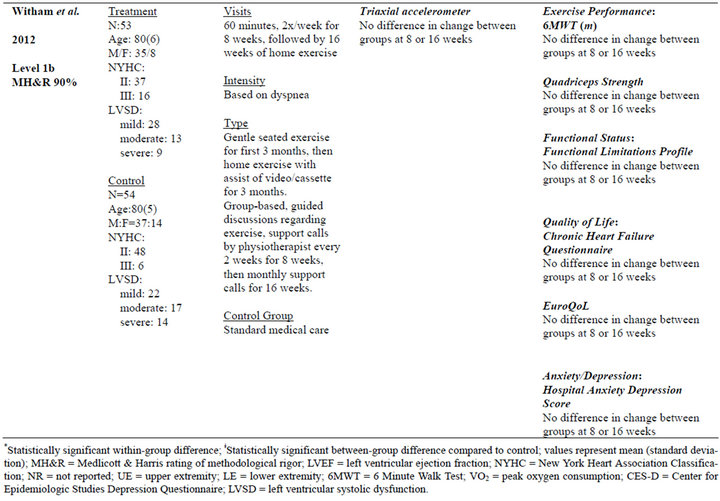

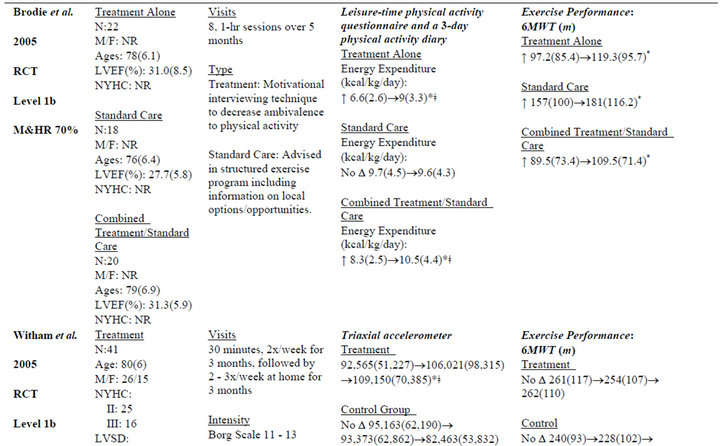
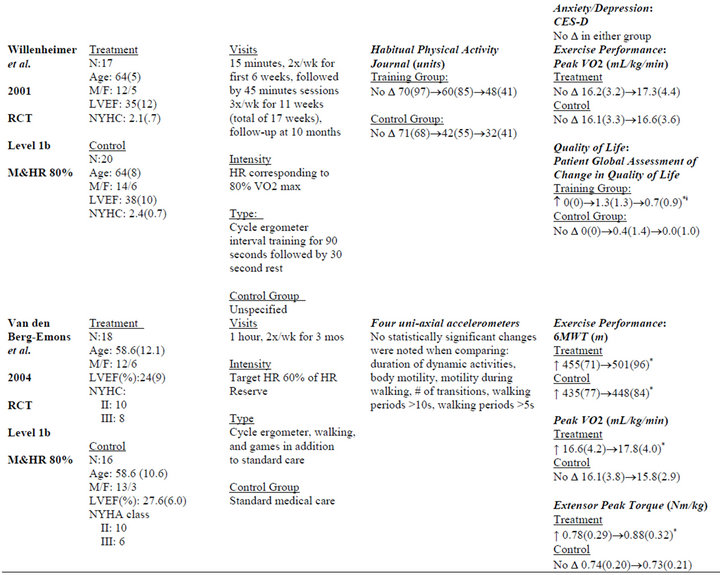

Table 2. Extracted data for studies in individuals with chronic heart failure.
3.1.4. Follow-Up
Only two studies [39,41] measured long-term results (6- month or greater follow-up). The majority [37,38,40, 42,43] of studies had no follow-up after intervention concluded.
3.1.5. Intervention Type and Dosage
Five studies [38-43] used exercise interventions, one study [37] used psychosocial intervention, and two studies [42,43] used an exercise intervention with a psychosocial component. The average length of intervention was 3.33 months, with a range of 1 month [39] to 6 months [38,43]. Frequency of intervention ranged from two times per week [40] to seven times per week [39], with the other four studies at 2 - 3 times per week.
