Open Journal of Biophysics
Vol.06 No.04(2016), Article ID:71661,108 pages
10.4236/ojbiphy.2016.64012
Motoyosi Sugita―A “Widely Unknown” Japanese Thermodynamicist Who Explored the 4th Law of Thermodynamics for Creation of the Theory of Life
Kazumoto Iguchi
Kazumoto Iguchi Research Laboratory (KIRL), Tokushima, Japan

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 6, 2016; Accepted: October 28, 2016; Published: October 31, 2016
ABSTRACT
The purpose of this paper is to introduce to you, the Western people, nowadays a “widely unknown” Japanese thermodynamicist by the name of Motoyosi Sugita and his study on the thermodynamics of transient phenomena and his theory of life. This is because although he was one of the top theoretical physicists in Japan before, during and after WWII and after WWII he promoted the establishment of the biophysical society of Japan as one of the founding members, he himself and his studies themselves have seemed to be totally forgotten nowadays in spite that his study was absolutely important for the study of life. Therefore, in this paper I would like to present what kind of person he was and what he studied in physics as a review on the physics work of Motoyosi Sugita for the first time. I will follow his past studies to introduce his ideas in theoretical physics as well as in biophysics as follows: He proposed the bright ideas such as the quasi-static change in the broad sense, the virtual heat, and the field of chemical potential etc. in order to establish his own theory of thermodynamics of transient phenomena, as the generalization of the Onsager-Prigogine’s theory of the irreversible processes. By the concept of the field of chemical potential that acquired the nonlinear transport, he was seemingly successful to exceed and go beyond the scope of Onsager and Prigogine. Once he established his thermodynamics, he explored the existence of the 4th law of thermodynamics for the foundation of theory of life. He applied it to broad categories of transient phenomena including life and life being such as the theory of metabolism. He regarded the 4th law of thermodynamics as the maximum principle in transient phenomena. He tried to prove it all life long. Since I have recently found that his maximum principle can be included in more general maximum principle, which was known as the Pontryagin’s maximum principle in the theory of optimal control, I would like to explain such theories produced by Motoyosi Sugita as detailed as possible. And also I have put short history of Motoyosi Sugita’s personal life in order for you to know him well. I hope that this article helps you to know this wonderful man and understand what he did in the past, which was totally forgotten in the world and even in Japan.
Keywords:
Unknown Japanese Thermodynamicist, Motoyosi Sugita, Thermodynamics of Transient Phenomena, Virtual Heat, Broad Quasi-Static Change, Chemical Potential, Field of Chemical Potential, Diffusion Phenomena, Number of Partition, Dissipation Function, Onsager’s Theory of Irreversible Processes, Prigogine’s Least Production of Entropy, 4th Law of Thermodynamics, Maximum Principle, Pontryagin’s Maximum Principle, Bellman’s Optimality Principle, Theory of Metabolism, Theory of Life, Cybernetics

1. Introduction
Who knows Motoyosi Sugita? Who is Motoyosi Sugita?
The name of Motoyosi Sugita (see Figure 1) is “widely unknown” all over the world today. It is so as well even in Japan nowadays. In this paper I would like to introduce you to this important Japanese theoretical physicist.
Figure 1. Dr. Motoyosi Sugita (Born August 1905-Died 14 January 1990). The picture was taken at the age of 29 in front of his house in Tokyo, Japan in Summer in 1934. His son Yūkiti was born in May in this year. Yūkiti passed away on the 2nd of August in 2012 (By courtesy of Ms. Setsu Honda).
I did neither know his name nor his work until this spring in 2016. As I started recently writing a paper on the theory of thermodynamics in the irreversible processes, I found him, this brightest fellow in the early stage of Japan after WWII. Actually he was one of the top figures in the theoretical physicists in Japan right after WWII.
Motoyosi Sugita founded the Japanese Biophysics society as one of the first founding members. To start up the society, in order to show how the scientific society of Bio- physics was important to the Japanese Government, they presented a book on Biophysics as the proceedings of the first meeting among the Japanese Biophysicists [1] . In the part III of this book, Motoyosi Sugita wrote a review, “The Biological Open Systems and Fluid Equilibrium”, where his use of the words “Fluid equilibrium” was meant the so-called “Dynamical equilibrium”. It was his life-time objective to construct “Thermodynamics of life”.
As I read any one of his articles, I have been impressed by his very deep thought on Life as well as Thermodynamics. His ideas seem to me very important and crucial for understanding the physical aspects of life, and therefore, very prompt for making a breakthrough in the research of theoretical biology. Thus, in this paper I would like to summarize what I have studied from his works.
1.1. Birth
Motoyosi Sugita was born in Yatsushiro-machi in Kumamoto prefecture in Japan on August in 1905. Exact day of the born was not known so far. His father was Heishiro Sugita and his mother Haya Sugita. They had the first boy, but the boy was gone by sick before Motoyosi was born. So, Motoyosi became the officially first son of their family. His father Heishiro was the school teacher, later became a principal so that he went many places for teaching as a principal. During Heishiro was spending time at Kumamoto with his mother, Motoyosi was born.
1.2. Education
He graduated from the elementary school attached to the Kochi Teachers College and entered the Dai-ichi Junior High School in Kochi prefecture in 1919.
In 1921 he was admitted to the Konan Junior High School in Kobe, Hyogo Prefecture, moved from the Junior High School in Kochi. At this time most of good high schools in Japan had both junior high school and high school as one school system for 5 - 6 years. The Konan High School was one of them.
In 1926, he graduated from the physical science division in the Konan High School and entered the Department of Physics, the Faculty of Science, the Tokyo Imperial University.
In 1929, he graduated from the Department of Physics, the Faculty of Science, the Tokyo Imperial University. The Tokyo University at that time was under the old system of the Great Empire of Japan, which was totally different from the modern university system of Japan after WWII. Hence, the Tokyo Imperial University was perfectly different from the Tokyo University nowadays. The former was only for the top rank brilliant students selected from them in the Japanese society at that time.
1.3. Working
In 1929, He was enrolled as a researcher at the Institute for Electricity (Denki- Shikenjo), the Ministry of Traffic and Postal Affairs (Teishin-sho).
In September in 1934, he became a teacher at the School for the Japanese Navy Organization.
In August in 1941, he retired the School for the Japanese Navy Organization and became a researcher in Kobayasi Institute in Tokyo (see Figure 2). Here he published his first text book “Thermodynamics New Lecture” in 1942 [2] . He became a lecturer at the Department of Industrial Management, the Tokyo Commercial University.
In March in 1944, he became a professor at the Department of Industrial Man- agement, the Tokyo Commercial University. In April, he also worked as a lecturer at the Tsudajyuku University.
In June in 1949, he became a professor at the Department of Economics, the Hitotsubasi University (see Figure 3). As a concurrent post he became a professor at the Department of Commercial Science, the Hitotsubasi University (until it was re- pealed by the school system change by the Government in 1951).
In September in 1949, he earned the Ph.D. in Science from the Kyoto University by the doctoral thesis “Thermodynamics of Transient Phenomena”, whose partial fulfil- ment was published as a book “Thermodynamics of Transient Phenomena” from Iwanami-shoten in 1950 [3] .
In April in 1953, as concurrent posts, he became a professor at the Department of Sociology, and at the Department of Economics, the Hitotsubasi University. At the same time, he was promoted to be a lecturer for Condensed Matter Physics, at the Department of Engineering, the Meiji University.
Figure 2. Personnels of “Kobayasi Riken” in 1945. The picture was probably taken in front of the Kobayasi Institute (Kobayasi Rigaku Kenkyujo, shortly Kobayasi Riken), Tokyo, Japan, in March in 1945, right after WWII. Dr. Motoyosi Sugita is the second person from the left in the second row (By courtesy of the Kobayasi Rigaku Kenkyujo).
Figure 3. Prof. Motoyosi Sugita. The picture was probably taken at the age of 74 in the home office of his house, Tokyo, Japan in 1979 (By courtesy of Misuzu Shobo).
In September in 1956, he became a professor both for the Tokyo Commercial University and Hitotsubashi University until it was repealed by the school system change by the Government in 1962.
In April in 1959, he became a lecturer for the intensive lecture for Modern Technology at the Department of Management Sicence, the Konan University and a lecturer for the intensive lecture for the General Commercial Engineering, at the Department of Economics, the Ooita University.
1.4. Marrige
Motoyosi Sugita married Ms. Grace Sakae Oyama in 1933 (see Figure 4). Grace is her Canadian name. She was a Japanese originated Canadian whose ancestry was Christian and immigrated from Hirosaki, Aomori, Japan. She was born in Toronto, Canada. She graduated from the Victoria High School in Toronto. She entered the Nursing School there and graduated at the top of the school. She became the first nurse of the Japanese- Canadians in Canada.
She came to Japan for marriage with Motoyosi Sugita, leaving her family in Canada in 1933. During WWII, she spent very sad time because Japan and Canada became enemy each other, and her family in Canada was forced to be sent to the concentration camps in Canada. Long after WWII, when they visited USA and Canada for attending the International conferences for biophysics and bioengineering, she was able to meet her family members in Canada for the first time in 28 years.
They had one son, Yūkiti. Yūkiti went to Indonesia for his business after Motoyosi and Sakae died. However, he failed his business and he returned back to Japan. He spent his final days in his family’s summer house in Hokuto-shi, Yamanashi, Japan. Yūkiti died on 2 August 2012. Only one relative of Motoyosi Sugita’s family is Ms. Setsu Honda who lives in Hirosaki, Aomori, Japan. Other relatives are now living only in
Figure 4. Prof. Motoyosi Sugita and his wife, Grace Sakae Sugita. The picture was taken at his age of 56 in front of her parents’ home in Hirosaki, Aomori, Japan in July, 1961, when they visited there for their greeting to the family right before they went to attend the Conferences in Canada and USA. During the visit abroad she was able to meet her family and relatives in Canada for the first time in 28 years since she came to Japan for her marriage with him (By courtesy of Ms. Setsu Honda).
Canada.
The above information was sent as a letter from Ms. Setsu Honda as her courtesy. I really appreciate it from the bottom of my heart.
1.5. Visits Abroad
In July in 1961, he visited the United States of America and Canada for three months. He attended the 4th International Conference on the Medical Electronics held at New York and the International Conference on Mathematical Biology held at North Carolina.
In August in 1965, he visited the U.S.S.R., Austria, Italy, France, England, West Germany, and Denmark for three months. He attended the International Conference on Molecular Biology held at Napoli, Italy and the second International Conference on Biometrics held at Helgoländ, West Germany.
At the time, he became the president for Bioengineering of the Japanese Society for Medical and Biological Engineering (until 1967).
In 1967, he visited the U.S.S.R., Sweden, West Germany, Netherlands, Belgium, France, Swiss, Austria for three months. He attended the 7th International Conference on Medical Electronics held at Stockholm, Sweden and the 3rd International Con- ference on Biometrics held at Helgoländ, West Germany.
In 1969, he retired from the Hitotsubashi University and became a professor emeritus (see Figure 5).
On the 14th day of January in 1990, he passed away at the age of 85.
Figure 5. Prof. Motoyosi Sugita. The picture was taken at his age of 80 in front of his second house in Hokuto-shi, Yamanashi, Japan in August in 1985 (By courtesy of Ms. Setsu Honda).
1.6. Publications
Motoyosi Sugita published many textbooks [1] - [6] as well as the general books, which were all written in Japanese such as Soceity and Cybernetics [7] , What is Cybernetics? [8] , W. Heitler: Thinking and Wanderings [9] , What is Information Science? [10] , Society and Theory of Systems [11] , Recommendation of Engineering Thinking [12] , The Function of Academics and Creation [13] .
At the same time he published many important scientific papers. Before WWII, he published papers written in German [14] - [21] . After WWII, he published many papers in the Japanese science journals for the public such as Kagaku (meaning Science) and Seibutsu Kagaku (meaning Bioscience) [22] - [35] and Iryo Denshi to Seitai Kogaku (meaning Medical Electronics and Bioengineering) [36] [37] . He published many Japanese articles in his working place reports: in the journal of Kobayasi Institute such as the Bulletin of Kobayasi Institute [38] - [59] ; and in his Hitotsubashi University journals such as the Annals of Hitotsubashi University [60] [61] [62] , Hitotsubashi Ronso [63] [64] [65] [66] [67] , the Bulletin of Hitotsubashi University [68] - [75] . He also published many papers written in English in Western Journals such as the Journal of Physical Society of Japan [76] [77] [78] [79] [80] and the Journal of Theoretical Biology [81] - [91] , as in the references. And also he published many other papers on Physics education and Mathematics education as well, which are neither included nor listed in this paper. You can just see them in the National Diet Library, “Kokkai Toshokan”, of Japan [92] .
1.7. The Research History of Motoyosi Sugita
As early as in 1930’s before WWII, he started to study physics. During this time, at first he seemed to spend much time to translate German physics papers written in Germany (Deutschland) such as Carl Wagner [93] and Georg Siemens [94] into the Japanese and published the articles to the Journal of the Mathematical and Physical Society of Japan (Su-butsu Gakkai Shi). Once he found the concept of the virtual heat, he applied it to the thermodynamics of transient phenomena, and in doing so, he published papers in German in the Japanese journals [14] - [21] .
Thus he seemed to be an expert for the German language in the Japanese physics society at that time before WWII, since in the Japanese education system at that time the Japanese education system had been admirringly adopted from the German system as the first foreign language in the schools in Japan. And surely before WWII, Germany was one of the top countries in sciences including Chemistry and Physics at that time.
Although it has been perfectly forgotten already, Japan was a leading long-time economical supporting country for Germany that was economically totally broken by the WWI. Many Japanese business men privately supported the German society as well. A famous example was Hajime Hoshi who was one of the richest fellows in Japan at that time and he was the founder of the Hoshi Pharmaceutical Company and the Hoshi College of Pharmacy. Hajime Hoshi had supported the Chemical Society of Germany for a long time until Germany would recover [95] up to the era of the Adolf Hitler’s Third Reich of Germany.
Nearly ten years before WWII, Sugita published several famous papers in German as well as in English in the Japanese journals [14] - [21] as mentioned above. However, after WWII his works seemed to be ignored in the Japanese physics society. Because since then, the Japanese education systems totally changed to fit with the English-based society of U.S.A. from the German-based society of Deutschland before WWII. This changed to adopt English as the first foreign language instead of Germany in the schools.
Motoyosi Sugita studied the foundation of thermodynamics for biological systems [2] , and continued it after WWII. From the line of his German physics study which was the top physics country at that time mentioned above, he studied the theory of the German physicists, Becker and Döring [96] and Volmer [97] and an American physicist Frenkel [98] on the cluster growth in the metastable phases in supersaturated vapors.
As early as in 1948 right after the damage of WWII slightly reduced in the society, Motoyosi Sugita published an important paper that discussed the relationship between the metastable (or quasi-static) phenomena in thermodynamics and biological pheno- mena in the Japanese journal, Kagaku [23] . It was also published in the textbook entitled by Thermodynamics of Transient Phenomena [3] .
As the Japanese society was coming back till 1950 he published a more fundamental paper in a Japanese journal, Seibutsu Kagaku [29] . After a long study on the theory of thermodynamics in the transient phenomena such as life, he first postulated that there might exist the 4th law of thermodynamics; otherwise one cannot understand biological phenomena. He stated his considerations on it in §5 entitled by “Can one consider the 4th law of thermodynamics?”. I would like to quote here in the corresponding part from its English version [60] as follows:
…By the way, let us think here the circumstance deeply. According to the 2nd law of thermodynamics, Gibbs’ free energy, G, of the world has tendency to decrease in isothermal and isometric change. On the other hand, we find the tendency that the velocity of decreasing of G, i.e.,  wants to take a large value as far as possible. This might be a general principle of nature which I should like to call temporarily the 4th law of thermodynamics.
wants to take a large value as far as possible. This might be a general principle of nature which I should like to call temporarily the 4th law of thermodynamics.
The foundation of such a large principle will be discussed later, and we can suggest here that it is very important and beneficial idea that the nature of the transient phenomena as well as the living system may be clarified and explained uniformly by this principle.
There are many delicate problems concerning human thought if we propose to clarify the nature of life on the basis of physical and chemistry. In any way the matter looks like as if it were concerned in the 4th law. …
Hence, following the line of thought of Motoyosi Sugita [29] , I can summarize the laws of thermodynamics as follows:
1) The first law (W. Thomson’s principle): The Gibbs free energy G is conserved in a closed system; .
.
2) The second law (Clausius’s principle): The entropy S always increases in any process; .
.
3) The third law (Nernst’s theorem): The entropy approaches zero as the absolute temperature T approaches zero; .
.
4) The 4th law (M. Sugita’s postulate): The decreasing rate of the Gibbs free energy always takes the maximum in any process; 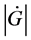 = max, where
= max, where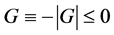 .
.
Fortunately Sugita published the above paper one year later in English [60] . But it was unfortunate since the journal of the Hitotsubashi university (to which he belonged) that he published was not famous at all among Western physicists as well as the Japanese physicists. And also it has not been available to the public for so long until recently after the internet service was provided.
In 1953 Motoyosi Sugita has found the way to apply the theory of thermodynamics of transient phenomena to more realistic biosystems such as metabolic systems [41] [42] [43] [44] [45] [76] [77] [78] [79] . From this stage his research entered the second stage to construct the thermodynamics of life. Step by step his way of thinking became cybernetics-like, where the feedback control systems played an important role in his theory [46] - [59] [81] [83] - [91] . One of them was cited in Steuart Kauffman’s famous book, The Origins of Order [99] .
Since no computer system was easily available for the bio-systematic calculations in Japan at that time, Motoyosi Sugita collaborated with electrical engineers to construct analog-digital computer circuits for their calculations. They simulated the circuits to obtain the solutions of their-own models of the metabolic control systems. These ideas were summarized as books [1] [5] [6] .
After retiring the Hitotsubashi University, Motoyosi Sugita became to write and publish many general books to the community [7] - [13] .
Although he published many papers and textbooks in science as well as many general books in Japanese, he published only about ten papers in English by unknown reasons. That is why he was so unknown in the Western countries as well as in Japan. Hence, nobody knew him nowadays, and so did I, in spite of his extremely important contributions to the thermodynamics theory.
In this paper I would like to review some important consequences of his theory and discuss the maximum principle in the open non-equilibrium systems as the foundation for the 4th law of thermodynamics to the readers especially in the Western countries.
In Section 2, I will show the bright ideas of Motoyosi Sugita such as the concepts of the broad quasi-static change, the irreversible cycle, and the virtual heat.
In Section 3, I will discuss the Motoyosi Sugita’s approach to the diffusion pheno- mena as the first successful application of his concepts.
In Section 4, I will review the theory of phase change and condensation as a preparation for understanding the following sections.
In Section 5, I will present the theory of thermodynamics of transient phenomena of Motoyosi Sugita, where his theory of chemical reactions will be shown using the concept of the field of chemical potential.
In Section 6, I will show the Motoyosi Sugita’s concept of the maximum principle in the transient phenomena. Here  = max conjecture will be discussed, which is a demonstration for the existence of the 4th law of thermodynamics.
= max conjecture will be discussed, which is a demonstration for the existence of the 4th law of thermodynamics.
In Section 7, I will compare the work of Motoyosi Sugita and those of Lars Onsager and Ilya Prigogine. I hope that the content of this section will be shared with the Western people.
In Section 8, I will discuss the maximum principle of Motoyosi Sugita and that of Pontryagin as well as the Bellman’s principle of optimality. This includes my own theory of the application of the Pontryagin’s maximum principle to thermodynamics. Therefore, I believe that this section is as my emphasis most important among other things.
In Section 9, I will show the Motoyosi Sugita’s theory of metabolism which is the first application of his maximum principle to theory of life. He spent many years as many as 20 years for studying this problem from many sides repeatedly.
In Section 10, I will present the Motoyosi Sugita’s way of thinking on the theory of life. This opens up the thermodynamics of life or life being as well as the network thermodynamics.
In Section 11, as the final section, a simple summary will be made.
2. The Bright Ideas of Motoyosi Sugita
A couple of years after Onsager published his seminal papers on the reciprocal relations in the irreversible processes in 1931 [100] [101] , Motoyosi Sugita published the theory of thermoelectric effects and the Kelvin’s relation in 1933 [17] . This was much later published in Japanese in the Japanese journal during WWII [20] [21] and included in his new text book of theromodynamics, “Netsu Rikigaku Shinko”, meaning Thermo- dynamics New Lecture [2] . In this research he introduced the concept of broad quasi- static change, the virtual heat, and the irreversible cyclic processes in order to describe the irreversible changes in thermodynamics of transient phenomena.
2.1. Sugita’s Concept of the Broad Quasi-Static Change
Motoyosi Sugita [2] [3] meant the quasi-static change “in the broad sense” by the naming of the broad quasi-static change. The broad quasi-static change is defined when the following conditions may be assumed:
 (2.1)
(2.1)
where 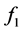 and
and 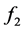 are some functions of V and T, respectively.
are some functions of V and T, respectively.
These are a generalization of the conditions for the case of ideal gases where
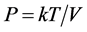 and
and 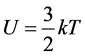 are satisfied with k being the Boltzmann constant. When
are satisfied with k being the Boltzmann constant. When
these conditions are assumed to be satisfied, one can almost follow the standard approach of the quasi-static processes in thermodynamics which means that the process is indefinitely very slow. Indeed, even in the non-idealistic cases the treatment of the quasi-static change in the normal sense has been applied and given plausible results.
This means that the local equilibrium can be satisfied even in the non-equilibrium states, where the broad quasi-static change makes sense if one excludes the relaxation phenomena in which conditions 1) and 2) are not satisfied. Unless the relaxation phenomena are considered, the broad quasi-static change can be applied to most of irreversible processes. The concept of the broad quasi-static change has the amazing possibility of development, once it is combined to statistical mechanics.
Suppose that many macroscopic parts are in the equilibrium state. Let us denote by 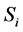 the entropy of that i-th small part and by
the entropy of that i-th small part and by 

Therefore, if we use

then we have

This means that when one considers the broad quasi-static change in the irreversible processes, one should not take or need not take the W or Z over the entire phase space. One must cut off the phase space into the small pieces that are in local equilibrium, and concatenate them to cover the whole phase space. This is the meaning of the local equilibrium in the point of view of Motoyosi Sugita.
2.2. Sugita’s Concept of the Virtual Heat
Another assumption that forms the theory of irreversible processes is the concept of the virtual heat. Let us consider the case when the heat 


is satisfied, where 




Substituting Equation (2.5) into Equation (2.A), we obtain

This 


where

Now, from the concept of the broad quasi-static change, 

Here we never think as if

holds true.
If we think of the adiabatic change without input and output of heat, i.e., 

Hence,

What is important here is the distinction between 

In Equation (2.5), if the work 

For the outside of the system, the reservoir gives the heat 


Therefore, for the whole system we have

Thus, the Sugita’s concept of the virtual heat is very natural and important when one considers the thermodynamics of transient phenomena.
2.3. Sugita’s Concept of the Irreversible Cycle
Let us now consider the Sugita’s concept of the irreversible cycle. Let us denote by 




since the reservoir gives the heat 
Let us denote respectively by T and S the temperature and the entropy of the system (working material) that performs the cycle. Then we have

since the system receives the heat 



After finishing the cyclic process, the system has to come back to the initial stage of the process (i.e., the initial condition) such that it yields

Hence, the total entropy change in the cycle is given by

On the other hand, for reversible processes, since

one has

Thus, the entropy change occurs only in the thermal reservoir outside the system in the process of the irreversible cycle, such that Equation (2.18) cannot conflict with the second law of thermodynamics. The equality of Equation (2.18) has a great meaning
that the integration of 
irreversible cycle. Therefore, one can treat quantitatively the thermodynamics of the irreversible cycle in the same as that of the reversible cycle.
I would like to note that this aspect of the Sugita’s concept of the irreversible cycle is different from that of the Prigogine’s concept of the irreversible cycle, where the entropy change of the system is treated as a quantity that always increases during the process such that 

Now let us consider the change in the thermal reservoir. The heat 


since 

called the reduced heat. However, the reduced heat is not a real heat for the reservoir, since the sign of it is reverse to that of entropy of the reservoir [Equation (2.22)]. Thus, although the concept of the reduced heat plays a historical role, it is not so important as a physical quantity. From Equation (2.19) together with Equation (2.15) the following holds

This is nothing but the Clausius’ inequality for the irreversible cycle, where the equality holds true for reversible processes. This can be regarded as the generalization of the standard proof for the Clausius’ inequality for the irreversible cycles [2] [3] [4] [113] .
Historically speaking, Clausius accomplished to derive this relation for the first time. From the fact that the equality holds true in the reversible processes, he showed that

represented a closed curve representing any thermodynamic cycle by a staircase with adiabatic curves and isothermal curves. Regarding the cycle as a combination of many infinitesimal Carnot cycles, for the high temperature sources, denote by 




Now if the intervals could be made so as to be infinitesimally small, then Equation (2.24) becomes

This is the Clausius’ inequality derived from himself [113] .
However, once we look at the expressions in Equation (2.24), the sign of the reduced
heat 

quasi-static reversible processes it becomes exactly the entropy change of the working material and hence the equality of the above Equation (2.25) can hold. From this situation, usually it has been thought that one cannot treat the theory quantitatively since the inequality holds in the irreversible processes or when one seeks for the
entropy by

because one escapes from the complexity of the irreversible processes such that one need not take into account the working material, and because one discusses the cycle process only considering the lost heats. On the other hand, although not always but when the process can be regarded as the broad quasi-static change, and when the true character of the virtual heat is clearly known such as the friction heat or thermal conduction, we can take 

Since the state goes back to the initial state after the completion of the cycle, it seems trivial that the entropy goes back to its initial value as well. Since it was said that in the irreversible processes, one cannot say anything about the entropy in the midst of the process, we could not have said anything about like the above. Now we should note that 
In order to derive the entropy, one must consider the quasi-static reversible processes and define the heat of the processes in the standard point of view as usual. But once one defines the entropy under the quasi-static change, one can use the relation 




This is the argument of Motoyosi Sugita for the concepts of the broad quasi-static change, the virtual heat and the irreversible cycles in the thermal processes. He applied these concepts to the various physical systems such as the thermoelectric effects of Lord Kelvin [2] [3] [4] [14] [15] [17] [20] [21] as well as many biological systems [38] - [91] .
2.4. Application to the Kelvin’s Relation in the Thermoelectric Effect
As an application of the above results, we become able to treat the problem of thermoelectricity (see Figure 6), which had been thought to be difficult to consider. Now, let us denote by (1) the high temperature part with the temperature 




Seeing from the outside of (1), the ejected heat per second 

Next, let us denote the resistance by




This is the so-called Peltier heat. If we take 

Figure 6. The system of thermoelectric effects.

For the part (2), we similarly obtain

where the minus sign in the left hand side comes from the reverse direction of the current i in the circuit.
Next consider the part 





This is coreesponding to the quantity


Similarly for the wire B and considering the part

where the sign in the right hand side is due to the condition that the direction of the temperature is reversed to the direction of the current i.
In the stationary state, the sum of the heats that this system absorbs equals the work that the electromotive force E can do. Hence, one has

which is the equation corresponding to the relation (2.B) but its integrated form, where the integration of 


Next, suppose that the relation 



It is obvious when the thermoelectric couple is in the stationary state. Then the entropy change occurs only in the thermal reservoir (or one may think the one-round of electron through the circuit as a cycle.) From Equation (2.32) and Equation (2.33), the Kelvin’s relation is given by

So far we are based on the Clausius’ inequality:

Now, rewriting




The derivation of the above result Equation (2.37) is as follows: Denote by 





For the wire A we find

By integration by parts we obtain

On the other hand, for the wire B we similarly obtain


respectively. Here we think that 



Therefore, based on the inequality (2.37), one cannot derive the Kelvin’s relation, as long as the thermal conduction and Joule’s heat can be neglected. Namely, to take Equation (2.37) as the base is not wrong but not sufficient [3] . As Boltzmann [114] had shown the following relation

this cannot exactly yield the Kelvin’s relation.
Now, from Equation (2.36) the linear term of 

This means that if Equation (2.36) is added a small quantity less than 0, then Equation (2.37) forms an inequality. Therefore, it is obvious that one cannot derive the desired result from it. On the other hand, we obtain Equation (2.36) by not only considering the heat flowing in from the outside of the system but also considering the heat that the system absorbs. And by this we can derive the Kelvin’s relation exactly.
In summary, the point of view of Motoyosi Sugita lies in the fact that one can obtain Equation (2.36) even for the irreversible cycles. Now, the entropy increase can be obtained immediately from Equation (2.46). Motoyosi Sugita [2] [3] [4] [20] [21] pointed out the insufficiency of the Tolman’s argument [115] in the same problem.
3. Sugita’s Approach to the Diffusion Phenomena
Let us next consider the Motoyosi Sugita’s approach to the diffusion phenomena [3] . In order to see how the concepts of the broad quasi-static change and the virtual heat work in each physical problem, he applied them to the diffusion problem [3] . The diffusion phenomena are really important phenomena when we construct the theory of thermodynamics in transient phenomena. To do so, I would like to follow his argument in his test book [3] .
3.1. Langevin Equation
Now let us consider an ideal gas that is constructed from the mixing of the two species of molecules 1 and 2. For the sake of simplicity, we assume that the density gradient only exists in x direction. This is equivalent to consider the one-dimensional diffusion problem along x direction.
Let us denote by 




Hence,

Now, let us denote by


where

Or if we use



Now taking as 

where

then the above equation looks like the Langevin equation type such as

So far we have considered the case of ideal gases. However, for more general cases, if we use

the right hand side of Equation (3.6) can be rewritten as

or if we use 

As Sugita pointed out, the Langevin equation is the equation of motion that one thinks as if the stationary motion is considered statistically so that the effect of accele- ration cannot play a role to the averaged velocity, and the random forces come from like-particles in thermal motions are statistically averaged.
Next let us explain that when the work done by the resistance of friction 
3.2. Mixing Entropy and Free Energy
Now let us denote by 





where 

If the equation is reversely seen, then the chemical potential has to be defined by

Now, since the G is decreasing by the mixing, from Equation (3.9) we have

This means that in the mixing system of ideal gases, the mixing entropy is increasing by the mixing of the molecules and by it G is decreasing. And although the decrease of G is the decrease per second in the irreversible process, we cannot necessarily know but surely know the change in time of G in the midst of the process.
Based on the Langevin equation Equation (3.6'), suppose that the molecule is forced to move by 
For the sake of simplicity let us limit ourselves to the ideal gases. Then, only the energy per molecule 


If we assume the process is an isothermal change, then replace 

The first term in the right hand side vanishes if the boundary condition is taken. In the second term in the right hand side, since we have the continuity equation:

if we change 


On the other hand, in the ideal system the change in G only occurs in the change of entropy. Therefore, if we rewrite 


Hence, this agrees with Equation (3.11). From considering the above situation, we can recognize that the assumption that the force acting on the diffusion particle is given by Equation (3.6’) is not inconvenient.
Let us consider the case of the non-ideal systems [28] . In this case the chemical potential 




Therefore, once we add the heats due to Equation (3.15) and Equation (3.16) to the above increase of entropy due to

This is not inconsistency but rather everything is consistent very much. If we compare the above with Equation (3.16), then

This means that 



3.3. How to Count the Number of Partition
For this title, Motoyosi Sugita used the word the number of complexion. The number of complexion is nothing more than the number of partition in classical statistical mechanics or the number of sates in quantum statistical mechanics in the modern terminology [116] [117] . When the system is in equilibrium, the number of ways that particles interchange their positions in the system is nothing but the number of complexion in his terminology. In this equilibrium case, one can definitely define the chemical potential such as Equation (3.8).
Can such a treatment be allowed even in the midst of the non-equilibrium process? This was the Motoyosi Sugita’s problem. Obviously, it is allowed for the equilibrium state of the process. But it is not trivial for the non-equilibrium state in the irreversible or transient phenomena.
Suppose that the previous argument that provides Equation (3.8) is correct [28] . Assume that 

where




Hence, by taking the functional derivative for the above with respect to


The second term in the right hand side comes from the mixing entropy of the system. Therefore, it corresponds to what one assumes that the number of partition at an instantaneous time in the midst of the process is given by

If we take




Now, suppose that the whole region of space is divided into small parts of 



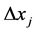
Next, if we remove the partitions and wait for a short time so that the diffusion occurs, and if we place the partitions in the system once again, then the distribution of the molecules becomes different concentrations of 
In this way, as the distribution of concentrations is continuously changing by the diffusion, the corresponding region in phase space is also moving continuously through the region corresponding to each state in the midst of the irreversible process and going into the region corresponding to its final equilibrium state. Once the true equilibrium is achieved, not changing or mixing the molecules within the region 
There is no limit for time in the equilibrium state, since each process is reversible so that an infinite time can be spent. Therefore, even if molecules are far apart to each other, the state that the molecules exchange their positions can be realized by the ergodic assumption. When the diffusion occurs, 


In other words, the molecules within 
On the other hand, for each diffusion molecule, it should not move with the constant velocity 



In summary, the above argument is the Motoyosi Sugita’s argument on the application of the concepts of the broad quasi-static change and the virtual heat to the diffusion phenomena [3] . He also applied his brilliant concepts to many other systems such as the osmotic pressure for the cell membranes. However, I would like to skip this here.
4. The Theory of Phase Change and Condensation
Before going to discuss the Motoyosi Sugita’s theory of thermodynamics of transient phenomena in detail, let us look at the theory of nucleation or condensation in 1930’s [96] [97] [98] as an excursion.
His theory was motivated to be apply it to construct the thermodynamic theory or thermodynamics of life or life being [3] . In order to construct thermodynamics of life or life being, he presented mainly three big concepts:
1) the “field”of chemical potential;
2) the generalized nonlinear Ohm’s law;
3) the maximum principle in transient phenomena.
He extracted these concepts from intensively studying the theory of phase change and condensation or nucleation originated by the German physicists, Becker and Döring [96] and Volmer [97] and an American Physicist, Frenkel [98] before WWII. Therefore let us first review some of the early theory of nucleation phenomena as a prototype or precursor of the theory of thermodynamics of transient phenomena of Motoyosi Sugita.
4.1. Frenkel’s Theory on Nucleation in the Supersaturated State
When Mayer [120] studied statistical mechanically the nature of vapor, he considered clusters that are aggregated into groups of several molecules in addition to single molecules. Frenkel [98] simplified this idea to phenomenologically represent such groups of several molecules as spherical clusters with a certain radius.
Let us now denote by (n) a cluster with n molecules. Denote by 



If we define by 
then

Next, define by 

then the Gibbs free energy G of the whole vapor is given by

where T is the temperature of the system, k is the Boltzmann constant. Here 
When the mixing entropy between single molecules and clusters with different sizes should be evaluated, the expression of mixing entropy for molecules of identical sizes is assumed and used to this case. This seems a problem. And as n becomes large, the expression for the mixing entropy must be modified. Furthermore, we cannot imagine that big liquid drops can float in the vapor. Therefore, the above expression of the Gibbs free energy is not exact quantitatively. However, if we restrict ourselves to the phenomenological argument, then it seems sufficient to approximately represent the system by Equation (4.4).
On the other hand, let us denote by 

Suppose that this mixture of clusters in vapor lies in equilibrium. In this case, seeking for the maximum of G with taking the variations of 


or

This is the equation that determines the distribution of (n)-clusters first derived by Frenkel [98] .
This is the distribution of concentrations of (n) clusters in the equilibrium state in the saturated and the supersaturated vapors. It means as follows: When some part of the gas conforms the mixture of (n)-clusters and when the mixture is included in the system, the entropy becomes larger than the one when all the molecules stay as single molecules in the vapor. If we look into each molecule, 


4.2. Supersaturated Vapors
Next following the argument of Frenkel [98] , let us consider the supersaturated vapor. When the vapor is supersaturated, since

where if we denote by 


Considering Equation (4.8), as n becomes large, 
Thus one can understand the behavior of the supersaturated vapor system pheno- menologically. If


Motoyosi Sugita pointed out the following [3] [23] : As stated in the previous section, one can assume that even in the transient phenomena, the expression of 


Now, the cause that interrupts the changeover from the vapor phase to the liquid phase is the lack of the surface area which leads to the condensation. In order that the liquid phase emerges in the supersaturated vapor phase, it has to pass through the state of extremely small liquid drops in the midst of the process. Large clusters are not included in the midst of the growth. Very small is the rate that clusters gradually grow as the condensation occurs at the surface of small (n) clusters. Therefore, the system is at a standstill as a vapor. According to Frenkel [98] , this is the metastable state. The distribution of 
Motoyosi Sugita noted here the following: When the Gibbs free energy for the entire system G is decreasing as the second law of thermodynamics shows, there appears some part which has larger Gibbs free energy, and this supports the place where con- densation occurs, and it determines the rate of the change in the transient phenomena.
The reason why clusters have large 
Next, let us differentiate Equation (4.8) with respect to n and evaluate the maximum.
Substituting the value of 


the liquid, M the molecular weight and 


On the other hand, let us denote by 

From this we find the Kelvin’s equation:

And if the maximum of 


where K is the maximum size of the clusters. According to Frenkel [98] , the cluster of this size is the nucleus of condensation. The range that 




In the above argument, we have assumed that in the metastable state the rate of growth of cluster is small enough. And although the system is not in equilibrium, we have treated it as if it were in equilibrium. This provides the variation principle

4.3. Kinetics of Cluster Growth
According to Becker and Döring [96] , the growth of the cluster is carried out in the following process:

Here 1 stands for a single molecule and it collides with (n), the cluster consisting of n molecules and condensates into


and so on, the probability of these processes must be very small enough compared with that of the collision process of Equation (4.14), since triple collisions or the collision between (3) and 1 are molecular dynamically very few events such that they loose the meaning to form
Volmer [97] slightly improved the calculation of Becker and Döring [96] . In his calculation he denotes by J the rate of the process of Equation (4.14), which is the total number of clusters per time from 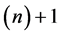


where 



Now in equilibrium of the growth process, both the forward and backward processes are attained such as

This provides

where N is the total number of molecules in the system such that


In the saturated vapor, it is no wander that Equation (4.18) must hold valid as well. However, even in the supersaturated vapor, the rate J is expected to be small such that


In summary the above theory is the theory of condensation in the supersaturated state originated by Becker and Döring [96] and completed by Volmer [97] and Frenkel [98] in the Western countries before WWII.
4.4. Thermodynamics of Kinetics of Cluster Growth
Motoyosi Sugita investigated the above theories of Becker and Döring [96] , Volmer [97] and Frenkel [98] very carefully.
In considering the kinetics of the cluster growth thermodynamically, since the half of Equation (4.18) could be valid even for the non-equilibrium state and then it holds as well, he rewrote Equation (4.18) as follows:

Denote now by 

concentration 1, respectively. Then J is given as

Let us introduce the concept of the chemical resistance 

and let us define the chemical potential for a single molecule in cluster (n) in the midst of the growth process by

Then Equation (4.19) can be rewritten as

The above equation looks like the Ohm’s law of


Now, from this point of view the growth process of clusters

can be regarded as a system of resistances in series in an electrical circuit following the Ohm’s law. In the nucleation process the resistance 
Motoyosi Sugita noted the following: The mathematical form of Equation (4.22) is much more convenient than that of Becker etc. It is not the equation to show the destiny of the growth and the decomposition of clusters. Each cluster emerges as a result of statistical and thermal fluctuations and the system moves towards its dynamic equilibrium by following the field of force as the needles of a clock move. This idea becomes very important when we think of crystal growth and life being. What is important here is not the quantitative treatment but the qualitative treatment of the theory. In order to introduce the concept of the field of chemical potential, i.e., the m-field, the expression of J was written in the form of Equation (4.22) in terms of the chemical potentials 



5. Thermodynamics of Transient Phenomena of Motoyosi Sugita
As is noticed in the above, the theoretical framework of the above theory is very general. Becker and Döring [96] as well as Volmer [97] applied it to the two-dimensional crystal growth on the surface of crystal. Indeed Motoyosi Sugita applied it to many physical systems in the broad quasi-static change or in the metastable state from physics to biology [4] [5] [6] [41] - [91] . In this section, I would like to focus our attention on the application to chemical reactions.
5.1. Motoyosi Sugita’s Theory of Chemical Reactions
Let us consider the chemical reaction of the following form [104] :

where 




On the other hand, Motoyosi Sugita [3] [60] frequently used the following expression for it:

where 



Denote by 

species in mole (simply called the mole numbers). Let us denote by 





where 



reaction or degree of achievement and the reaction rate by Theophile De Donder [102] [103] [104] , respectively.
On the other hand, Motoyosi Sugita used 


where i and j stand for the reacting and produced molecules. In this way Motoyosi Sugita emphasized the change of molecular numbers in molecular statistics [3] [5] [60] , while the De Donder’s school emphasized the change in the reaction equations [102] [103] [104] .
Following Motoyosi Sugita’s theory [5] , let us define the Gibbs free energy G as

where 



It means that if the amount of 

is done such that the Gibbs free energy increases. Since the sum in the Gibbs free energy G is linear in

Using the reaction equation of Equation (5.4), we have

where if 

where 

This is nothing but the Affinity introduced by De Donder [102] and Progogine [103] [104] before WWII as well as Marcelin [122] and Jouguet [123] even before WWI in Western countries.
From the knowledge of thermodynamics, G becomes the minimum when the system goes to chemical equilibrium. Hence, in chemical equilibrium,



This yields the following criterion for the direction of the reaction of Equation (5.2):

This is the criterion given through the concept of affinity.
Let us denote by 

Simply suppose that the mixing entropy is approximately given as

where 


Substituting these into 

This yields

where we have defined as

Rewriting Equation (5.18), the law of mass action in the chemical equilibrium is given by

where 
Now let us consider when the system is not in equilibrium. In this case, Equation (5.4) yields the rate equation:

where 




Here if we may follow the argument of Prigogine et al. [103] [104] , then we may define as

where

5.2. Motoyosi Sugita’s Concept of the Generalized Nonlinear Ohm’s Law
Following the theory of Motoyosi Sugita [5] , let us define the chemical resistance 

Here I would like to note the following: If we rewrite as

respectively, then we can derive the relation of detailed balance:

Thus, the equation for 
Motoyosi Sugita jumped over the standard way of thought and he assumed that the chemical potential is meaningful even when the system is not in equilibrium yet. Therefore, he assumed for all components of the molecules

Using Equation (5.28) together with Equation (5.25), he was able to rewrite Equation (5.24) as follows:


Applying these into Equation (5.21) or Equation (5.23) and rewriting
was able to derive the following:

This expression has the generalized nonlinear form of the Ohm’s law:
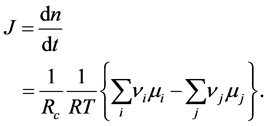
Now we are able to know the following relation:

where 

5.3. Motoyosi Sugita’s Concept of the Field of Chemical Potential
Equation (5.31) was first introduced by Motoyosi Sugita long long time ago [23] [29] [60] . He was inspired by the expresson of Equation (4.22). It was derived by Becker and Döring [96] , Volmer [97] and Frenkel [98] (shortly represent BDVF). They studied the theory of condensation considering the nucleation of clusters in the supersaturated state. Therefore, it describes the non-equilibrium state in the irreversible process of condensation.
Motoyosi Sugita recognized that when chemical equilibrium is slightly broken or when chemical reactions are going on, the physical conditions in chemical reactions are the same as those in condensation as well as nucleation. Thus I would like to express schematically the relationship between the Sugita’s J and the BDVF's J in the following:

The mathematical form in 





As was discussed before in the previous section, in Equation (5.35) one can derive the above expression for J without any problem. However, the expression for 

As an example, let us apply the concept to the condensation on the surface (of either liquid or solid). Denote by J the number of molecules per second that collide with the surface. Denote by 


Here 









Motoyosi Sugita recognized that this kind of rule for phase change seems very similar to that of the Gibbs’ phase rule for the equilibrium state [126] . In the former phase change occurs as a consequence of the broad quasi-static change in the irreversible process of transient phenomena, while in the latter phase change occurs as a con- sequence of the realization of equilibrium state. Thus there is a conceptual difference between them such as the former is time-dependent and the latter is not time- dependent. However, Motoyosi Sugita postulated the validity of the application of the concept of the field of chemical potential to many biological nonuniform systems such as polymers or macromolecules in protoplasm in cells.
Denote by K a part in a nonuniform system and denote by 






and if the chemical reaction in equilibrium in the part K is given by

then the condition

must be satisfied. On the other hand, if the equilibrium is not yet attained, then the kinetic rate equation like Equation (5.35) and Equation (5.36) should be applied to describe the system. Even for dynamic systems such as the system of life or life being, if the system is in dynamic equilibrium such as fluid equilibrium or chemical equilibrium, then we may assume that Equation (5.39) is approximately satisfied in the sense of broad quasi-static change and of local equilibrium. This is the concept of the field of chemical potential introduced by Motoyosi Sugita long ago.
Now, let us turn back to the reason how Motoyosi Sugita noticed the concept of field of chemical potential, for a while. As mentioned in the previous sections, he studied the the quasi-static change in classical thermodynamics in Japan before WWII, where Japan was very isolated from Western countries occasionally dashing to the war. He found the way of exceeding it and called it the broad quasi-static change in quasi- thermodynamics. He understood that chemical potential 

In my opinion, it is obvious that his way of thought came from this experience in his physics study, and then he applied the concept to other physical and chemical examples such as chemical reactions. The first look of Motoyosi Sugita for this discovery seems to be the following simple equation:

where 
So, Motoyosi Sugita stepped forward to go beyond the equilibrium thermodynamics to the quasi thermodynamics of transient phenomena. Thus, although we think of chemical potential such as a numerical value for the equilibrium state in the standard point of view of thermodynamics, Motoyosi Sugita never thought like this but he always thought that chemical potential is a field defined on space-time such as the field in field theory even for dynamic, nonuniform, irreversible, non-reproducible and transient phenomena. This is his philosophy on the field of chemical potential.
5.4. Relationship between Cooperative Phenomena and Chemical Potential
Motoyosi Sugita further mentioned that the chemical potential plays an important role when the system undergoes phase change. It is not well-known in the recent modern text books in thermodynamics [103] [104] [105] [106] [108] [116] . This is the cooperative effect when the system undergoes a phase change in the irreversible process in transient phenomena. In other words, it can be dubbed the much more modern word, induction-association principle for the phenomena. The word inducetion- association principle was first introduced by Gilbert N. Ling [129] [130] and has been advocated by Gerald H. Pollack [131] [132] for a long time.
This very particular aspect of the phenomena is the following: When the system faces a phase change, if it undergoes the phase change, then it cannot occur so literally, however. Rather, it never occurs even when the temperature of the system already goes below the critical temperature at which the phase change is supposed to occur. This is the supersaturation phenomena, discussed before. At this moment, in order to make the system undergo the phase change, a nucleus or a stimulus, i.e., a kind of trigger is needed for the phase change. Otherwise, it stays still. Conversely speaking, even if the stimulus of a trigger is very very small or microscopically small, the entire system undergoes the phase change. Hence, the effect is very cooperative. This phenomenon is not perfectly understood yet even nowadays.
In order to understand this type of phenomenon, Motoyosi Sugita emphasized the importance of the concept of the field of chemical potential, namely the m-field. According to Frenkel [98] , even in solid, partially melt parts are included near melting point. But the parts do not develop so easily even below the melting point. He put it the name pre-melting. Motoyosi Sugita postulated that in such a case the m-field plays an important role. He imagined that the m-field is always fluctuating thermally or locally under various conditions. One good example is the cluster growth in the previous section. Local raise of the value of m-field promotes the local phase change in the system until the system goes to equilibrium. A local variation of m-field initiates the action at a distance to another point in the system. It is long-range interaction. Thus, local variation of m-field acts as a trigger for the phase change through the long-range interaction of m-field. This is the nucleation in the supersaturated phases.
In this way, the system can communicate through the m-field in the system just like when the electrical potential does. This means that m-field extends the lines of force as an induction of the generalized potential, the m-field. Mathematically, it may be con- sidered as the gradient of the m-field. Therefore, the idea of induction-association principle of Gilbert N. Ling seems very similar to that of cooperativeness of m-field of Motoyosi Siguta.
The cause of such lines of force comes from the m-field. The m-field consists of the mixing entropy term. Hence, the lines of force or the long-range interaction between the parts in the system appears as the consequence of the mixing entropy, e.g., the last term in Equation (5.40). In the standard viewpoint this nature of long-range interaction emerges as a consequence of electric effect. However, Motoyosi Sugita extended the concept such that so is true for the m-field as well. On the other hand, the action of energy is not long-range but local or short-range. Thus, the induction-action principle of Gilbert N. Ling and the cooperative effect of Motoyosi Sugita comes from the mixing entropy, not from the energy of the system.
Can one think of the field in life being as the m-field? He sometimes asked such a question. In such biomaterial or life or life being, the m-field is not made of a single component but is constructed by a huge number of components. Obviously, the structure of the m-field becomes very complex. If so, then it would be very very difficult to calculate the entropy term, calculating the partition number such as in Equation (3.23), since no simple formula exist for such complicated molecular systems. However, in principle and ideally, we can think of the Gibbs free energy G, the number of partition W and the sum of states Z, etc. Then we can expect that the system can move towards the direction of 
His conclusion is as follows: The m-field is very long-range enough to affect each other. A slight variation such as temperature fluctuation can be eliminated by the cooperativeness of the entire system. Therefore, when one has to apply thermo- dynamics to the system in considering the entire system as a whole. Especially the second law of thermodynamics is such a law. One must be very careful when he applies the second law of thermodynamics to the partial systems. It is sometimes said the following: Since life is not a closed system, we cannot apply the second law of thermodynamics to life. This seems a ridiculous idea, since it misses the point. While one takes into account the Gibbs free energy, one may consider the partial systems, under the condition that temperatue is constant, one may consider the other parts as thermal sources by which some parts can be affected with other parts through the thermal communication to each other.
5.5. The m-Field, as an Invisible Force
In the previous example of the cluster growth in the supersaturated phase, the clusters grow like this:

Here in between the nearest states of clusters 


The field of chemical potential of 






In Equation (4.8) as n becomes large, 
After the growth processes of non-equilibrium thermodynamics are finished, once such clusters or complicated life being develop their structures, the fully developed structures of such clusters or fully developed complicated structures of life being consist of very large Gibbs free energy by definition. This complicated situation seems to contradict the laws of thermodynamics.
Thus, Motoyosi Sugita noticed that there might exist some kind of hidden law of thermodynamics in addition to the three laws of thermodynamics. He postulated that there might exist the 4th law of thermodynamics which dominates the speed of the transient phenomena as mentioned in the introduction.
6. Motoyosi Sugita’s Concept of the Maximum Principle in Transient Phenomena
In this section, let us consider the most important contribution of Motoyosi Sugita in my viewpoint. As discussed in the previous section, the concept of the filed of chemical potential is quite important when we considerate the non-equilibrium processes.
6.1. Motoyosi Sugita's 
As early as in 1950 Motoyosi Sugita wrote a paper entitled in Japanese, Biological Thermodynamics and its Method, which was published one year later from the Annals of the Hitotsubashi University, entitled in English Thermodynamical Method in Biology [60] . He stated in Japanese on the existence of the 4th law of thermodynamics [29] as the paragraph quoted in Introduction. And also he first applied his theory of thermodynamics in the transient phenomena to the theory of metabolism.
For the reason why Motoyosi Sugita believed the existence of the 4th law of thermodynamics, he listed several examples that seem to be related to this 4th law as follows:
Here let us see many instances suggesting this large principle of thermodynamics.
1) The cascade principle(Stufenregel) found by W. Ostwald [97] shows that the nature has the tendencies as if it wanted to take the pass of smaller resistance or make a de tour and want to establish the equilibrium as fast as possible.
2) Generalizing further the rule described above, it might be said that the nature prefers the line of the least resistance, if there are ways side by side for the equilibrium.
a) According to Volmer [97] , for instance, the crystal formation shows that such a pass is taken actually.
b) Eyring and others [124] [125] called such a process rate determining.
c) Electric current in conductor takes the distribution that heat loss is minimum if the total current takes a given value. Therefore the heat generation must be maximum if the potential difference will be taken as constant. Therefore, if a cell is applied to drive the current, it will take the distribution to dissipate the free energy of the cell as fast as possible.
d) Onsager [100] [101] has derived his reciprocal relation from the principle of least dissipation function. This principle might be considered to the maximum velocity of entropy increase which will be discussed later.
3) If a new passage is built independently which has less resistance than others already existing, then the circumstance above described, that might be the 4th law of thermodynamics, may also be seen from our common sense.
a) The new way may be considered having delicate catalytic action, therefore, large free energy of activation or small entropy. The free energy of activation determines the rate of development of such a passage acting as if the initial cost is to construct a highway. That is why the construction of the way of small resistance is retarded. Nevertheless, it becomes rate determining when it is performed and the old ways become only bypass or will be ruined.
b) The idea of natural selection or struggle for life of biology may be considered as having the relation to this principle. That is the free energy discharged through the old passage is used to the free energy of activation of new way, and the material itself constituting the old way may be useful also as the material of construction (see (v) of VI).
c) Such a circumstance like natural selection can be seen also in the inorganic worlds. For instance, let us observe the nuclear formation of ice in supersaturated water vapor under freezing point, and containing super-cooled water droplets. If the crystal nucleus is formed, not only the condensation occurs on this nucleus, but the super-cooled droplets vaporize and disappear. This is the consequence of the 4th law and the same phenomena can be seen on the discharged plate of PbSO4 of battery and also in the case of recrystallization of metals and others, and they are playing a role to promote the tendency to the thermodynamic equilibrium.
Thus from the early beginning of his research he recognized and imagined the existence of the 4th law of thermodynamics, where he expected that some kind of the generalization of the least dissipation of energy of Onsager could be necessary. Therefore, I would like to call his expectation the Motoyosi Sugita’s 
In the next section of that paper [29] [60] , VI. Mathematical Theory and Conclusion, he sketched the outline of the 4th law of thermodynamics as follows:
1) First, on the base of microscopic reversibility, Onsager [100] [101] has shown that

where 

2) On the same base as Onsager, Landau and Lifshitz have shown that

in their statistical physics [134] .
3) Let us denote by 



where 



4) Let us consider quasi-chemical processes

between the components. This describes a set of chemical reactions such as chain reaction, whose set is denoted by s. It means that there are many chemical reactions that consist of a finite number set of molecules 

5) Let us denote by 


Through this linear transformation, the variables 



6) Substituting Equation (6.6) into Equation (6.3), we find

where we have defined the affinity 

I would like to note here that there is a sign mistake for Equation (6.79) in the English version of this paper [60] .
7) From the rate theory of chemical reaction, 

where 
8) Inserting Equation (6.9) into Equation (6.7), we can see that 



The procedure, which neglected the higher term, corresponds with the cut off method discussed in Section 2. The summation of the right hand side of Equation (6.10) may be interpreted as

9) The reversal of the m-field can be interpreted if we consider the transition from the stage

In the above paper [29] [60] Motoyosi Sugita was not able to present the detail of the proof of the conjecture. It was limited to suggest the existence. However, in the succeeding papers [40] - [46] [48] [61] [75] [77] [78] [79] he argued the sketch of the conjecture and frequently tried to prove it.
6.2. Relationship between the Boltzmann’s H-Function and the m-Field
In order to investigate the Motoyosi Sugita’s conjecture, the so-called Boltzmann’s H-function plays an important role. So, let us first consider the relationship between the Boltzmann’s H-function and the m-Field for the molecular statistics. For this purpose I would like to restrict ourselves to consider the system of chemical reactions only. However, this way of thinking can be generalized to other physical, chemical and biological systems as well.
Since there is a basic idea for proving the conjecture in [75] , I would like to follow it here. Motoyosi Sugita first defines the Boltzmann’s H-function for chemical reactions by

where 


And if we write 

where the equality holds true only when
Next, let us consider the derivative of H-function with respect to time along the course of time development. Then, Motoyosi Sugita considers the following:

Let us consider the chemical reaction equations such as Equation (5.21) for chemical reactions of Equation (6.5), for our case here. Associated with the choice of the reaction Equation (6.6), we can write the chemical reaction equations in the following:

where P and R mean production and reduction in the chemical reactions, respectively, and 

where



and

respectively. On the other hand the affinity for each chemical reaction is defined by Equation (6.8). The chemical equilibrium values 

Obviously this produces the law of mass action such as Equation (5.22) for each chemical reaction:

Substituting all into Equation (6.15) using the definition of chemical potentials Equation (6.4), finally Motoyosi Sugita ends up with

This is nothing but Equation (6.10), where 
Theorem 1 (Boltzmann’s H-theorem).