3.1.6. Daily Activity Measures and Outcomes
Several different daily activity measures were utilized. Four [38,40,42,43] of the seven studies used accelerometry, while the other three [37,39,41] used either a questionnaire or an activity diary. One study [38] used doubly-labeled water in conjunction with accelerometry. Only two studies [37,42] showed between-group improvements in daily activity. The remaining five studies [38-41,43] demonstrated no improvements in daily activity.
3.1.7. Exercise Performance Measures and Outcomes
Measurements included the 6MWT [37,38,40,42,43], peak VO2 [38,40,41], heart rate [39], and various muscular performance tests. Two studies [38,40] demonstrated improvements across multiple measures of exercise performance. The most commonly used measures were peak VO2 [38,40,41] and the 6MWT [37,38,40, 42,43]. Significant between-group changes in 6MWT [38], peak VO2 [38,40], exercise duration [38], and peak torque [40] were found in two of seven studies.
3.1.8. Quality of Life Measures and Outcomes
Five studies [38,40-43] measured quality of life (QOL). Measures included: Minnesota Living with Heart Failure Questionnaire [38], Medical Outcomes Study-SF 36 [38], Philadelphia Geriatric Morale Score [42], Chronic Heart Failure Questionnaire [42,43], Patient Global Assessment of Change in Quality of Life [41], and EuroQoL [43]. Significant between-group differences as a result of the intervention were only found in one study using the Patient Global Assessment of Change in Quality of Life [41].
3.1.9. Anxiety/Depression Measures and Outcomes
Three studies [38,42,43] measured anxiety/depression outcomes. Depression/anxiety measures included the Center for Epidemiologic Studies Depression Questionnaire [38] and the Hospital Anxiety and Depression Scale [42,43]. No changes in anxiety/depression were found.
3.1.10. Functional Status Measures and Outcomes
Three studies measured functional status [38,42,43]. Measures included the Function Status Assessment [38] and the modified Functional Limitations Profile [42,43]. No study reported significant between-group differences in any of the aforementioned measures.
3.1.11. Concurrent Changes between Daily Activity and Secondary Outcomes
Figure 2 summarizes the relationship between changes in daily activity concurrent with changes in other seconddary outcome measures by plotting the within-group effect sizes of the intervention groups for all measures across all seven studies. Overall, a positive linear relationship did not exist, with the greatest changes in daily activity not occurring concurrently with changes in other measures. Furthermore, only one study [37] demonstrated a within-group effect size greater than 0.50 for daily activity.
When assessing concurrent changes in daily activity and anxiety/depression, there were no consistent results; two studies [38,43] showed no changes in daily activity or anxiety/depression, another study [42] demonstrated an increase in daily activity without a concurrent improvement in anxiety/depression, while yet another study
[40] showed improvement in anxiety/depression without an improvement in daily activity. No studies found concurrent change in daily activity and anxiety/depression.
In evaluating concurrent change in daily activity and QOL, three studies [38,40,43] showed no change in either daily activity or QOL. One study [41] showed increased daily activity but no improvement in QOL. Another study [41] showed improvement in QOL but no increase in daily activity. No studies found concurrent change in daily activity and QOL.
In the assessment of concurrent change in daily activity and functional status, three studies [38,41,43] found no change in daily activity or functional status. Two studies [37,42] found increased daily activity without an increase in activity participation. No study showed an increase in daily activity and activity participation concurrently.
In comparing concurrent changes among daily activity and exercise performance, three studies [39,41,43] showed no change in daily activity or exercise performance. Two studies [37,42] showed increases in daily activity without associated changes in exercise performance. Two studies [38,39] demonstrated improvements in exercise performance but no increase in daily activity. No studies found concurrent change in daily activity and exercise performance.
3.2. COPD
One hundred and eighty four unique articles were found using the search strategy previously outlined. Ultimately, nine studies [44-52] met the inclusion criteria for this review: three randomized control trials [45,47,48], one
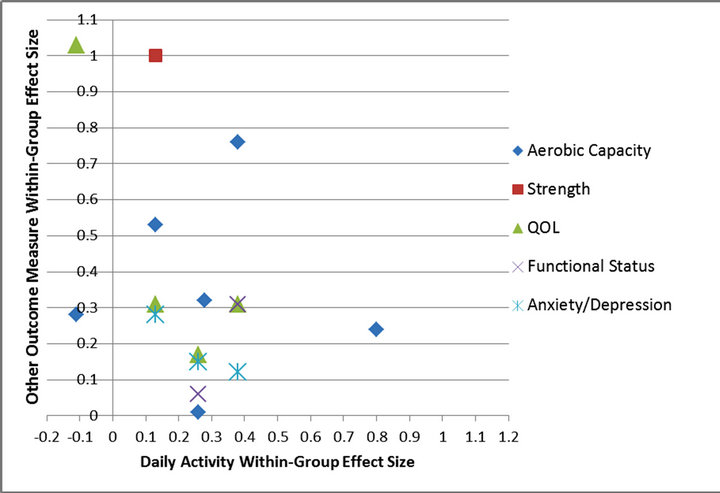
Figure 2. Concurrent intervention within-group effect sizes for daily activity and other outcome measures in CHF studies.
cohort study [44], and five case control studies [44, 46,49,51,52] (Figure 3). Methodological rigor scores ranged from 40% [51] to 90% [45]. One study [44] was rated as “strong”, seven studies [44,46-50,52] were judged to be “moderate”, and one study [50] was determined to be “weak”. Of these nine studies, two [47,49] were unique to this search from the previous systematic review and meta-analysis by Ng et al. [30]. As discussed previously, Ng et al. [30] concluded that exercise training had little-to-no effect on physical activity in patients with COPD. For further details regarding the results and details of these seven included studies, refer to the full text article by Ng et al. [30].
The two additional articles found by the present literature search reported similar conclusions. Slinde et al. [49] explored how total daily energy expenditure changes when underweight patients with COPD enter a physiotherapy program. Fourteen subjects meeting the inclusion criteria of severe COPD, as indicated by FEV1 less than 50%, and body mass-index of less than twentyone kg/m2, completed the study. In total, subjects attended eight, 90-minute physical therapy sessions consisting of moderate physical activity and an educational intervention. The authors concluded, based on accelerometer and doubly-labeled water measurements, that the mean total energy expenditure actually decreased 6% during the intervention period as compared to the control, with between-group results not statistically significant.
Probst et al. [47] compared the effect of a high intensity, whole-body endurance and strength program to a low-intensity calisthenics-and-breathing exercise program to improve daily activity. Forty individuals with

Figure 3. Search results for chronic obstructive pulmonary disease.
COPD were randomized into two equal treatments groups and completed 36 therapy sessions over a 12 week period. Based on accelerometry, Probst et al. [47] concluded that neither highnor low-intensity treatment had a significant impact on improving daily energy expenditure.
Concurrent Changes between Daily Activity and Secondary Outcomes
Figure 4 summarizes the relationship between changes in daily activity concurrent with changes in other seconddary outcome measures by plotting the within-group effect sizes of the intervention groups for all measures across all nine studies. Overall, a positive linear relationship did not exist, with the greatest changes in daily activity not occurring concurrently with changes in other measures. Furthermore, only one study [45] demonstrated a withingroup effect size greater than 0.50 for daily activity, with small-to-no effects in all other outcome measures when daily activity effect sizes where greater than 0.50.
When assessing concurrent changes in daily activity and anxiety/depression, one study [50] showed no changes in daily activity or anxiety/depression and another demonstrated a large effect on daily activity but no effect on anxiety/depression [45]. Only one study [52] showed concurrent increase in daily activity and improved anxiety/depression.
With regard to concurrent changes in daily activity and QOL, four studies [45,47,48,50] showed no change in either daily activity or QOL. One study [44] showed improved QOL with no increase in daily activity. Two studies [46,52] demonstrated concurrent change in increased daily activity and improved QOL.
In the assessment of concurrent change in daily activity and functional status, four studies [45,47,48,50] found no change in daily activity and participation. One study [44] showed increased functional status with no positive effect on daily activity. Two studies [46,52] showed concurrent change in increased daily activity and improved functional status.
In comparing concurrent changes among daily activity and exercise performance, two studies [45,48] showed no change in daily activity or exercise performance. One study [52] showed increased daily activity without associated improvement in exercise performance. Three studies [44,47,50] demonstrated improvements in exercise performance but no increase in daily activity. Only one study [46] found a concurrent increase in daily activity and improved exercise performance.