Let us now prove the H-theorem. Following the similar argument of Motoyosi Sugita [75] , we find the following:

where 

then the summand looks like

Since
The equality holds only for the equilibrium. Hence, the theorem is proved.
6.3. Motoyosi Sugita’s Idea for the Proof of the Conjecture
Motoyosi Sugita also considered more general case of the nonlinear processes, which may be represented by the following equations:

where x means a vector of 



The stability of this system is investigated by the Lyapunov theorem. Denote by


where we have assumed that 

Now the simplest Liapunov function is defined as

By differentiating this with respect to t, we obtain

If all real parts of the eigenvalues of the Jacobian matrix 

Motoyosi Sugita applied this theorem to the Boltzmann’s H-function. And he proved the H-theorem is valid if the Lyapunov’s theorem holds.
Let us define the general Boltzmann’s H-function:

By differentiating the above equation, we immediately obtain

Motoyosi Sugita proved a mathematical theorem:
Theorem 2. If 


where 

Let us follow his proof, which is short. By assumption, we expand 


Let us define 



Using Equation (6.37), the Jacobian can be Taylor expanded in the following:

The remained procedure is to use Equation (6.35) and Equation (6.39) and to derive the right hand of Equation (6.34).

Hence, the theorem is proved. This is nothing more than the mean value theorem in the analysis for the analytic functions with many variables.
Using this theorem, Equation (6.33) turns out to be the following:

Now if all the real parts of 

have the same sign. Therefore, we can obtain the following:

By differentiating Equation (6.33) with respect to t, we obtain

The second term in the above is always positive. Let us now substitute Equation (6.29) into the first term, we find

By definition

the Liapunov theorem is valid, all the real parts of eigenvalues of the Jacobian are negative. Hence, by multiplication, Equation (6.44) is always positive. From this fact, we obtain the following property of the H-function:
Theorem 3.

Let us define the entropy production 

where k is the Boltzmann constant. Therefore, from Theorem 3 we immediately yield the following theorem:
Theorem 4.

This is nothing but the theorem of the minimum entropy production or the Prigogine’s principle of minimum entropy production [103] [104] .
In summary, this is the outline for the proof of the conjecture proposed by Motoyosi Sugita [75] . He tried again and again to prove this conjecture from various point of view. However, the general proof has never been done in his life time.
7. The Ideas of Motoyosi Sugita as a Specific Development of Lars Onsager’s Lifework
In 1951 Motoyosi Sugita first presented the theory of the maximum principle in transient phenomena such as those discussed in the previous section [38] . This paper was entitled as The Maximum Principle in the Transient Phenomena and the Application to Biology in Japanese. In this paper he first stated his vision and idea on the maximum principle in the transient phenomena. He argued the relationship between his idea of maximum principle and the existing old ideas such as the maximum-minimum principle in the Joule heat, the Boltzmann’s principle in the theory of gases, and the Onsager’s principle of the least dissipation of energy in the theory of irreversible processes [100] [101] . He finally applied his idea to many biological systems such as the thermodynamics of metabolism, the relationship between the maximum principle and the metabolism, the origins of life, and the dynamic equilibrium, the relaxation oscillations, the wholeness of life, etc.
In the succeeding paper in 1952, he further studied the maximum principle in relation to the Boltzmann’s H-theorem [40] . This paper was entitle as The Relationship between the Boltzmann’s H-Theorem and the Dissipation Function in Japanese. This paper is a really instructive one. As is discussed in the previous section, his theory preceded the times of Prigogine [103] [104] . So, in this section I would like to present his comparison between the Motoyosi Sugita’s theory and the Prigogine’s theory as well as Onsager’s theory [100] [101] and Katchalsky’s theory [105] [106] [108] . Fortunately for the Western people, these Japanese papers were summarized as the English versions [77] - [79] .
7.1. Relationship between the Boltzmann’s H-Function and the Irreversible Work
As is shown in the previous section, we have obtained the Boltzmann’s H-function, especially for the case of chemical reactions. Motoyosi Sugita first applied his idea of the virtual heat that has been discussed in the section II to the irreversible work of the system.
In order to see the difference between the method of Motoyosi Sugita and that of Ilya Prigogine more easily, let us change the notation of Motoyosi Sugita to adjust with that of Prigogine. Let us denote by i the internal system which is doing the irreversible work. Let us denote by e the external thermal reservoir, where we assume that no irreversible work has been done. By definition, we have

If the process is the broad quasi-static change (under the isothermal and isopressure), then we have

which is equivalent to

This is not satisfied when the irreversible work exists. In this case we have

which is the isothermal irreversible work. Or equivalently,

On the other hand, since the heat 

Now we assume that there is no heat exchange otherwise, the total entropy of the system is given by

Since there is no irreversible work in the reservoir e,



This suggests that there exists the maximum principle in the transient phenomena in terms of the Boltzmann’s H-function. Hence, his conjecture is crucially important in the theory of non-equilibrium thermodynamics in the irreversible processes in the transient phenomena such as life. It also suggests the existence of the 4th law of thermodynamics.
On the other hand, Prigogine only showed that for the internal system

This means that the entropy of the internal system 

However, as Motoyosi Sugita discussed long long ago, no matter what the irreversible process is taken into account, the following must be satisfied when the internal process is cyclic since the final state must come back to the initial state after one cycle:

On the other hand, as is shown in Section 2 [see Equation (2.19) and Equation (2.23)], we must have for the external reservoir after one cycle

This is the advantage of the Motoyosi Sugita’s concept of the virtual heat for the irreversible processes in the transient phenomena.
7.2. Relationship between the Boltzmann’s H-Function and the Dissipation Function
This argument can be generalized to the systems of flow dynamics or fluid dynamics. In this case there is matter exchange between the reservoir e and the system i. Let us denote by 




This 



or equivalently

This simple looking but very important relation was independently rediscovered by Katchalsky much later [105] [106] [108] . What is important here is that the above equation is valid even for open systems such as the flow or fluid dynamics as well as life systems.
Now Motoyosi Sugita imposes the maximum principle for this relation as follows: Suppose that 






maximum under the restriction that
Theorem 5 (Motoyosi Sugita’s Maxmum Principle) Under the constraint

Thus Motoyosi Sugita challenged us who can prove this principle in general. Whoever can do so, then the 4th law of thermodynamics is proven.
7.3. Relationship between Motoyosi Sugita’s Theory and Lars Onsager’s Theory
Following the idea of Lars Onsager [100] [101] [118] [119] , the entropy change 

where 

Let us define the state variables,

where 



Equation (7.19) together with Equation (7.18) yields

where

Here 

Considering Equation (7.20) together with Equation (7.19), Onsager postulates the following variation principle:

Or equivalently,

Here Onsager assumes that the quadratic dissipation function 

where 
By the variation principle for Equation (7.22) or Equation (7.22'), we have to consider the following variational equation:

From this, we obtain

Substituting Equation (7.23) into Equation (7.25), we obtain the famous linear relation:

where the coefficients 

This is the Onsager’s reciprocal theorem. Solving Equation (7.26) for

where


The variational principle of the above equation is given as

Apart from the energy dissipation 

Since for the isothermal system the internal energy is kept constant, the rate of the Gibbs free energy 



as expected, where 
Now I would like to note that the variational principle of either Equation (7.24) or Equation (7.30) falls into the Motoyosi Sugita’s maximum principle discussed before [see Equation (7.16)]. In the above case of Onsager’s minimum or maximum principle, Onsager implicitly assumed that there exists a constant M such that







Next let us consider the relationship between the Motoyosi Sugita’s m-field and the the above Onsager’s theory. As is shown in the above Onsager’s relation between the generalized forces and the generalized flows(or currents) are linear [see Equation (7.26) and Equation (7.28)]. However, in the Motoyosi Sugita’s theory it is not so but it is nonlinear. Going back to Equation (6.10), we hold the following relation:

where from Equation (5.31) or Equation (6.17), 

Let us suppose that the system is nearly in the thermodynamic equilibrium as was considered by Onsager. If we assume

where 


where 


respectively. Then,

Or inversely,

Formally solving the above for

where 

might be obtained if 


which can be written in the quadratic form like Equation (7.23) if the terms of the higher order of 



where 

7.4. Relationship between Motoyosi Sugita’s Theory and Ilya Prigogine’s Theory
Following the idea of Prigogine [102] [103] [104] , Prigogine assumes that the entropy increase of the system 

where Prigogine’s 




while the affinity 

where 

This looks different but it is equivalent to Equation (7.33). The correspondence between the Motoyosi Sugita’s theory and the Ilya Prigogine’s theory is as follows:
Sugita (1951) [38] Prigogine (1999) [104]

Thus I can conclude that Motoyosi Sugita succeeded in formulating the theory of non equilibrium thermodynamics long before it was reformulated again and intensively applied by the Prigogine’s school. Unfortunately, since such papers were written first in Japanese and published in Japanese journals such as the Bulletin of Kobayasi Institute and the Journals of the Hitotsubashi University, the contents of his theory have never been appreciated worldwide. This was really unfortunate for us to study his theories. This is one of the reasons why I am writing this paper.
8. The Relationship between the Motoyosi Sugita’s Maximum Principle and the Pontryagin’s Maximum Principle
Now I would like to prove the conjecture of Motoyosi Sugita’s Maximum Principle, using the optimal control theory [135] - [141] . From this, the relationship between the Motoyosi Sugita’s maximum principle and the Pontryagin’s maximum principle becomes clear. I would be able to conclude that the Motoyosi Sugita’s Maximum Principle is nothing but the Pontryagin’s maximum principle in the theory of optimal control.
As I have written in the introduction, I became aware of Motoyosi Sugita’s work in this Spring in 2016. Two years before this year, I have written a couple of papers on the application of the optimal control theory to thermodynamics [142] [143] . Therefore, at that time I did not know the research work of Motoyosi Sugita at all. However, once I became familiar with his work on the maximum principle that is given in Equation (7.16), I became sure that his maximum principle is nothing more than that of Pontryagin [135] [136] such that I can prove it using the theory of optimal control. This approach will be a generalization of the Motoyosi Sugita’s proof given in Section 6. And it will fill in the lack of proof for the conjecture of Motoyosi Sugita with a rigorous one.
Before doing so, I would like to present my philosophy for the problem, since my motivation has come from the very different viewpoint from that of Motoyosi Sugita. I would like to show it in the next subsection first.
8.1. Attractiveness of the Formulation of Classical Mechanics
What is most attractive in the theoretical framework of classical mechanics is as follows:
We believe that the energy is conserved in any mechanical problem, unless there is no dissipation of energy. This is the concept of energy conservation law. Based upon this energy conservation law, we assume that the initial energy is given in the problem for the mechanical system such as a pendulum or a spring. So, as long as there is no dissipation of energy, once the initial energy is given to the system, then it moves automatically and forever. This is our understanding on the physics of macroscopic mechanical objects.
As we know in classical mechanics, all variables in the system are mechanical variables such as the coordinates whose vector is given as 


On the other hand, in our problem of non-equilibrium thermodynamics for the systems of life or living things, the system is described by dynamical change of the densities of ions, atoms, molecules, etc. under chemical reactions. So, the densities are given as a sum of the sets of classical particles or objects. At a given time the system is determined by the instantaneous values of the densities in the system. Since the system is dominated by the densities, we may call them the state variables. Thus, we have to treat the macroscopic state variables as the new type of mechanical variables in the dynamical systems.
This means that we regard the biologically living macroscopic system as a classical mechanical system given by regarding the state variables as the mechanical variables. This point of view is interesting, since we can regard the living objects as classical mechanical objects. As if a pendulum moved automatically following the energy conservation law, the macroscopic biological system would move automatically following some unknown law of physics. If such a new type of law exists, it will be very nice. I would like to find such new principle of conservation law. This is our goal here.
8.2. Modern Control Theory and Pontryagin’s Maximum Principle
The above vision of mine seems quite similar to that of Motoyosi Sugita. What I stated as the unknown law of physics is absolutely what Motoyosi Sugita stated as the 4th law of thermodynamics long ago. Since there are many detailed mathematical proofs for the Pontryagin's maximum principle, I would like to skip such proofs in this paper, but only show the essence of the proof. If you want to see such proofs, then I would like to recommend you to consult other books [135] - [141] and my papers [142] [143] .
In this section, we are going to consider the essential concepts and the formalism of the so-called Pontryagin’s theory of optimal control [135] [136] for the later purposes. This theory is the totally new type of extensions of the standard control theory [144] which is based upon the negative feedback mechanisms before 1960. Since then, the Pontryagin’s theory was called the modern control theory, while the old control theory was called the classical control theory. This reminds us of what happened in the discovery of quantum mechanics.
On the other hand, theoretically speaking, the Pontryagin’s theory of optimal control is the natural extension of the formalisms of Hamilton’s principle and the least action principle in classical mechanics [134] . It was totally a revolution in theoretical physics as well. However, much has long been not so well-known in physics society. It seems because the revolution has occurred in the optimal control theory and the automatic control theory in engineering community around the year of 1960 and because the value of scientists of USSR was intentionally and absolutely ignored by the Western scientists at that time in the era of the cold war between USSR and USA. This was very unfortunate.
8.3. Equations of Motion for the Open Dynamical System
Let us denote by 




namely,

As in the case of classical mechanics, once we regard the state variables x as the classical variables, we can define a Hamiltonian. Let us denote by 


According to the Pontryagin’s theory of the optimal control [135] [136] , we can prove the Hamilton equation:


In order to escape from the confusion between the standard Hamiltonian in classical mechanics due to Hamilton and the Pontryagin’s Hamiltonian in the optimal control theory, we would like to use the Pontryaginian or Pontryagin’s Hamiltonian for the latter. This is because they are totally different from each other in a physical unit. Hamiltonian is given in units of energy [Joules], while Pontryaginian is given in units
of work rate, 

Equation (8.4) provides nothing but the original equations of Equation (8.1) by definition. Equation (8.5) is something else. But it can be regarded as a generalization of the speed of change in the Gibbs free energy,
To understand this point, let us suppose that 



Substituting this in the above original Pontryaginian of

This is nothing but the standard time-derivative of Gibbs free energy: Since




This expression means that the rate of Gibbs free energy is conserved under the time-development of the system. This proves the first part of the conjecture for the maximum principle of Motoyosi Sugita, where he assumed that M = constant for

absolute value of the Hamiltonian 
8.4. Proof of the New Conservation Law
The general proof of the conservation of the Pontryagin’s Hamiltonian is quite complex. It is not so convenient to describe the detail in short here. Since the proof is given in the text book of Pontryagin et al. [135] [136] , we skip the detail. Therefore, I would like to describe the essence of the proof.
As before, we start with the dynamics given by Equation (8.1) [or Equation (8.2)]. Let us find the equilibrium state taking the variation 

where 


Substituting the above into Equation (8.1), we can expand the original dynamical equations with respect to

The matrix 
Next, let us define the adjoint matrix,

And let us define the following dynamical equations for the new functions,

where


Let us prove that the above Pontryaginian is a constant of motion in the nonlinear dynamical systems for the state variables. Please do not confuse that this problem is a problem for mechanical variables in classical mechanics. Although the Pontryagin’s Hamiltonian is mathematically analogous to the Hamiltonian, it is not the same physical quantity; the former represents the work rate (i.e., the power) and the latter the energy in our choice, as was mentioned before.
Differentiating with respect to time, we have

And we have

for

Here we have assumed that the extremum condition for 


By definition, this is equivalent to the following optimal condition:

where the maximum condition [141] is also given by

If the condition is for the minimum then the inequality has to be reversed.
Let us now impose

which is nothing but Equation (8.13), since if we take its transpose then we have

This equation is called the adjoint equation for the original nonlinear dynamical equations of Equation (8.11). The new set of variables 

Substituting Equation (8.21) into Equation (8.17), we finally obtain

This means that 

Hence, we have proven that the Pontryagin’s Hamiltonian (the Pontryaginian) is conserved in the course of time-development. Thus, the proof is obtained.
This Pontryaginian in the nonlinear systems with the state variables plays an important role of the Hamiltonian in classical mechanics. Physically speaking, this means that as long as the Power is fixed as a conserved quantity, there exists an optimal process that preserves the power.
8.5. Comparison with the Prigogine’s Method
The above approach is quite analogous to the Prigogine’s method in the nonlinear systems [103] [104] . The Prigogine’s method for the stability of the nonlinear dynamics is nothing more than the Lyapunov’s method in mathematics.
In this method, we first assume that the left hand sides of Equation (8.1) or Equation (8.2) are all zeros. This provides the following:

Suppose that Equation (8.25) can be solved. Solving Equation (8.25) for 



Similar to Equation (8.9), we expand the state vector as

Substituting this into Equation (8.2), we similarly obtain
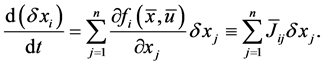
For the special values of

Then substituting the above into Equation (8.28), we obtain

This yields the characteristic equation:

By investigating the characteristics of
In this way, we can understand that the Pontryagin’s method in the optimal control theory is a natural generalization of the Prigogine’s method in nonlinear theory.
8.6. Generalization of the Pontryagin’s Hamiltonian to the System with a Constraint
In the above, we have proven that the Pontryagin’s Hamiltonian with state variables in nonlinear dynamics plays the role of the Hamiltonian of mechanical variables in classical dynamics. And we have shown that the Pontryagin’s Hamiltonian is a constant of motion of the dynamical system, i.e., a conserved quantity. However, we have not yet proven that the Pontryagin’s Hamiltonian takes its maximum value in the region of the admissible control parameter vectors. And we have not yet show that the principle works as well, even when there is a constraint of the system. This constraint is an- alogous to the constraint that we know as the least action principle through the Lagrangian L in classical mechanics. We are now going to consider these problems.
Suppose that there is a constraint in the system such as

where the time-development of the system obeys Equation (8.1) and 

Let us now impose that this constraint takes the minimum value in the course of the time-development of the system in between 





The physical meaning of this is the following: We evaluate the functional 




In this more general case than the previous one, we can define Pontryagin’s Hamiltonian 

Obviously this has the form of the Hamiltonian in classical mechanics such as




Let us now suppose the following new variable 

By differentiation we find

Let us define a fictitious parameter

Then we can represent the above Equation (8.34) more compactly such as

However, at this time the system must obey the following nonlinear dynamics:

As before, we then have the equations of motion similar to Equation (8.4) and Equation (8.5):


where




where the maximum condition [141] is also given by

If we take 
8.7. Pontryagin’s Maximum Principle
Now we can summarize the very important theorem which is known as the Pontryagin’s maximum principle in the optimal control theory [135] [136] . This theorem is described as follows:
Theorem 6 (Pontryagin’s Maximum Principle) Let us suppose that the dynamical system is described by the nonlinear dynamical equations:

for
Let 










One necessary condition that control 




(1) For all t in time interval




(2) 


In practice, if 

for




The proof of the Pontryagin’s maximum principle is very complicated but is given in detail in the literature [135] [136] . So, we have omitted the proof here. However, the result is quite simple enough for us to apply to physical problems.
Let us go back to the case of the nonlinear dynamics with a constraint 


Hence, we have the following Pontryagin’s maximum principle for this case: at some control vector value

8.8. The 4th Law of Thermodynamics as the Motoyosi Sugita’s Maximum Principle
Now, let us go back to the maximum principle of Motoyosi Sugita [see Equation (7.16)]. We can restate it as follows:
Theorem 7 (Modern Version of the Motoyosi Sugita’s Maxmum Principle) Suppose
that the rate of the Gibbs free energy 


where the state variables are assumed to obey the following dynamical equations:

Then, if the system advances under the optimal control of the control variables u, then there exists a maximum of the Hamiltonian 

And in this moment, always the following equations hold: The equations of motion:

for

and the maximum condition [141] :

Here in Equation (8.49) if 

The above approach of Pontryagin’s maximum principle is very general and therefore it should not be restricted within thermodynamics. However, I would like to see the relationship between the Motoyosi Sugita’s maximum principle and the Pontryagin’s maximum principle.
Let us apply the above method to thermodynamics especially for the isothermal system where T = const. In order to do it, we must assume that the dissipated energy from the system is becoming the virtual heat such that

where 

or equivalently

Therefore, once we regard this variational constraint as 

where we have taken as
then we must regard the Hamiltonian 

yields

This Hamiltonian was first found by the author two yeas ago [142] [143] .
Thus, as long as we take the extremum using Equation (8.46), we have to obtain the following simple relation

This is the most general expression for the Gibbs equation generalized from the standard one in the textbook of thermodynamics:

Furthermore, if we impose the quadratic relation for the dissipation function of Equation (8.50) then we substitute it into the above. We finally obtain the following relation:

8.9. Relationship between the Pontryagin’s Maximum Principle and the Bellman’s Principle of Optimality
Now I would like to make a comment on the relationship between the Pontryagin’s maximum principle and the Bellman’s principle of optimality (see the details in Appendix). Although both theories seem to treat the same kind of optimal problem, the apparent looking of the results is very different. Even though I can say that they are almost equivalent concepts, it is far from being trivial. Therefore, I would like to clarify this problem. This was first done by Pontryagin et al. [135] [136] .
Bellman simply assumes that there is a dynamical process whose the time develop- ment of the system is given by a time t in between the initial time 



Suppose that the system of the nonlinear equation of Equation (8.2) would be solved under the optimal control u for



Let us define 



Differentiating this with respect to t, we then derive the following:

For the optimal control, we have to take the optimal condition for the control parameter u. So, we finally obtain the optimality relation:

where U stands for the space of admissible control. This is the result that we apply the principle of dynamic programming to the system of the nonlinear equations.
Next, let us define the function

Differentiating this with respect

where we have used the trivial relation 

Since

Then, if we define

then Equation (8.65) turns out to be the adjoint equation:

On the other hand, the Equation (8.61) can be written as

Since the left hand side of the above equation is nothing but the Pontryagin’s Hamiltonian

from using the Bellman’s dynamic programming.
Thus, as was shown by the Pontryagin’s group [135] [136] , the maximum principle of Lev Semyonovich Pontryagin in the modern control theory is essentially equivalent to the optimality principle of Richard Bellmann in the modern control theory.
9. The Motoyosi Sugita’s Theory of Metabolism: The First Application of the Maximum Principle to Life
Around the year of 1951 Motoyosi Sugita found an idea that he should apply his theory of the maximum principle to the theory of metabolism of life [38] . This was intensively studied and published in the Japanese journals [30] [31] [32] [33] [34] [41] [42] [43] [44] [45] as well as in English [61] [77] [78] [79] . Much later he generalized the idea to more complex systems of life where the control or regularity comes into the system and fortunately these were published in English [62] [80] - [87] .
In this section I would like to introduce to you the earliest version of his theory of metabolism as the application of the maximum principle.
9.1. Combined Chemical Reactions
Let us denote by 



where r is the number of chemical reactions and 


This has to mean

If we denote by 

This is the characteristics of life. When the system is not in the stationary state, the life itself automatically performs the cycle and adapts itself with adjusting this cycle by interacting with the external systems. Since it is not in stationary in this case, we have
On the other hand, when we consider the stationary state, since it is not in equilibrium, local entropy production exists due to


where 

Now if we assume that there is no dissipation outside the body of life, then the right hand side of Equation (9.2) represents the dissipation of the Gibbs free energy. The first term means that supply from the outside to the system and waste from the system to the outside. If the system of life is in the stationary state, then they are compensate to each other. Because since


where the first term represents the catabolism and the second term the anabolism.
In chemical reactions in life there seem to exist three types of chemical reactions such as (i) consumption, (ii) supply, and (iii) reproduction. Thus we would like to represent them as follows:

Figure 7. Schematic diagram of chemical reactions in life.
where 







In the system of life, the above reaction are not performed independently, but they should be performed at the same time. Therefore, we can assume the following reactions:

Now we assume that the reaction rates (speeds) are defined by




where


and 












Thus, once we consider in this way, the reactions are performed automatically such that the Gibbs free energies ejected from the reactions of (ii) are used for the reproduction reactions of (iii) and the dissipations of the Gibbs free energies from reproductions (namely, the Gibbs free energy produced by catabolism) are used for the progression of this combination of the chemical reaction of Equation (9.5).
9.2. Reactions of Metabolism and Maximum Principle
For the sake of simplicity, we assume the following:


This means that the Gibbs free energies produced from (ii) supply are all used for (iii) reproduction. From this situation, large energy and negative entropy are produced and they become the energy and entropy for activation. That is, so-called negative entropy has to be interpreted as activation entropy. In order that smart give and receive of the Gibbs free energies can be performed, it is not possible if the activated complex of the reacting components of the materials of (i), (ii) and (iii) takes common feature with large entropy, but it can be possible if it makes the organization with small entropy. Here is the role of proteins. Therefore, such materials like proteins thermodynamically collapse and they proceed the reactions of Equation (9.5) by give and take of the Gibbs free energy before their collapse, and constantly reproduce the materials with holding the materials that collapse are going to collapse. We have to interpret the concept of negative entropy in this meaning. The point of view of Schrödinger [147] and Brillouin [148] has a danger to recognize the negative entropy as a permanent existence.
According to Equation (9.10), the consumption (or exhaustion) per second asso- ciated with 


On the other hand, the reproduction per second is given by

Now when the system is in the stationary state, we have to hold the following relation:

As is discussed in the section VII, Motoyosi Sugita applied the method of maximum principle of Equation (7.16) to the above problem. Assuming that 




Now the relationship between 


Here multiplying 

Comparing Equation (9.16) with Equation (9.13), we obtain

or

This is the detailed balance equation in the process of the chemical reaction s.
From Equation (9.18) together with the help of Equation (9.1'), if we can know the ratios of 


To understand this point, let us assume that there is a material K such that it is produced by the rate of 


As is shown in Figure 8, suppose that 

Figure 8. Schematic diagram of combined chemical reactions in metabolism of life. 
the difference of chemical potential in each reaction is written as

Or if we use Equation (9.19) then

Motoyosi Sugita points out that if we write as

then Equation (9.21) has the same form of the equation of simple reproduction in economics, where 
Similarly to what was discussed on Equation (9.18), what we can say about Equation (9.21) is the following: If we are able to know the ratio between each reaction rate in the metabolism in the body with keeping the life body in the stationary state, then we are able to find the relation between the chemical potentials. From this we are able to measure the negentropy in the living state by the kinematical method. On the other hand, if we want to measure it by the usual chemical method then we have to make the living system in the equilibrium state and hence inevitably we have to kill the life. However, if we seek for the inter-relationship between the chemical rates with keeping the living chemical reactions, then we can measure the free energy of the system that is in the living state.
As a special example for the above theory of Motoyosi Sugita, he discussed the combined chemical reactions of ATP and proteins. Suppose that all protein reactions are averaged for all proteins conceptually such that we can assume that there is only one protein. Denote by 




where 




Figure 9. Schematic diagram of combined chemical reactions of Protein and ATP in metabolism of life.
Thus, when we think in this way, the negentropy (=negative entropy) is not permanently existing, but constantly produced by the reaction (iii), and constantly increasing by the reaction (i). If one doubts why entropy cannot increase in life body, then one forgets the combination of reactions (iii) and (i). Even though

9.3. Analogy between Thermodynamics in the Transient Phenomena and Theory of Metabolism
In the year of 1953, Motoyosi Sugita noticed some analogy between the thermal engine and the chemical engine [30] [31] [32] [33] [34] [41] [42] [43] [44] [45] [77] [78] [79] . He recognized that it is more convenient for us to consider the Gibbs free energy than to consider energy. This is because when we consider the balance of chemical energies in life phenomena, the energy is immortal forever but the free energy can be dissipated. He thought that this point is very important for life.
Suppose that there is a life system that eats foods and discharges excreta. Let us denote by 



Now the balance of the Gibbs free energy must satisfy the following relation:

This equation corresponds to Equation (2.6) or Equation (3.19) in the thermal system such that 




This corresponds to the Gibbs free energy change in the thermal reservoir for the thermal system such that 


Since D in the life system corresponds to 
Let us now take the steady state into consideration. In the steady state

Let us consider the detailed balance of Gibbs free energy. A part of Gibbs free energy 

Figure 10. Schematic diagram of thermodynamics of life as the flow model for the balance of Gibbs free energy.
Thus, we must hold the following:

Then we obtain

Let us define the following relation:
Then we have

9.4. Balance of Substances in Life
As a generalization of the concept of metabolic reaction, Motoyosi Sugita considered the network of the flows of substances in life systems.
Let us denote by 
Catabolism occurs in the directions from more complex molecules to simpler molecules, while Anabolism occurs from simpler molecules to more complex molecules. For the sake of simplicity, let us write the anabolism direction as in a series of





This network is schematically shown in Figure 11.
Figure 11. Schematic diagram of the network of chemical reactions in life system. Solid arrows mean the catabolic reactions and dotted arrows mean the anabolic reactions.
In the stationary state we must have the following as usual:

Now let us consider the weight increase 




where w is the weight of the body. Let us denote by 





respectively.
9.5. Gibbs Free Energy of Life
As described before, let us denote by 



Let us relate the quantities given by Equations (9.24)-(9.26) with the quantities given by Equations (9.32)-(9.36). Let us define the chemical potential that X belongs to the state i by

From this we can define the rate of the Gibbs free energy of the life system by

Now substituting Equation (9.32) into 

This can be converted into the following form:

where 



respectively. In Equation (9.40), 








Now if we define D as

then we obtain

This is nothing but Equation (9.29). In the stationary state it provides the following:

Thus, we have been able to represent the quantities for the life system such as







This is the essence of the Motoyosi Sugita’s theory of metabolic network system, which was entitled as Metabolic Turnover of Entropy and Energy and its Mathematical Analysis in Life I-V. It was first published in Japanese in the Busseiron Kenkyu [30] [31] [32] [33] , which was also published in English in the Journal of Physical Society of Japan [61] [77] [78] [79] , and later the generalized version of the theory was published in Japanese in the Bulletin of Kobayasi Institute as well as in the Busseiron Kenkyu [34] [41] [42] [43] [44] [45] . I would like to strongly recommend the Western people to read his theory published as the English papers [61] [77] [78] [79] .
9.6. The Birth of Network Thermodynamics
The above Motoyosi Sugita’s theory of metabolic network in the life-being system is much earlier than the network thermodynamics that was founded by Aharon Katchalsky’s group [106] [108] as earlier as 20 years. As one of the coworker of Lars Onsager, Aharon Katchalsky with his students George F. Oster, and Alan S. Perelson independently developed the extension of the Onsager’s theory of irreversible processes to the theory of life system. They found the relationship between the network of chemical reaction in biosystems and that of electrical circuit in electronics. Hence, they named their theory the network thermodynamics.
The starting point of Aharon Katchalsky’s group is Equation (7.32):

where in order to adjust with their notation, I have used 

Let us suppose that there is a biochemical network system that is represented by a network graph such as Figure 11. Let us assume that we define chemical potential 



By using this problem-setting, they found the Kirchhoff’s law in biosystems, where there are the Kirchhoff's current law (KCL) and Kirchhoff’s voltage law (KVL). The KCL is the conservation law of the currents on a node such that the total amount of incoming currents to the node must be the same as the outgoing currents from the node. The KVL is the conservation of the voltages (or the potentials) along any closed circuit in the network graph. Obviously these are very common knowledge in the elementary circuit theory or the elementary electromagnetism. Hence there is no need to explain much more. However, although there is a very famous theorem in the circuit theory that is called the Tellegen’s theorem [149] - [154] , this is not so well known among physicists except some experts in electronics, although it is a natural gen- eralization of the theorem of the Joule’s least heat in the steady current or the Thomson’s theorem in the static electric field [155] .
The Tellegen’s theorem states the following: Let us denote by 





then the following relation must be satisfied:

where t stands for the transpose of the vector. This simple and rather looking trivial relation of Equation (9.49) is called the Tellegen’s theorem. And it is really a very powerful tool in the electrical circuit theory [149] - [154] .
What is important here is that Aharon Katchalsky’s group has found that Equation (9.46) in the biochemical network system is equivalent to Equation (9.49) in electronic circuit systems. Let us represent the 



The rate of the Gibbs free energy of the system, 

From Equation (9.46) we obtain

If we explicitly write down the KCL and the KVL for a given network graph and if we use the relations of the KCL and the KVL, then we can prove that the above Equation (9.52) can be converted into the form of Equation (9.49). This is the findings of Aharon Katchalsky’s group [106] - [112] .
I would like to put a comment here. There is a difference between the Motoyosi Sugita’s approach and the Katchalsky’s approach. In the former, Motoyosi Sugita used the concept of field of chemical potential and therefore, the current flow 

while in the latter Katchalsky’s group used the Onsager’s linear relation or the De Donder’s linear relation:

where T is the temperature of the system, k is the Boltzmann constant, 


the above chemical potentials are defined as

Very very sadly Aharon Katchalsky(Aharon Katzir) was killed by terrorism of the Japanese red army at the Ben Gurion International Airport in 1972. He was one of the victims when 26 people were killed by the attack. I feel very sorry for that. I would like to state that Rest in Peace to him and others. Therefore, actually the year of 1972 is the end of his theory. Later on, Perelson and Oster had followed the line of Katchalsky straightforwardly and extended their theory to more complex network systems [106] - [112] . However, their theory became very pure mathematical so that none can easily follow them. Hence, the Katchalsky theory seems dead at that time. However, recently some people noticed the importance of the Katchalsky’s work and applied to the membrane system of life [156] [157] and the complex metabolic network [158] [159] as Motoyosi Sugita did it long long ago as early as 65 years. This would be regarded as some revival for their theory.
As was discussed in Section 7, the reason why we must have Equation (9.46) lies on the fact that there exists the 4th law of thermodynamics in nature from the view point of Motoyosi Sugita. It is realized as the maximum principle for the transient pheno- mena such as life phenomena. The concept of the transient phenomena is much broad concept than that of the irreversible processes studied by Lars Onsager and Ilya Prigogine as well as Aharon Katchalsky. The maximum principle for the transient phenomena always works as the 4th law of thermodynamics in biological phenomena such as metabolism. So, the equation such as Equation (9.46) appears as the Tellegen’s theorem in the circuit theory and in the biological network theory as well.
10. The Motoyosi Sugita’s Theory of Thermodynamics of Life
10.1. The Phenomena of Life and Its Analogy to Social Economics
In the English paper of 1954 [61] , Motoyosi Sugita pointed out the resemblance of the phenomena of life and the social economics, since the life phenomena can be regarded as the society of molecules in cells or that of cells as follows.
1) Our body consumes various organic and inorganic substances, some of which are produced in our body, like hormone, enzymes, protein, nuclear acid, fats and others, and some of which are taken from the external world by the work of our muscles and digestive organs, like inorganic salt, vitamins, amino acids and others.
2) These substances are useful to maintain life. The idea of nutritive value is well known but quantitatively the value of caloric units is mainly taken into account. The nutritive value of vitamins, iron and other inorganic substances and some amino acids is also taken into account but only qualitatively. There may exist the idea corresponding to utility or welfare function in economics which may be treated analytically and quantitatively.
3) There is consumption of Gibbs free energy (shortly say, F. E.) to produce or absorb the necessary substances and consumption is required by production and intake. Even the absorption of glucose, which is the last stage of digested starch is carried out by the investment of F. E. of ATP, an ester of phosphoric acid of high energy. Therefore, in the case of famine or when ill-fed, our organ loses the power to digest or absorb nutritious substances due to the lack of F. E., which corresponds to initial cost. On the contrary, the function of intestinal absorption will be dangerously damaged if over-fed.
The above is shown in Figure 12, in which the energy is fed back to take the chemical energy from the external world. This feeding back is similar to business life, in which an enterprise is sometimes suppressed by the lack of the initial cost. Indeed our
Figure 12. Schematic diagram of thermodynamics of life as the syphon model for the balance of Gibbs free energy. This is equivalent to the diagram of Figure 10.
body corresponds to a factory and ATP to capital.
4) There is the balance of the need and the supply. Superfluous protein, for instance, loses its amino-group and changes into carbon-hydrates corresponding to consumer’s goods. On the other hand, the protein of our tissues, which corresponds to producer’s goods, is destroyed by lack of protein, and the material is used to construct the other necessary part.
According to Professor Kida the relatively short legs of the Japanese are due to the lack of protein of high quality in food during growth. The body seems to lack protein to build legs, for we must use the material to construct the necessary part of our organs. Medical science may be considered good management in the balance of matter and F. E.
5) Our body corresponds to our system of industry. Various substances are produced in every part of our body and supplied to other parts. On the other hand, the parts are also supplied from other parts. There is an exchange and economy of matter and energy. For instance, the production of protein corresponds to the first department of pro- ducer’s goods. In this case as well ATP as protein is consumed. The consumption of the latter corresponds to the depreciation of producer’s goods, in this case the chemical apparatus made of protein.
The ATP which is consumed, is reproduced again in our body and carbon-hydrates, protein and ATP are consumed for reproduction. Here, the carbon-hydrates corre- spond to consumer’s goods and the reproduction of ATP to the second department of economics. Therefore, there is a close analogy between the two fields. For instance, labour is reproduced by the consumption of goods, just as carbon-hydrates in food. This fact is important from the point of view of methodology (see Figure 9).
On the other hand the consumption of protein which is an example of catabolism, corresponds to depreciation which is repaired by anabolism.
6) Depreciation and the repair is the general aspect of life. For instance, reproduction is the turnover of the body itself, which depreciates during life, especially by re- production itself.
If we take, however, the history of man-kind into account, depreciation in the individual body is repaired by other bodies. Therefore, those who enjoy youth enjoy the turnover of the individual body.
Therefore, one of the most prominent aspects of life is the turnover of molecules of cells and of the individual body, so that the world of the living organism is repaired and steadily maintained. This is very important from the point of view of thermo-dynamics, for the F. E. on earth is constantly consumed by organisms.
The steadiness is similar to that of the river, which consumes the potential energy of water and also maintains steadiness on the balance of water.
In a similar manner the depreciation of the apparatus of a chemical plant, the value of N. E. (negative entropy) of our body is also depreciated (c.f., see Section 9). On the other hand this value of N. E. regulates the value of F. E. of activation of bio-chemical reactions. Therefore, the catalytic action of the organs, corresponding to the function of the chemical plant, is also depreciated and repaired. In this respect the writer has introduced the idea of the metabolic turnover of N. E., corresponding to the depre- ciation and the repair of producer’s goods in economics.
7) Besides the feed back of F. E. there is the circulation of matter in our body, for instance the chemical cycle of ATPADP or the reduction and oxidation of enzymes. Figure 13 shows the circulation of phosphates of adenosine in which ATP is included. The circulation is very complicated, in general, but is schematized in Figure 7. This is similar to the circulation of paper money in our society. In a similar manner, the matter of high energy is taken from the external world and excreted, so that our body corresponds to a pipe and is called an open system. But it is not an open system like the pipe through which the water of a tank flows.
8) The feedback of matter and energy is very similar to the management of our social life. Chemical processes in our body are combined like the system of gears (c.f., see Section 9), and, if we wish to promote a process, the effect is fed back and produces sometimes unexpected results. Here is the difference of the biochemical change from that in vitro. Therefore, if the knowledge of chemistry in vitro is applied mechanically the effect may be contrary to expectation, as in the controlled social economy.
There is bad circulation in our body. For instance, the appetite is diminished, if health is destroyed, and health is disturbed if the appetite is diminished. Good management by the physician will eliminate bad circulation.
9) There is the balance and stability of matter and F. E., in our metabolism. If the balance is disturbed,the function of our body is disturbed. We have seen that our body resembled a pipe, through which the matter of high chemical energy flows and the matter of low chemical energy is excreted. The balance seems to be favorable to the flow of matter (see Figure 14). In social life the balance of production and consumption is favourable to the movement of goods. There is the recovering action in our body as well
Figure 13. Schematic diagram of the circulation of phosphates of adenosine in our body [160] . ATP: Adenosine triphosphate; ADP: Adenosine diphosphate; AMP: Adenosine monophosphate.
Figure 14. Schematic diagram of the current flow. (a) Balanced flow. There is no oscillation. (b) Unbalanced flow. There is oscillation perpendicular to the flowing direction.
as in our society. If the balance is disturbed, and cannot be recovered, a catastrophe occurs and finally death of our body.
Prof. Bertalanffy [161] [162] [163] called the balance of our body dynamic stability or equilibrium. From the point of view of thermodynamics, this is not thermal equilibrium. But stability can be seen in many transient phenomena of the inanimate world. I have studied such phenomena from the point of view of molecular statistics and noticed the stable equilibrium of the second coordinate which will be discussed later (c.f., see Section 9) in connection with the maximum principle.
10.2. Motoyosi Sugita’s Inclination and Devotion to Incorporate Cybernetics of Norbert Wiener
After 1957 when I was born in Kofu, Japan, Motoyosi Sugita turned the direction of his bio-theoretical work to incorporate the cybernetics of Norbert Wiener [164] [165] [166] . There seem to be several reasons for it.
1) First, Motoyosi Sugita got stuck on his way to prove the maximum principle for the existence of the 4th law of thermodynamics around 1957;
2) Second, there suddenly emerged a world-wide upheaval of cybernetics of Norbert Wiener around the time;
3) Third, he became to see more importance on the regulation such as feedback control introduced by Norbert Wiener and digital control than on the nonequilibrium thermodynamics in the transient phenomena;
4) Fourth, among other things, his concept of field of chemical potential [see Equation (6.9), Equation (7.36), Equation (9.6)]:

was very easy to adjust with cybernetics. Here the chemical resistance 
the role of the regulation nozzle in the regulator process.
5) Fifth, the concept of cybernetics is so broad that it fascinated him very much, since he was a full time professor for economics and economical management in the Hitotsubashi University which is one of the top national universities for the humanities course in Japan.
6) Sixth, his time was a bit earlier than the era of modern optimal control theory. I believe that this would be the main reason for that.
As is known that cybernetics as well as feedback control of Norbert Wiener belong to the classical control theory, we know the modern control theory such as L. Pontryagin’s maximum principle [see Section 8] and Richard Bellman’s optimality principle (see Appendix) nowadays. Motoyosi Sugita was born in the age of classical control theory much earlier than that of modern control theory. Therefore, he seemed unfamiliar with what happened in the control theory around 1960 when Pontryagin’s revolution occurred. So, he would like to catch up the Norbert Wiener’s theory for his theory of thermodynamics of life.
The above situation seemed to stimulate Motoyosi Sugita to write many books on the application of cybernetics to the theory of life or life being [5] [6] [7] [8] [10] [11] [12] . For the English world, he published many papers on it around 1960-1975 [62] [80] - [87] . Only one of them (1963’s paper) was cited in Stuart A. Kauffman’s The Origins of Order [99] .
Although this research theme is very fascinated, this is out of scope of this paper. Otherwise, more several hundreds pages would be needed to do it. This work would be for you, the readers, probably not for me, I hope!
11. Conclusions
In conclusion, I have introduced the personal history of Motoyosi Sugita in Section 1. Here I summarized his birth, education, working, marriage, visits, publications, as well as his research history, etc.
In Section 2, I have shortly summarized his bright ideas of the concept of broad quasi-static change, the concept of virtual heat, and the concept of irreversible cycle. And I have shown his application of them to a certain classical phenomenon in physics such as the Kelvin’s thermoelectric effect.
In Section 3, as another example for his application to other classical phenomena in physics, I have discussed the diffusion phenomena. Here the Langevin equation, the mixing entropy and free energy, the number of partition have been discussed.
In Section 4, the theory of condensation in the supersaturated state developed by Becker-Döring, Volmer, and Frenkel has been discussed. I explained that this theory promoted Motoyosi Sugita to recognize the importance of the concept of the field of chemical potential.
In Section 5, I have summarized Motoyosi Sugita’s thermodynamics of transient phenomena. Here for the concrete understanding, the theory has been applied to the chemical reactions only. Considering the system of chemical reaction network, the concept of the field of chemical potential, the relationship between cooperative phenomena and the chemical potential have been discussed.
In Section 6, I summarized the maximum principle of Motoyosi Sugita in the transient phenomena. Here I have discussed 
In Section 7, I have argued the relationship between the Motoyosi Sugita’s theory and the theories of Lars Onsager and Ilya Prigogine. Here in general, both of them are almost identical and therefore they established the same type of theory independently. However, while the Onsager-Prigogine’s theory was limited within the linear theory, Motoyosi Sugita’s theory went far beyond the linear theory using the concept of the field of chemical potential.
In Section 8, I have discussed the relationship between the maximim principle of Motoyosi Sugita and that of Pontryagin in the modern optimal control theory. I have shown that the Motoyosi Sugita’s approach can be absorbed into the more broad category of Pontryagi’s theory. And therefore, the way to prove his conjecture lies on the fact that the Pontryagin’s maximum principle can be regarded as a key to prove the existence of the 4th law of thermodynamics when it is applied to non-equilibrium thermodynamics in the transient phenomena. I also have shown the Bellman’s principle of optimality (see Appendix).
In Section 9, I have shortly summarized the first application of the Motoyosi Sugita’s maximum principle to the theory of metabolism. Here I have discussed the combined chemical reactions, reactions of metabolism, and the maximum principle, the analogy between thermodynamics in the transient phenomena and the theory of metabolism, the balance equation of substances, the Gibbs free energy of life, the birth of network thermodynamics, respectively.
In Section 10, I have shortly introduced the Motoyosi Sugita’s theory of life. Here I have shown his way of thinking on the topic, quoting his main explanation on his motivation. Finally, I have presented the Motoyosi Sugita’s work of theory of life adapting cybernetics of Norbert Wiener after 1957.
Looking back at my long journey to introduce the widely unknown Japanese thermodynamicist, Motoyosi Sugita, to the Western world (namely, the English- reading people) as well as to the young generations of biophysical scientists in Japan, what I have done here seems far from to be perfect. However, being insufficient is much better than nothing to do. All that glitters is not gold. If what I have done here would help you to understand the great work of Motoyosi Sugita, then I could sleep well. This is only just a beginning for the construction of theory of life.
Acknowledgements
I would like to thank Dr. Jewgeni Starikow, Dr. Hiroaki S. Yamada and Dr. Hirohisa Tamagawa for useful communications and for their help in collecting Dr. Motoyosi Sugita’s very old research papers. I would like to thank Dr. Kazuhiko Aomoto for his useful comments and suggestions on mathematics. I would like to express my special thanks to Ms. Noriko Terui of Sōtōshu Hōjōji for providing me many books and scientific papers written by Dr. Motoyosi Sugita. I have no word to express my sincere thanks to Ms. Noriko Terui when I had to find the relative of Dr. Motoyosi Sugita and his wife. I was totally and perfectly owed by her for that. And I finally found Ms. Setsu Honda as the Motoyosi Sugita’s last relative. I would like to thank Ms. Setsu Honda for private communications on the history of Dr. Motoyosi Sugita and his family and for providing me several pictures of Motoyosi Sugita and accepting them for presenting in public. I learned from her that Dr. and Mrs. Sugita had only one son Yūkichi who passed away in the 2nd day of August 2012 and hence there is no descendant for them. I would like to thank my son Jōji Iguchi for finding first Dr. Motoyosi Sugita’s textbook of thermodynamics in Kobe University and thank my elder son Issaku Iguchi for his encouragement. Finally I would like to thank my wife Kazuko Iguchi for her constant support and encouragement and for sharing our life for a long time. I could really enjoy writing this paper.
Cite this paper
Iguchi, K. (2016) Motoyosi Sugita―A “Widely Unknown” Japanese Thermodynamicist Who Explored the 4th Law of Thermodynamics for Creation of the Theory of Life. Open Journal of Biophysics, 6, 125-232. http://dx.doi.org/10.4236/ojbiphy.2016.64012
References
- 1. Oka, S., Kubo, H. and Sugita, M. (1966) Physics Aspects of Life Phenomena. Unoshoten, Tokyo. (In Japanese)
- 2. Sugita, M. (1942) Thermodynamics New Lectures. Chijinshokan, Tokyo. (In Japanese)
- 3. Sugita, M. (1950) Thermodynamics of Transient Phenomena. Iwanami-shoten, Tokyo. (In Japanese)
- 4. Sugita, M. (1957) Thermodynamics and Molecular Statistics. Nankodo, Tokyo. (In Japanese)
- 5. Sugita, M. and Okayama, S. (1970) Information Science. Asakura-shoten, Tokyo. (In Japanese)
- 6. Sugita, M. (1979) Bionics and Theory of Life. Gakkai-shuppan Center, Tokyo. (In Japanese)
- 7. Sugita, M. (1966) Society and Cybernetics. Misuzu-shobo, Tokyo. (In Japanese)
- 8. Sugita, M. (1973) What Is Cybernetics? Hosei University Press, Tokyo. (In Japanese)
- 9. Sugita, M. and Heitler, W. (1975) Thinking and Wanderings (Translated by Motoyosi Sugita from Germany to Japanese). Kyoritsu-shuppan, Tokyo. (In Japanese)
- 10. Sugita, M. (1976) What Is Information Science? Jikkyo-shuppan, Tokyo. (In Japanese)
- 11. Sugita, M. (1976) Society and Theory of Systems. Misuzu-shobo, Tokyo. (In Japanese)
- 12. Sugita, M. (1977) Recommendation of Engineering Thought. Ootsuki-shoten, Tokyo. (In Japanese)
- 13. Sugita, M. (1979) The Function of Academics and Creation. Ootsuki-shoten, Tokyo. (In Japanese)
- 14. Sugita, M. (1932) über die Theomodynamik der nicht reversiblen Erscheinungen I und II. Journal of the Physical Society of Japan, 14, 579-591.
- 15. Sugita, M. (1932) über die Theomodynamik der nicht reversiblen Ernscheinungen II ter Teil. über die Thermoelektrizität. Journal of the Physical Society of Japan, 14, 636-648.
- 16. Sugita, M. (1933) Bemerkung über die Planckschen Beteis des zweiten Würmesatzes. Journal of the Physical Society of Japan, 15, 108-113.
- 17. Sugita, M. (1933) über die Theomodynamik der nicht reversiblen Ernscheinungen III ter Teil. über die Boltzmannsche Relation in der Thermoelectrizität. Journal of the Physical Society of Japan, 15, 12-29.
- 18. Sugita, M. (1934) On the Theory of Temperature Ionization of Gas. Part I. On the Quantization of the Atom. The Explanation of the Atomic Continuous Spectrum of Hydrogen. Journal of the Physical Society of Japan, 16, 254-264.
- 19. Sugita, M. (1934) On the Theory of Temperature Ionization of Gas. Part II. The General Theory and Relation between Temperature and Pressure Ionization. Journal of the Physical Society of Japan, 16, 401-413.
- 20. Sugita, M. (1942) Rapid Fluid Motion of Gases and Thermodynamics. Ooyo Butsuri, No. 3, 135-139. (In Japanese)
- 21. Sugita, M. (1942) Thermoelectricity and the Clausius’ Inequality. Journal of the Physical Society of Japan, 16, 4-6. (In Japanese)
- 22. Sugita, M. (1946) Application of Scaling Law for Rapid Heating and Quenching by Liquid Thermal Sources. Kagaku, 17, 118-119. (In Japanese)
- 23. Sugita, M. (1948) Thermodynamics of Metastable Phenomena and Biological Phenomena. Kagaku, 18, 202-209. (In Japanese)
- 24. Sugita, M. (1948) Theory of Dielectrics under the Fluctuating Electric Field I. Busseiron Kenkyu, 10, 26-32. (In Japanese)
- 25. Sugita, M. (1948) Theory of Dielectrics under the Fluctuating Electric Field II. Busseiron Kenkyu, 10, 33-36. (In Japanese)
- 26. Sugita, M. (1948) Theory of Dielectrics under the Fluctuating Electric Field III. Busseiron Kenkyu, 11, 36-46. (In Japanese)
- 27. Sugita, M. (1948) Theory of Dielectrics under the Fluctuating Electric Field IV. Busseiron Kenkyu, 12, 8-14. (In Japanese)
- 28. Sugita, M. (1949) Interpretation of Mixing Entropy. Nihon Butsuri-gakkai Shi, 4, 134-136. (In Japanese)
- 29. Sugita, M. (1950) Biological Thermodynamics and its Method. Seibutsu Kagaku, 2, 1-10. (In Japanese)
- 30. Sugita, M. (1950) A Tentative Plan for Analyzing Metabolic Reactions. I. Busseiron Kenkyu, 1950, 15-26. (In Japanese)
- 31. Sugita, M. (1953) A Tentative Plan for Analyzing Metabolic Reactions. II. (Depreciation and Entropy Metabolism of Life Seen as a Chemical Equipment). Busseiron Kenkyu, 1953, 99-120. (In Japanese)
- 32. Sugita, M. (1953) A Tentative Plan for Analyzing Metabolic Reactions. III. (On the Origins of Life). Busseiron Kenkyu, 1953, 121-128. (In Japanese)
- 33. Sugita, M. (1953) A Tentative Plan for Analyzing Metabolic Reactions. IV. (Thermodynamic Analysis of Growth and Infirmity of Old Age). Busseiron Kenkyu, 1953, 114-120. (In Japanese)
- 34. Sugita, M. (1955) A Tentative Plan for Analyzing Metabolic Reactions. V. (Analysis of Growth Curves and Metabolic Rotations). Busseiron Kenkyu, 1955, 6-17. (In Japanese)
- 35. Sugita, M. (1968) The Idea of Bionics applied to the Mathematical Model of Economics. Keizai Kenkyu, 19, 363-368. (In Japanese)
- 36. Sugita, M. (1971) Cells viewed as Molecular Automata. Iryodenshi to Seitai Kogaku, 9, 48-53. (In Japanese)
- 37. Sugita, M. (1975) Molecular Biology by Mathematical Engineering Description. Iryodenshi to Seitai Kogaku, 13, 1-8. (In Japanese)
- 38. Sugita, M. (1951) The Maximum Principle in the Transient Phenomena and Its Applications to Biology. Bull. Kobayasi Institute, 1, 88-101. (In Japanese, Abstract in English)
- 39. Sugita, M. (1951) On the Idea of Negative Entropy. Bull. Kobayasi Institute, 1, 271-277. (In Japanese, Abstract in English)
- 40. Sugita, M. (1952) The Relationship between the Boltzamann’s H Theorem and the Dissipation Function. Bull. Kobayasi Institute, 2, 128--136. (In Japanese, Abstract in English)
- 41. Sugita, M. (1952) Entropy Metabolism in Life and Its Mathematical Analysis I. Bull. Kobayasi Institute, 3, 42--53. (In Japanese, Abstract in English)
- 42. Sugita, M. (1954) Entropy Metabolism in Life and Its Mathematical Analysis II. Mathematics of Tracer in Isotopes. Bull. Kobayasi Institute, 4, 199-208. (In Japanese, Abstract in English)
- 43. Sugita, M. (1954) Entropy Metabolism in Life and Its Mathematical Analysis III. On the Quasi Thermodynamic Method. Bull. Kobayasi Institute, 4, 209-221. (In Japanese, Abstract in English)
- 44. Sugita, M. (1954) Entropy Metabolism in Life and Its Mathematical Analysis IV. Thermodynamics of Metabolism. Bull. Kobayasi Institute, 4, 222-230. (In Japanese, Abstract in English)
- 45. Sugita, M. (1955) Entropy Metabolism in Life and its Mathematical Analysis V. Analysis of Growth Curves and Thermodynamics of Cell Division. Bull. Kobayasi Institute, 5, 23-33. (In Japanese, Abstract in English)
- 46. Sugita, M. (1955) Model Circuits of Nonstationary Statistical Theory and Its Application to the Law of Detailed Balance. Bull. Kobayasi Institute, 5, 167-170. (In Japanese, Abstract in English)
- 47. Sugita, M. (1955) Thermodynamic Consideration for the Oparin’s Theory of Origin of Life. Bull. Kobayasi Institute, 5, 171-183. (From 171 to 176 in English, and Otherwise in Japanese)
- 48. Sugita, M. (1957) On the Maximum Principle in Fluid Systems (Including Biosystems) and the Condition of Quasi Equilibrium. Bull. Kobayasi Institute, 7, 1-8. (In Japanese, Abstract in English)
- 49. Sugita, M. (1958) Informative Correlation in the Irreversible Processes and Life Phenomena. Bull. Kobayasi Institute, 8, 159-173. (In Japanese, Abstract in English)
- 50. Fukuda, N. and Sugita, M. (1959) The Unified Interpretation about Metabolism of Elements in Life Systems and Its Distributions. Bull. Kobayasi Institute, 9, 1-11. (In Japanese, Abstract and Introduction in English)
- 51. Sugita, M. (1960) Linear Programming and Mechanical Modeling Solutions. Bull. Kobayasi Institute, 10, 1-3. (In Japanese, Abstract in English)
- 52. Fukuda, N. and Sugita, M. (1960) The Unified Interpretation about Metabolism of Elements in Life Systems and Its Distributions (Supplement). Bull. Kobayasi Institute, 10, 4-10. (In Japanese, Abstract in English)
- 53. Sugita, M. (1960) Biological Reactions and Digital Equivalent Circuits. Bull. Kobayasi Institute, 10, 163-170. (In Japanese, Abstract in English)
- 54. Fukuda, N., Sugita, M. and Ogawa, Y. (1961) Efforts of Numerical Experiments in the Areas of Biology (As Iron Metabolism as an Example). Bull. Kobayasi Institute, 11, 14-18. (In Japanese, Abstract in English)
- 55. Sugita, M. (1962) Analysis Using Dogital Circuits and Molecular Biology. Bull. Kobayasi Institute, 12, 23-28. (In Japanese, Abstract in English)
- 56. Sugita, M. (1962) Analysis using Digital Circuits and Molecular Biology II. A Review on the Real Examples. Bull. Kobayasi Institute, 12, 89-92. (In Japanese, Abstract in English)
- 57. Sugita, M. (1962) Simulations of Metabolic Systems by a Combination of Digital Circuits and Analogue Computers. Bull. Kobayasi Institute, 12, 159-162. (In Japanese, Abstract in English)
- 58. Sugita, M. and Fukuda, N. (1963) Analysis using Digital Circuits and Molecular Biology III. Stationary States and Its Switching of Regulation Genes and Working Genes. Bull. Kobayasi Institute, 13, 27-35. (In Japanese, Abstract in English)
- 59. Sugita, M. (1967) Unification of Continuous and Finite Mathematics and Simulation of Biological System Using Hybrid Computing System. Bull. Kobayasi Institute, 16, 13-19. (In Japanese, Abstract in English)
- 60. Sugita, M. (1951) Thermodynamical Method in Biology. Annals of Hitotsubashi Acad., 1, 9-21. (In English)
https://hermes-ir.lib.hit-u.ac.jp/rs/handle/10086/11787 - 61. Sugita, M. (1954) Mathematical Analysis of the Metabolic and Analogy of Economics. Annals of Hitotsubashi Acad., 4, 163-182. (In English)
https://hermes-ir.lib.hit-u.ac.jp/rs/handle/10086/11827 - 62. Sugita, M. and Fukuda, N. (1960) Mathematical Theory of Metabolism and Isotope Kinetics, Especially Using the Analogue Computer for the Analysis of Iodine Metabolism in Thyroid Gland. Hitotsubashi Journal of Arts and Sciences, 1, 62-81. (In English)
https://hermes-ir.lib.hit-u.ac.jp/rs/handle/10086/4740 - 63. Sugita, M. (1949) Natural Phenomena and Fitness. Hitotsubashi Ronsou., 22, 447-481. (In Japanese)
https://hdl.handle.net/10086/4627 - 64. Sugita, M. (1951) Natural Law Seeing from Biophysics and its Limitations. Hitotsubashi Ronsou., 25, 97-123. (In Japanese)
https://hdl.handle.net/10086/4499 - 65. Sugita, M. (1953) Balance in Need and Supply in Life and Economics. Hitotsubashi Ronsou., 29, 467-492. (In Japanese) https://hdl.handle.net/10086/4333
- 66. Sugita, M. (1954) The Way of Thinking of Labour Science and Thermodynamics. Hitotsubashi Ronsou., 32, 45-66. (In Japanese)
https://hdl.handle.net/10086/4256 - 67. Sugita, M. (1964) World View and Philosophy of Cybernetics. Hitotsubashi Ronsou., 52, 567-585. (In Japanese)
https://hdl.handle.net/10086/3044 - 68. Sugita, M. (1959) Theory of Information and Control (Cybernetics) and New Technological Thoughts. Bull. Hitotsubashi Acad., 2, 101-144. (In Japanese)
https://hdl.handle.net/10086/9653 - 69. Sugita, M. (1959) Origin of Life and Information Theory. Bull. Hitotsubashi Acad., 1, 444-484. (In Japanese)
https://hdl.handle.net/10086/10001 - 70. Sugita, M. (1960) Smuggling Method—A Hyper-Dynamical Model for Solving Problems of Linear Programming. Hitotsubashi Journal of Arts and Sciences, 1, 88-90. (In English)
https://hdl.handle.net/10086/4739 - 71. Sugita, M. (1961) Logical Approach in Biophysics, Functional Analysis of Chemical System in Vivo Using Digital Model and Logical Circuit. Bull. Hitotsubashi Acad., 3, 191-226. (In Japanese)
https://hdl.handle.net/10086/9983 - 72. Sugita, M. and Fukuda, N. (1963) Analysis of the Genetic Control of Microbial System using Logical Circuit and Analogue Computer. Bull. Hitotsubashi Acad., 5, 1-28. (In Japanese) https://hdl.handle.net/10086/9492
- 73. Sugita, M. (1966) Kinetic Theory and Mathematical Model of Cellular Metabolism. Bull. Hitotsubashi Acad., 8, 1-47. (In Japanese)
https://hdl.handle.net/10086/9482 - 74. Sugita, M. (1967) Mathematical Model of Cellular Division. Bull. Hitotsubashi Acad., 9, 1-29. (In Japanese)
https://hdl.handle.net/10086/9479 - 75. Sugita, M. and Fukuda, N. (1969) Stability of the Metabolic Systems and the Control by Information Molecules. Bull. Hitotsubashi Acad., 11, 1-34. (In Japanese)
https://hdl.handle.net/10086/9473 - 76. Saitô, N. and Sugita, M. (1952) Energy Dissipation and Entropy Production in Irreversible Processes of Dilute Systems. Journal of the Physical Society of Japan, 7, 554-559.
http://dx.doi.org/10.1143/JPSJ.7.554 - 77. Sugita, M. (1953) Thermodynamical Analysis of Life I. Thermodynamics of Transient Phenomena. Journal of the Physical Society of Japan, 8, 697-703.
http://dx.doi.org/10.1143/JPSJ.8.697 - 78. Sugita, M. (1953) Thermodynamical Analysis of Life II. On the Maximum Principle of Transient Phenomena. Journal of the Physical Society of Japan, 8, 704-709.
http://dx.doi.org/10.1143/JPSJ.8.704 - 79. Sugita, M. (1953) Thermodynamical Analysis of Life III. Mathematical Analysis of Metabolism. Journal of the Physical Society of Japan, 8, 709-718.
http://dx.doi.org/10.1143/JPSJ.8.709 - 80. Sugita, M. (1961) Feedback Mechanism of Chemical System from the Viewpoint of Information Technology. Progress of Theoretical Physics Supplements, 17, 143-161.
http://dx.doi.org/10.1143/PTPS.17.143 - 81. Fukuda, N. and Sugita, M. (1961) Mathematical Analysis of Metabolism using an Analogue Computer: I. Isotope Kinetics of Iodine Metabolism in the Thyroid Gland. Journal of Theoretical Biology, 1, 440-459.
- 82. Sugita, M. (1961) Functional Analysis of Chemical Systems in vivo using a Logical Circuit Equivalent. Journal of Theoretical Biology, 1, 415-430.
- 83. Sugita, M. (1963) Functional Analysis of Chemical Systems in Vivo Using a Logical Circuit Equivalent II. The Idea of a Molecular Automaton. Journal of Theoretical Biology, 4, 179-192. http://dx.doi.org/10.1016/0022-5193(63)90027-4
- 84. Sugita, M. and Fukuda, N. (1963) Functional Analysis of Chemical Systems in Vivo Using a Logical Circuit Equivalent III. Analysis using a Digital Circuit Combined with an Analogue Computer. Journal of Theoretical Biology, 5, 412.
http://dx.doi.org/10.1016/0022-5193(63)90087-0 - 85. Yǎs, M., Sugita, M. and Bensam, A. (1965) A Model of Cell Size Regulation. Journal of Theoretical Biology, 9, 444-470.
http://dx.doi.org/10.1016/0022-5193(65)90043-3 - 86. Sugita, M. (1966) Functional Analysis of Chemical Systems in vivo using a Logical Circuit Equivalent IV: Simulation of Cellular Control Systems using a Hybrid Computing System. Journal of Theoretical Biology, 13, 330-356.
http://dx.doi.org/10.1016/0022-5193(66)90026-9 - 87. Sugita, M. (1975) Functional Analysis of Chemical Systems in Vivo Using a Logical Circuit Equivalent V: Molecular Biological Interpretation of the Self-reproducing Automata Theory and Chemico-Physical Interpretation of Information in Biological Systems. Journal of Theoretical Biology, 53, 223-237.
http://dx.doi.org/10.1016/0022-5193(75)90113-7 - 88. Sugita, M. (1966) Kinetic Theory and Mathematical Model of Cell Metabolism. Helgoländer wissenschaftliche Meeresuntersuchungen, 14, 78-109.
http://dx.doi.org/10.1007/BF01611615 - 89. Sugita, M. (1967) A Hybrid Computing Model of the Cellular System. 7th Dig. Int. Conf. Med. Biol. Eng., Stockholm, 479.
- 90. Sugita, M. (1967) Cell Biology by Computer. Science, 157, 536.
http://dx.doi.org/10.1126/science.157.3788.536-a - 91. Sugita, M. (1968) Interaction of a Digital (Genetic) System with a Continuous (Enzymatic) One in the Cell. In: Locker, A. and Krueger, F., Eds., Quantitative Biology of Metabolism: Models of Metabolism, Metabolic Parameters, Damage to Metabolism, Metabolic Control, Springer, New York, 33-37.
http://dx.doi.org/10.1007/978-3-642-51065-6_4 - 92. The National Diet Library.
http://www.ndl.go.jp - 93. Wagner, C. (1929) über die thermodynamische Behandlung stationärer Zusübde in nicht isotermen Systemen. Ann. d. Phys., 3, 629. The Journal of Mathematical and Physical Society of Japan, 3, 419-429 (in Japanese).
- 94. Siemens, G. (1931) über die Vorgänge im elektromagnetischen Felde, dargestellt durch die Kraftlinien. Zeits. f. Phys., 66, 396. The Journal of Mathematical and Physical Society of Japan, 4, 402-406 (in Japanese).
- 95. Hoshi, S.-I. (1978) Meiji, Chichi and America (Meaning Meiji, Father and America). Shincho-sha, Tokyo. (In Japanese)
- 96. Becker, R. and Döring, W. (1935) Kinetische Behandlung der Keimbildung in übersättigten Däimpfen. Annalen der Physik, 5, 719-752. (In Germany) (The English Translation Exists in the Following: Becker, R. and Döring, W. Kinetic Treatment of the Nucleation in Supersaturated Vapors.
http://ufdc.ufl.edu/AA00006149/00001 - 97. Volmer, M. (1939) Kinetik der Phasenbildung. Dresden u., Leipzig. (Volmer, M. (1966) Kinetics of Phase Formation. Central Air Documents).
- 98. Frenkel, J. (1939) Statistical Theory of Condensation Phenomena. The Journal of Chemical Physics, 7, 200-201. Frenkel, J. (1939) A General Theory of Heterophase Fluctuations and Pretransition Phenomena. The Journal of Chemical Physics, 7, 200-201.
http://dx.doi.org/10.1063/1.1750413 - 99. Kauffman, S. (1993) The Origins of Order: Self-Organization and Selection in Evolution. Oxford University Press, Oxford.
- 100. Onsager, L. (1931) Reciprocal Relations in Irreversible Processes I. Physical Reviews, 37, 405-426.
http://dx.doi.org/10.1103/PhysRev.37.405 - 101. Onsager, L. (1931) Reciprocal Relations in Irreversible Processes II. Physical Reviews, 38, 2265-2279.
http://dx.doi.org/10.1103/PhysRev.38.2265 - 102. De Donder, T. and Van Rysselberghe, P. (1936) Thermodynamic Theory of Affinity. Vol. 1, Stanford University Press, New York.
- 103. Glansdorff, P. and Prigogine, I. (1971) Thermodynamics Theory of Strucuture, Stability and Fluctuations. Wiley-Interscience, London and Japan.
- 104. Prigogine, I. and Kondeputi, D. (1999) Modern Thermodynamics. Editions Odile Jacob, Paris. (In Japanese, This Book Was Translated from French into Japanese).
- 105. Katchalsky, A. and Curran, P.F. (1965) Nonequilibrium Thermodynamics in Biophysics. Harvard University Press, Cambridge.
http://dx.doi.org/10.4159/harvard.9780674494121 - 106. Oster, G.F., Perelson, A.S. and Katchalsky, M.A. (1970) Network Thermodynamics. Nature, 234, 393-399.
http://dx.doi.org/10.1038/234393a0 - 107. Oster, G.F. and Desoer, C.A. (1971) Tellegen’s Theorem and Thermodynamic Inequalities. Journal of Theoretical Biology, 32, 219-241.
http://dx.doi.org/10.1016/0022-5193(71)90162-7 - 108. Oster, G.F., Perelson, A.S. and Katchalsky, A. (1973) Network Thermodynamics: Dynamic Modelling of Biophysical Systems. Quarterly Reviews of Biophysics, 6, 1-134 (Translated into Japanese by Imai, Y., Murakami, M. and Yoshida, H., Kitami-shobo, Tokyo, 1980).
- 109. Oster, G.F. and Perelson, A.S. (1973) Systems, Circuits and Thermodynamics. Israel Journal of Chemistry, 11, 445-478. http://dx.doi.org/10.1002/ijch.197300041
- 110. Oster, G.F. and Perelson, A.S. (1974) Chemical Reaction Dynamics. Part I: Geometrical Structure. Archive for Rational Mechanics and Analysis, 55, 210-274.
http://dx.doi.org/10.1007/BF00281751 - 111. Perelson, A.S. and Oster, G.F. (1974) Chemical Reaction Dynamics. Part II: Reaction Networks. Archive for Rational Mechanics and Analysis, 57, 31-97.
http://dx.doi.org/10.1007/BF00287096 - 112. Perelson, A.S. (1975) Network Thermodynamics. Biophysical Journal, 15, 667-685.
http://dx.doi.org/10.1016/S0006-3495(75)85847-4 - 113. Clausius, R. (1854) über eine veränderte Form des zweiten Hauptsatzes der mechanischen Wärmetheorie. Ann. d. Phys., 93, 481-506. Collected Papers of Thermal Theories of Clausius: Thermodynamic Theory as the Origins of Entropy. Translated into Japanese by Yagi, E., et al., Tokai University Press, Tokyo, 2013.
- 114. Boltzmann, L. (1909) Zur Theorie der thermoelektrischen Ersheinungen. Wiss. Abh. , 3, S321.
- 115. Tolman, R.C. and Fine, P.C. (1948) On the Irreversible Production of Entropy. Reviews of Modern Physics, 20, 51.
http://dx.doi.org/10.1103/RevModPhys.20.51 - 116. Huang, K. (1987) Statistical Mechanics. 2nd Edition, John Wiley and Soncs, New York.
- 117. Husimi, K., Shōji, I., Nakano, H. and Nishiyama, T. (2010) Quantum Statistical Mechanics. Reissue Edition, Kyoritsu-Shuppan, Tokyo. (In Japanese)
- 118. Onsager, L. and Machlup, S. (1953) Fluctuations and Irreversible Processes. Physical Reviews, 91, 1505-1512.
http://dx.doi.org/10.1103/PhysRev.91.1505 - 119. Machlup, S. and Onsager, L. (1953) Reciprocal Relations in Irreversible Processes II. Systems with Kinetic Energy. Physical Reviews, 91, 1512-1515.
http://dx.doi.org/10.1103/PhysRev.91.1512 - 120. Mayer, J.E. (1937) The Statistical Mechanics of Condensing Systems. I. The Journal of Chemical Physics, 5, 67-73. http://dx.doi.org/10.1063/1.1749933
- 121. Ostwald, W. (1914) The Principles of Inorganic Chemistry. Macmillan, New York.
- 122. Marcelin, R. (1914) Expression des vitesses de transformation des systèmes physico-chimiques en fonction de l’affinité. Comptes Rendus Hebdomadaires de l’Académie des Sciences, 158, 116-118.
- 123. Jouguet, Z.E. (1926) Velocity of Reaction and Thermodynamics. Annales de Physique, 5, 5.
- 124. Eyring, H. (1935) The Activated Complex in Chemical Reactions. The Journal of Chemical Physics, 3, 107-115.
http://dx.doi.org/10.1063/1.1749604 - 125. Glasstone, S., Eyring, H. and Laidler, K.J. (1941) The Theory of Rate Processes. McGraw-Hill, New York.
- 126. Gibbs, J.W. (1878) On the Equilibrium of Heterogeneous Substances (Abstract). Amer. J. Sci., 96, 441-458. Gibbs, J.W. (1878) On the Equilibrium of Heterogeneous Substances (Full Paper). Transactions of the Connechcut Academy, III, 57--353.
- 127. Debye, P. and Hückel, E. (1923) De la theorie des electrolytes. I. Abaissement du point de congelation et phenomenes associes. Phys. Zeit., 24, 185-206.
- 128. Onsager, L. and Samaras, N.N. (1934) The Surface Tension of Debye—Hückel Electrolytes. The Journal of Chemical Physics, 2, 528-536.
http://dx.doi.org/10.1063/1.1749522 - 129. Ling, G.N. (1992) A Revolution in Physiology of the Living Cell. Krieger Publishing Company, New York.
- 130. Ling, G.N. (2001) Life at the Cell and Below-Cell Level: The Hidden History of a Fundamental Revolution in Biology. Pacific Press, Inc., New York.
- 131. Pollack, G.H. (2001) Cells, Gels and the Engines of Life. Ebner & Sons, Seattle.
- 132. Pollack, G.H. (2013) The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor. Ebner & Sons, Seattle.
- 133. Rayleigh, L. (1873) Note on the Numerical Calculation of the Roots of Fluctuating Functions. Proceedings London Mathematical Society, 4, 357-363.
http://dx.doi.org/10.1112/plms/s1-5.1.119 - 134. Landau, L.D. and Lifshitz, E.M. (1959) Statistical Physics. Pergamon Press, New York.
- 135. Pontryagin, L.S., Boltyanskii, V.G., Gamkrelidze, R.V. and Mishchenko, E.F. (1962) Mathematical Theory of Optimal Processes. Wiley, New York (Translated into Japanese by Sekine, T., SogoTosho, Tokyo, 1967).
- 136. Boltianskii, V.G. (1966) Mathematical Methods of Optimal Control. In: Neustadt, L.W., Ed., Izdat, Nauka, Moscow (English Translated by Trigogoff, K.N., Interscience Publishers, New York, 1967; Translated into Japanese by T. Sekine. Sogo Tosho, Tokyo, 1968).
- 137. (1967) Introduction to Mathematical Theory of Control Processes. Academic Press, New York.
- 138. Bellman, R. and Kalaba, R. (1965) Dynamic Programming and Modern Control Theory. Academic Press, New York.
- 139. Tou, J.T. (1964) Modern Control Theory. MacGraw-Hill Book Company, New York.
- 140. Schultz, D.G and Melsa, J.L. (1967) State Functions and Linear Control Systems. MacGraw-Hill Book Company, New York.
- 141. Sussmann, H.J and Willems, J.C. (1997) 300 Years of Optimal Control: From the Brachystochrone to the Maximum Principle. IEEE Control Systems, 17, 32-44.
http://dx.doi.org/10.1109/37.588098 - 142. Iguchi, K. (2014) Theory of Non-Equilibrium Thermodynamics in the Optimal Control Processes Part I: Mathematical Formulation. Unpublished.
http://www.stannet.ne.jp/kazumoto/iguchi-noneqthermostat.pdf - 143. Iguchi, K. (2014) Theory of Non-Equilibrium Thermodynamics in the Optimal Control Processes Part II: Physical Approaches to the Pontryagin's Maximum Principle. Unpublished.
http://www.stannet.ne.jp/kazumoto/iguchi-noneqthermostat2.pdf - 144. Gibson, J.E. (1963) Nonlinear Automatic Control. McGraw-Hill Book Company, New York (Translated into Japanese by Horii, T., Corona-sha, Tokyo).
- 145. Feynman, R.P. and Hibbs, A.R. (1965) Quantum Mechanics and Path Integral. MacGraw-Hill Book Company, New York.
- 146. Kubo, R., Ichimura, H., Tsuboi, T. and Hashizume, N. (1961) Thermodynamics and Statistical Mechanics. Shokabo, Tokyo. (In Japanese)
- 147. Schrödinger, E. (1993) What Is Life? The Physical Aspect of the Living Cell and Mind. Schrödinger, E. (1992) What Is Life? With Mind and Matter and Autobiographical Sketches. Cambridge University Press, Cambridge.
- 148. Brillouin, L. (1950) Thermodynamics and Information Theory. America Scientist, 37, 594-599. Kagaku, 20, 216. (In Japanese)
- 149. Tellegen, B.D.H. (1952) A General Network Theorem, with Applications. Philips Res. Rep., 7, 259-296.
- 150. Brayton, R.K. and Moser, J.K. (1964) A Theory of Nonlinear Networks, I. Quarterly of Applied Mathematics, 22, 1-33.
- 151. Brayton, R.K. and Moser, J.K. (1964) A Theory of Nonlinear Networks, II. Quarterly of Applied Mathematics, 22, 453-491.
- 152. Branin Jr., F.H. (1966) The Algebraic-Topological Basis for Network Analogies and the Vector Calculus in Symposium on Generalized Networks. IBM Tech. Rep. TROO 855, No. 16, 453-491.
- 153. Desoer, C. and Kuh, E.S. (1969) Basic Circuit Theory. MacGraw-Hill, New York.
- 154. Penfield, P., Spence, R. and Duinker, S. (1970) Tellegen’s Theorem and Electrical Networks. MIT Press, Cambridge.
- 155. Jackson, J.D. (1999) Classical Electrodynamics. Wiley, New York.
- 156. Imai, Y. (1989) Membrane Transport System Modeled by Network Thermodynamics. Journal of Membrane Science, 41, 3-21.
http://dx.doi.org/10.1016/S0376-7388(00)82387-X - 157. Imai, Y. (1996) Power Balance Equation of Membrane Transport System: A Basis for Network Thermodynamics. Maku, 21, 264-269.
http://dx.doi.org/10.5360/membrane.21.264 - 158. Beard, D.A., Liang, S.-D. and Qian, H. (2002) Energy Balance for Analysis of Complex Metabolic Networks. Biophysical Journal, 83, 79-86.
http://dx.doi.org/10.1016/S0006-3495(02)75150-3 - 159. Qian, H., Beard, D.A. and Liang, S.-D. (2003) Stoichiometric Network Theory for Nonequilibrium Biochemical Systems. European Journal of Biochemistry, 270, 415-421.
http://dx.doi.org/10.1046/j.1432-1033.2003.03357.x - 160. Potter, V.R. (1944) Biological Energy Transformations and Cancer Problems. Advances in Enzymology, 4, 201-256.
http://dx.doi.org/10.1002/9780470122495.ch6 - 161. von Bertalanffy, L. (1950) An Outline of General System Theory. British Journal for the Philosophy of Science, I, 134-165.
http://dx.doi.org/10.1093/bjps/i.2.134 - 162. von Bertalanffy, L. (1950) The Theory of Open Systems in Physics and Biology. Science, 111, 23-29.
http://dx.doi.org/10.1126/science.111.2872.23 - 163. von Bertalanffy, L. (1957) Quantitative Laws in Metabolism and Growth. The Quarterly Review of Biology, 32, 217-231.
http://dx.doi.org/10.1086/401873 - 164. Wiener, N. (1948) Cybernetics. Hermann, Paris.
- 165. Wiener, N. (1954) The Human Use of Human Beings. Cybernetics and Society, No. 320. Da Capo Press, Paris.
- 166. Wiener, N. (1961) Cybernetics or Control and Communication in the Animal and the Machine, Vol. 25. MIT Press, New York.
http://dx.doi.org/10.1037/13140-000
Appendix: The Bellman’s Theory of Optimality Principle
The Richard Bellman’s work in engineering mathematics and control theory has not so well-known in physics community as well [137] [138] . However, it is also very important for our purpose as well as so is Pontryagin’s theory. As is shown before, on the one hand, Pontryagin’s theory is the generalization of the Hamilton principle in classical mechanics to the theory of optimal control. On the other hand, Bellman’s theory is the generalization of the Hamilton-Jacobi theory in classical mechanics to the theory of optimal control, whose theory is called the dynamic programming in the control theory [137] [138] . From the quality and flavor of Bellman’s work and the age that the work was done, I feel like that he is “Richard Feynman” [145] in engineering mathematics. Even their faces are alike to each other as well.
1. Multistage Processes
The expression of the multistage processes is that in the optimal control theory and in the applied mathematics. It is nothing more than the recursive processes and the iteration processes in physical terminology.
Now, let us denote by a state vector x a point in an n-dimensional space. Next, let us consider that the point is transformed into another point 