4. DISCUSSION
The purpose of the present review was to evaluate the efficacy of exerciseand psychosocial-based intervenetions for improving daily activity in patients with COPD

Figure 4. Concurrent intervention within-group effect sizes for daily activity and other outcome measures in COPD studies.
and CHF. The results of the present review highlight several important limitations of the existing literature. More importantly, however, the present review provides insight into interventions utilized for disrupting the cycle of inactivity and deconditioning in individuals with COPD and CHF. Overall, the present results indicate that exercise-based interventions play a limited role in improving daily activity, and psychosocial-based interventions are a promising, but under-investigated, intervention.
With regard to limitations of the existing literature on interventions for improving daily activity in individuals with COPD and CHF, there are several noteworthy study design and measurement deficiencies. Although there were a greater number of studies of patients with COPD, few utilized a randomized design compared to all six of the studies in patients with CHF being randomized controlled trials. However, the measurement of daily activity in the CHF literature included an objective measurement (e.g. accelerometry) in only four studies, compared to objective measures being used in all nine in the COPD literature. It is therefore helpful to draw upon evidence from both populations when evaluating intervention efficacy. Indeed, similar findings were noted in both, with exercise-based interventions having little effect on daily activity.
With regard to the role of either exerciseand/or psychosocial-based interventions for disrupting the cycle of inactivity and deconditioning, psychosocial interventions appeared to have the greatest efficacy. Only four of nine studies [45,46,48,52] in patients with COPD showed within-group improvement in daily activity, and none demonstrated between-group changes. Similarly, two of seven studies [37,42] in patients with CHF resulted in statistically significant effects on daily activity. However, among the studies that demonstrated a statistically significant effect on daily activity, three of the four studies in those with COPD [45,46,48] and both of the studies in those with CHF [37,42] included a psychosocial component or were entirely a psychosocial-based intervention.
With regard to the cycle of inactivity and deconditioning, the results of the present study could be interpreted in two ways: 1) inactivity and deconditioning are not interrelated as previously proposed [8-12], or, 2) only directing interventions towards the deconditioning component of the cycle is insufficient for making changes in daily activity. In patients with CHF, Shoemaker et al. [53] observed that clinically meaningful changes in 6MWT performance were not accompanied by changes in daily activity over time (no intervention was provided). Furthermore, as noted in the present review, changes in exercise performance in response to exercise interventions did not occur concurrently with changes in daily activity (e.g. studies that demonstrated improved exercise performance did not improve in daily activity and vice versa). As previously discussed, nearly all of the studies in both populations that included at least a psychosocial intervention component resulted in improvements in daily activity.
Therefore, psychosocial-based interventions for improving daily activity, although relatively under-investigated in COPD and CHF, may be a promising new intervention. It is not clear whether psychosocial-based interventions alone or in combination with exercise-based interventions are most effective. Furthermore, it is not known which psychosocial interventions are effective, as highlighted by the 2005 and 2012 studies by Witham et al. The very low intensity, seated exercise intervention in the 2005 study that resulted in significant improvement in daily activity included subjects keeping “a diary of their daily activities [that was] used as a basis for a weekly telephone liaison [to] give encouragement and agree on new targets for daily walking activity.” The high intensity exercise intervention in the 2012 study that failed to demonstrate improvement in daily activity included “guided discussion sessions based on cognitive and behavioral techniques [that focused] on benefits of exercise [and] goals and how to work toward them” in addition to telephone calls every 2 weeks for the first 4 months followed by monthly phone calls for 4 months. Thus, the key components of psychosocial support or intervention for promoting changes in the habitual routines that impact daily activity are not clear.
Finally, the present review found that changes in QOL, anxiety/depression, and functional status did not occur concurrently with changes in daily activity. Although daily activity has been shown to be associated with anxiety/depression, QOL, and functional status [18,19,24,26- 28,54], it is not clear why changes in these measures did not consistently occur concurrently. No included study investigated the effect of pharmacologic or functional training interventions directed at improving anxiety/depression or functional status or how such interventions might impact daily activity. It is important to note that none of the included studies specifically accounted for baseline depression, anxiety, or low selfefficacy as possible factors that could confound response to intervention.
In summary, based on the results of the present systematic review, it can be concluded that exercise-based interventions alone are unable to promote an increase in daily activity within the COPD and CHF populations. Given the association between daily activity and other important outcomes such as hospitalization and mortality, clinicians should consider daily activity as clinical outcome, and should also consider the psychosocial needs of patients as they pertain to daily activity.
Future research should focus on expanding the application of psychosocial-based interventions in subjects with COPD and CHF. While current evidence of psychosocial-based intervention is promising, the volume of studies is limited. Future research should also compare the efficacy of exercise-based intervention versus psychosocial-based interventions versus a combination of exercise and psychosocial intervention to improve daily activity in individuals with COPD and CHF.
5. CONCLUSION
Exercise-based interventions serve a limited, if any, role in improving daily activity in individuals with COPD and CHF. Disrupting the cycle of inactivity and deconditioning requires more than just addressing the deconditioning aspect of this cycle. Psychosocial-based interventions are a promising, but under-investigated, intervention.
REFERENCES
- Garcia-Aymerich, J., Serra, I., Gomez, F.P., et al. (2009) Physical activity and clinical and functional status in COPD. CHEST, 136, 62-70. doi:10.1378/chest.08-2532
- Garcia-Aymerich, J., Lange, P., Benet, M., Schnohr, P., and Anto, J.M. (2006) Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax, 61, 772-778. doi:10.1136/thx.2006.060145
- Garcia-Aymerich, J., Farrero, E., Felez, M.A., et al. (2003) Risk factors of readmission to hospital for a COPD exacerbation: A prospective study. Thorax, 58, 100-105. doi:10.1136/thorax.58.2.100
- Garcia-Rio, F., Rojo, B., Casitas, R., et al. (2012) Prognostic value of the objective measurement of daily activity in patients with COPD. CHEST, 142, 338-346. doi:10.1378/chest.11-2014
- Shoemaker, M.J., Curtis, A., Vansgnes, E., Dickinson, M. and Paul, R. (2012) Analysis of daily activity from implanted cardiac defibrillators: The minimum clinically important difference and relationship to mortality/life expectancy. World Journal of Cardiovascular Diseases, 2, 129-135. doi:10.4236/wjcd.2012.23021
- Howell, J., Weisenberg, J., Kakade, A, et al. (2010) Maximum daily 6 minutes of activity: An index of functional capacity derived from actigraphy and its application to older adults with heart failure. Journal of the American Geriatrics Society, 58, 931-936. doi:10.1111/j.1532-5415.2010.02805.x
- Walsh, J., Charlesworth, A., Andrews, R., Hawkins, M., and Cowley, A. (1997) Relation of daily activity levels in patients with chronic heart failure to long-term prognosis. American Journal of Cardiology, 79, 1364-1369. doi:10.1016/S0002-9149(97)00141-0
- Hunt, S.A, Baker, D.W., Chin, M.H., et al. (2001) ACC/ AHA guidelines for the evaluation and management of chronic heart failure in the adult: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 38, 2101-2113. doi:10.1016/S0735-1097(01)01683-7
- Welsch, M.A. and Parish, T.R. (2004) The heart failure syndrome: Implications for exercise training. Cardiopulmonary Physical Therapy Journal, 15, 3-14.
- Belman, M.J. (1993) Exercise in patients with chronic obstructive pulmonary disease. Thorax, 48, 936-946. doi:10.1136/thx.48.9.936
- Gallagher, C.G. (1994) Exercise limitation and clinical exercise testing in chronic bstructive pulmonary disease. Clinics in Chest Medicine, 15, 305-326.
- O’Donnell, D.E., Bertley, J.C., Chau, L.K. and Webb, K.A. (1997) Qualitative aspects of exertional breathlessness in chronic airflow limitation: Pathophysiologic mechanisms. American Journal of Respiratory and Critical Care Medicine, 155, 109-115. doi:10.1164/ajrccm.155.1.9001298
- Petty, T.L. (2003) Definition, epidemiology, course and prognosis of COPD. Clinical Cornerstone, 5, 1-10. doi:10.1016/S1098-3597(03)90003-2
- Pitta, F., Troosters, T., Spruit, M.A., et al. (2005) Characteristics of physical activities on daily life in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 171, 972-977. doi:10.1164/rccm.200407-855OC
- Wasserman, K., Hansen, J.E., Sue, D.Y., Stringer, W.W. and Whipp, B.J. (2005) Principles of exercise testing and interpretation. Lippincott Williams & Wilkins, Philadelphia.