By repeating this process, the transformation from the n-th stage to the 

Thus, as the initial point flutters from point to point till it moves to the final point, we finally obtain an infinite series of the points. That is,

We call this multistage process. It is inconvenient to write many terms in the series at every time, we write it in the simplified way:

2. N-Stage Processes and Reduction of Data
Since we human being control the system in the optimal control theory, we consider the case of finite time. Therefore, we assume that there is an upper limit number N in the sequence. That is, we consider only the case:

Then, later we take the limiting process:

Now, we consider that there exists a function in this multistage process. In other words, we consider that a function g is written in the following:

This is thought of as a mapping from the coordinates 

As explicit examples, the following forms can be considered:


3. N-Stage Deterministic Process and Mathematical Representation of Policy
Let us consider the expression of the N-stage deterministic process in the optimal control theory and in the applied mathematics [137] [138] . It is nothing more than the recursive processes and the iteration processes in physical terminology.
Now, let us denote by a state vector x a point in an n-dimensional space. Let us denote by u the r-dimensional vectors for control variables that we determine at each stage. Then, an N-stage deterministic process is given as follows:

where we have defined

Let us now define the evaluation function or the performance index function by

In the theory of optimal control [135] [136] , the above external vector u is called the control vector or the control function. On the other hand, in the theory of dynamic programming [137] [138] , it is called the policy or decision.
4. Mathematical Representation of Policy
The evaluation function 




This is called the policy function. When the policy makes the evaluation function optimal, we may call it the optimal policy. And the optimality problem is that to determine the optimal policy by the multistage processes.
The above case of Equation (A.13) is most general, since it includes all the information of the past. Therefore, it is very complicated, since the policies in the past determine the present policy. So, we have to simplify the policy representation by restricting ourselves to consider only the case that the state is determined by the state in the past just before the present time, such as the law of causality. In this restricted case, we have

Or for a bit more complicated system, it is given as

where 

When we adopt the condition such as Equation (A.14) or Equation (A.15), the evaluation function 


5. Independency from the Past and Mathematical Representation of the Law of Causality
The multistage process (that is, the recursive process) always depends upon only the state one step before. Although the past, the present and the future are all connected in time series, the present state is determined by the past state one step before. This is the concept of the multistage process. Therefore, the present is nothing to do with all the past before the past one step before. In this sense, the present is independent of the past.
Mathematically, it is given by

which means

This is the so-called mathematical representation of the law of causality.
6. Mathematical Representation of the Law of Causality
We now define the mathematical representation of causality. Suppose that the state of the system is 




At this moment, we can separate the whole interval from the initial time 



In the first interval the state lies in 








7. Recursive Processes
When we consider the systems of engineering or physics, we evaluate a function that emerges by an engineering process or a physical process. We calculate the physical quantity, according to the process that the state of the system changes at a time. This becomes the main theme for us.
Now, we call such physical quantities the evaluating function or the performance index in the control theory. Let us denote it by

By the way, for the 


Therefore, the first relation becomes

This is a functional equation of
Assuming that the system start at the initial state x, if we want to find the maximum of the evaluation function

then we can derive

This is called the Bellman’s equation for dynamic programming.
8. Infinite Process, Time-Dependent Process, and Non-Stationary Process
The process with the limit 
And we consider that transformation function R is always the same so far. But we may think that the transformation function can change at each step. It is the time- dependent process. That is,

Furthermore, in the non-stationary process:

we have

If we can consider

then we have

9. Continuous Multistage Process
We have considered the discrete multistage processes so far. The discrete multistage processes that are generalized to the cases of the continuous time are the continuous multistage processes. In this case, if we divide the time very many:

then we can treat the process as if it is a discrete process, and then finally we take the time interval 

The transformation function 


We consider the evaluation function up to the linear term of 

Therefore, the recursive relation in this case is

Expanding both sides of the equation, and comparing the linear terms, we obtain the following:

This is the functional relation for the continuous multistage process. Physically considering, it is nothing but the Fokker-Planck equation [146] when the external force 

As is discussed before, if we want to obtain the maximum of the f, we need find the solution of the following equation:

This is called the Bellman’s differential equation.
10. Principle of Optimality and Bellman’s Optimality Equation
Bellman introduced the principle of optimality [137] [138] , which is described in the following principle:
Principle of optimality:
An optimal policy has the property that whatever the initial state and the initial decision are, the remaining decisions must constitute an optimal policy with regard to the state resulting from the first decision.
This principle is a very general property and has universality. And we can say that this principle of optimality is equally matched for the Dirac-Feynman’s principle for the path integral in physics [145] .
For example, we consider the following evaluation function:

This is the function that this physical quantity provides data when we decide whether or not the maximum effect is attained in the multistage deterministic process. We decide whether the process is effective or not through evaluating this function. Namely, we determine so that the function becomes maximum. Therefore, we denote by 

Since we assume that we decide the optimal policy at each step from the principle of optimality, we have

Comparing this for

The initial state is defined by

Similar functional equations are obtained for other multistage deterministic pro- cesses as well. In general we call these the Bellman’s optimality equation.
11. The Meaning of the Principle of Optimality and Dynamic Programming
The meaning of the principle of optimality and dynamic programming is as follows: In general, the evaluation function is a problem of the two-point boundary value of the initial and final states such that we have to consider all processes in between the interval.
In classical mechanics, we impose that the action function S becomes minimum between the initial and final states under the principle of least action. This provides the Euler-Lagrange equation for the orbit. Conversely, once the Euler-Lagrange equation is written down, we would like to solve the Euler-Lagrange equation. This provides the temporal motion of the orbit in between the two boundary times, which guarantees that the action S becomes always minimum.
On the other hand, in the theory of optimal control, we impose that the evaluation function 

In this sense, the Bellman's principle of optimality is the natural extension of the principle of least action in classical mechanics. In the automatic control engineering and the control theory, to determine the policy at each stage is called the dynamic programming. The Bellman’s dynamic programming provides the algorithms to give the optimal evaluation at each stage of the process.
Inversely, we can think that the Euler-Lagrange equation of motion is the algorithm that determines the orbit of the classical object in order to give the optimal action at each time. The evaluation function in classical mechanics is the action function and the evaluation function in the optimal control theory plays the same role as the action in classical mechanics.
12. Continuous Multistage Deterministic Process
We have considered the discrete multistage deterministic processes so far. The discrete multistage deterministic processes can be generalized the continuous multistage deter- ministic processes. In this case, if we divide the time interval into very many small intervals of

Then we can treat the process as if it is a discrete process, and at the end we take the limit of
Let us assume



where 


And we denote by 




We consider the evaluation function of Equation (A.39) up to the linear term of

Therefore, the recursive relation Equation (A.40) becomes

Expanding both sides of the equation up to the linear order of

This is the functional equation in the continuous multistage deterministic process. This is called the Bellman’s partial differential equation. Physically speaking, it is nothing but the Fokker-Planck equation [146] when the external force 

In the above we have considered the final time as a free parameter of T. However, since we can regard the initial time 


This corresponds to the Hamilton-Jacobi equation in classical mechanics, since the 


13. Relationship between the Variational Principle and the Dynamic Programming
At first glance, the functional equation of the dynamic programming for the continuous multistage deterministic process looks totally different from the Euler-Lagrange equation of motion in classical mechanics. We can prove that they are equivalent to each other, however.
For the sake of simplicity, let us consider the following evaluation function with one-dimensional state vector:

where

The state variable is 


Let us consider the problem to determine the initial tangent

In the first interval,

The principle of optimality is described as

Therefore, we have

This functional relation determines both the maximum of the integral and the policy function
Now, putting here 


This is the condition to get the maximum. To satisfy the principle of optimality is to require that always this condition is satisfied. Therefore, if we think that this condition is always satisfied, then we get rid of 

Differentiating both sides of Equation (A.56) with respect to time,

Similarly, differentiating both sides of Equation (A.57) with respect to q,

Comparing both results, since each right hand side is identical, we finally obtain the Euler-Lagrange equation:

In this case of the variational problem, we are not able to understand whether or not the original evaluation function takes the maximum value or the minimum value only from the extremum condition for the variation in the linear order. And the condition of Legendre is the condition that guarantees it; Namely, for the case of the maximum (minimum), we hold

In classical mechanics it is very difficult to put the restriction on the policy and it is not necessary to do so. However, in the dynamic programming and in the optimal control theory, there are various ranges and restrictions of the policy.
For example,

In such a case, since the principle of optimality is satisfied, we change the problem to the one that we seek for the maximum with a constraint. That is,

14. Geometrical Meaning of Dynamic Programming
The geometrical meaning of the dynamic programming is as follows: In classical mechanics we seek for the curve 
15. Hamilton-Jacobi Equation
When we apply the principle of optimality to classical mechanics, the problem of optimal control reduces to solve the Hamilton-Jacobi equation [137] [138] [139] [140] . Consider the action integral I:

where L is the Lagrangian. When the action integral takes the minimum, we write as

Similarly as before, we divide the whole interval of time into two intervals 


In the limit of

Differentiating inside 


And from this, we find



Now, this time if we apply the principle of optimality for the state x not at the initial time 




Thus, if we define the Hamiltonian H by

then we get

This is again the Hamiltonian-Jacobi equation in classical mechanics.
Thus, when the idea of the Bellman’s principle of optimality is applied to the special case of the action function in classical mechanics, then it reproduces to the usual the principle of least action. In this respect, the Bellman’s principle of optimality is thought of as being a natural generalization of the principle of least action.


