- Casaburi, R. (2005) Combination therapy for exercise tolerance in COPD. Thorax, 61, 551-552. doi:10.1136/thx.2006.058511
- American Thoracic Society, European Respiratory Society (1999) Skeletal muscle dysfunction in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 159, S1-S40. doi:10.1164/ajrccm.159.supplement_1.15945
- Yohannes, A.M., Baldwin, R.C. and Connolly, M.J. (2001) Mood disorders in elderly patients with chronic obstructive pulomonary disease. Reviews in Clinical Gerontology, 10, 191-202.
- Oka, R.K., Stotts, N.A., Dae, M.W., et al. (1993) Daily physical activity levels in congestive heart failure. American Journal of Cardiology, 71, 921-925. doi:10.1016/j.cardfail.2010.04.004
- van Gool, C.H., Kempen, G.I., Penninx, B.W., et al. (2003) Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: Results from the Longitudinal Aging Study Amsterdam. Age and Ageing, 32, 81-87. doi:10.1093/ageing/32.1.81
- Lamers, F., Jonkers, C.C., Bosma, H., et al. (2006) Effectiveness and cost-effectiveness of a minimal psychological intervention to reduce non-severe depression and chronically ill elderly patients: The design of a randomized control trial. BioMed Central Public Health, 6, 161- 170. doi:10.1186/1471-2458-6-161
- Rutledge, T., Reis, V.A., Linke, S.E., Greenberg, B.H. and Mills, P.J. (2006) Depression in heart failure a metaanalysis review of prevalence, intervention effects, and associations with clinical outcomes. Journal of American College of Cardiology, 48, 1527-1537. doi:10.1016/j.jacc.2006.06.055
- Phennix, B.W., Guralni, J.M., Ferrucci, L. et al. (1998) Depressive symptoms and physical decline in community-dwelling older persons. The Journal of the American Medical Association, 279, 1720-1726. doi:10.1001/jama.279.21.1720
- Patten, S.B., Williams, J.V.A., Lavorato, D.H. and Eliasziw, M. (2009) A longitudinal community study of major depression and physical activity. General Hospital Psychiatry, 31, 371-375. doi:10.1016/j.genhosppsych.2009.08.001
- Bandura, A. (1989) Human agency in social cognitive theory. American Psychologist, 44, 1175-1184. doi:10.1037/0003-066X.44.9.1175
- Oka, R.K., Gortner, S.R., Stotts, N.A. and Haskell, W.L. (1996) Predictors of physical activity with chronic heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. American Journal of Cardiology, 77, 159-163. doi:10.1016/S0002-9149(96)90588-3
- Arnold, R., Adelita V.R., DeJongste M.J., et al. (2005) The relationship between self-efficacy and self-reported physical functioning in chronic obstructive pulmonary disease and chronic heart failure. Behavioral Medicine, 31, 107-115. doi:10.3200/BMED.31.3.107-115
- Oka, R.K., DeMarco T. and Hakell, W.L. (2005) Effects of treadmill testing and exercise training on self-efficacy in patients with heart failure. European Journal of Cardiovascular Nursing, 4, 215-219. doi:10.1016/j.ejcnurse.2005.04.004
- Lox, C.L. and Freehill, A.J. (1999) Impact of pulmonary rehabilitation on self-efficacy, quality of life, and exercise tolerance. Rehabilitation Psychology, 44, 208-221. doi:10.1037/0090-5550.44.2.208
- Ng, C.L.W., Mackney, J., Jenkins, S. and Hill, K. (2012) Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chronic Respiratory Disease, 9, 17-26. doi:10.1177/1479972311430335
- van den Berg-Emons, R., Balk, A., Bussmann, H. and Stam, H. (2004) Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? European Journal of Heart Failure, 6, 95-100. doi:10.1016/j.ejheart.2003.10.005
- Mueller, L., Myers, J., Kottman, W., et al. (2007) Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clinical Rehabilitation, 21, 923-931. doi:10.1177/0269215507079097
- Gottlieb, S.S., Fisher, M.L., Freudenberger, R., et al. (1999) Effects of exercise training on peak performance and quality of life in congestive heart failure patients. Journal of Cardiac Failure, 5, 188-194. doi:10.1016/S1071-9164(99)90002-7
- Lorig, K.R., Sobel D.S., Stewart A.L., et al. (1999) Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization. Medical Care, 37, 5-14. http://www.jstor.org/stable/3767202 doi:10.1097/00005650-199901000-00003
- Strauss, S.E., Richardson, W.S. and Haynes, R.B. (2005) Evidence-based medicine: How to practice and teach EBM. 3rd Edition, Elsevier Churchill Livingstone, Edinburgh.
- Medlicott, M.S. and Harris, S.R. (2006) A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Physical Therapy, 86, 955-973.
- Brodie, D.A. and Inoue, I. (2005) Motivational interviewing to promote physical activity for people with chronic heart failure. Journal of Advanced Nursing, 50, 518-527. doi:10.1111/j.1365-2648.2005.03422.x
- Gottlieb, S.S., Fisher, M.L., Freudenberger, R., et al. (1999) Effects of Exercise Training on Peak Performance and Quality of Life in Congestive Heart Failure Patients. Journal of Cardiac Failure, 5, 188-194. doi:10.1016/S1071-9164(99)90002-7
- Mueller, L., Myers, J., Kottman, W., et al. (2007) Exercise capacity, physical activity patterns, and outcomes six years after cardiac rehabilitation in patients with heart failure. Clinical Rehabilitation, 21, 923-931. doi:10.1177/0269215507079097
- Van den Berg-Emons, R., Balk, A., Bussmann, H., and Stam, H. (2004) Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? European Journal of Heart Failure, 6, 95-100. doi:10.1016/j.ejheart.2003.10.005
- Willenheimer, R., Rydberg, E., Cline, C., et al. (2001) Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. International Journal of Cardiology, 77, 25-31. doi:10.1016/S0167-5273(00)00383-1
- Witham, M.D., Gray, J.M., Argo, I.S., et al. (2005) Effect of a seated exercise program to improve physical function and health status in frail patients ≥ 70 years of age with heart failure. American Journal of Cardiology, 95, 1120- 1124.doi:10.1016/j.amjcard.2005.01.031
- Witham, M.D., Fulton, R.L., Greig, C.A., et al. (2012) Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: A randomized controlled trial. Circulation: Heart Failure, 5, 209-216. doi:10.1161/CIRCHEARTFAILURE.111.963132
- Dallas, M.I., McCusker, C., Haggerty, M.C., et al. (2009) Using pedometers to monitor walking activity in outcome assessment for pulmonary rehabilitation. Chronic Respiratory Disease, 6, 217-222. doi: 10.1177/1479972309346760
- De Blok, B.M.J., De Greef, M.H.G., Ten Hacken, N.H.T., et al. (2006) The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: A pilot study. Patient Education Counseling, 61, 48-55. doi:10.1016/j.pec.2005.02.005
- Pitta, F., Troosters, T., Probst, V.S., et al. (2008) Are patients with COPD more active after pulmonary rehabilitation? CHEST, 134, 273-280. doi:10.1378/chest.07-2655
- Probst, V.S., Kovelis, D., Hernandes, N., et al. (2011) Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respiratory Care, 56, 1799-1807. doi:10.4187/respcare.01110
- Sewell, L., Singh, S.J., Williams, J.E.A., Collier, R. and Morgan, M.D.L. (2005) Can individualized rehabilitation improve functional independence in elderly patients with COPD? CHEST, 128, 1194-1200.
- Slinde, F., Kvarnhult, K., Grönberg, A.M., et al. (2006) Energy expenditure in underweight COPD patients before and during a physiotherapy program. European Journal of Clinical Nutrition, 60, 870-876. doi:10.1038/sj.ejcn.1602392
- Steele, B.G., Belza, B., Cain, K., et al. (2010) The impact of chronic obstructive pulmonary disease exacerbation on pulmonary rehabilitation participation and functional outcomes. Journal of Cardiopulmonary Rehabilitation and Prevention, 30, 53-60. doi:10.1097/HCR.0b013e3181c85845
- Steele, B.G., Belza, B., Hunziker, J., et al. (2003) Monitoring daily activity during pulmonary rehabilitation using a triaxial accelerometer. Journal of Cardiopulmonary Rehabilitation and Prevention, 23, 139-142.
- Walker, P.P., Burnett, A., Flavahan, P.W. and Calverley, P.M.A. (2008) Lower limb activity and its determinants in COPD. Thorax, 63, 683-689. doi:10.1136/thx.2007.087130
- Shoemaker, M.J., Curtis, A., Vansgnes, E. and Dickinson, M. (2013) Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulmonary Physical Therapy Journal, in press.
- US Department of Health and Human Services (1996) Physical activity and health: A report of the surgeon general. Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Healthy Promotion, Atlanta.
NOTES
*Corresponding author.

