Open Journal of Organic Polymer Materials
Vol.3 No.1(2013), Article ID:27194,8 pages DOI:10.4236/ojopm.2013.31004
Liquid Crystalline Polymers XIII Main Chain Thermotropic Copoly (Arylidene-Ether)s Containing 4-Teriary Butyl-Cyclohexanone Moiety Linked with Polymethylene Spacers
1Chemistry Department, College of Pharmacy, Al-Jouf University, Sakaka, Saudi Arabian
2Polymer Research Laboratory, Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
Email: kamalaly@yahoo.com
Received November 9, 2012; revised December 10, 2012; accepted December 23, 2012
Keywords: Liquid Crystal; Thermotropic; Synthesis; Characterization; Poly(Arylidene-Ether)s
ABSTRACT
A new homologous series of thermotropic liquid crystalline copoly(arylidene-ether)s based on 4-teriary butyl cyclohexanone moiety were synthesized by solution polycondensation of 4,4’-diformyl-α,ω-diphenoxyalkanes, Ia-d or 4,4’- diformyl-2,2’-dimethoxy-α,ω-diphenoxyalkanes IIa-d with the 4-teriary butyl-cyclohexanone III and cyclopentanone. A model compound IV was synthesized from the monomer III with benzaldehyde and characterized by elemental and spectral analyses. The inherent viscosities of the resulting polymers were in the range 0.22 - 0.92 dI/g. All the copoly(arylidene-ether)s were insoluble in common organic solvents but dissolved completely in concentrated H2SO4 and formic acid. The mesomorphic properties of these polymers were studied as a function of the diphenoxyalkane space length. Their thermotropic liquid crystalline properties were examined by DSC and optical polarizing microscopy and demonstrated that the resulting polymers form nematic mesophases over wide temperature ranges. The thermogravimetric analyses of those polymers were evaluated by TGA and DSC measurements and correlated to their structural units. X-ray analysis showed that copolymers having some degree of crystallinity in the region 2q = 5° - 60°. In addition, the morphological properties of selected examples were tested by Scanning electron microscopy.
1. Introduction
Thermotropic liquid crystalline behavior of polymeric materials is of considerable current interest, not only because of their potential as high-strength fibers, plastics, moldings etc. [1-3], but also because of their unique position in the theoretical scheme of structural order in liquid phases [4]. These thermotropic liquid crystalline (mesomorphic) polymers have two basic types [5]: one type has flexible aliphatic spacer groups, used to insulate the delicate intermolecular interactions in the liquid crystalline portions from small, but disruptive motions of the main chain. The other type of thermotropic mesogenic polymer is one in which the liquid crystalline moiety is incorporated in the main polymer chain. The introduction of long aliphatic segments “spacers” was avoided to retain the relative rigidity and high axial ratio of the macromolecules which seem to be necessary, not only to stabilize the mesophase, but also to obtain superior mechanical properties [6,7]. On the other hand, the anisotropy of the liquid crystalline mesophase offers the possibility of production of novel high performance materials, exhibiting excellent properties due to a proper arrangement of macromolecules in the mesophase during the processing. The spacer length can cause periodic changes in the thermodynamic properties of a material (in particular, the transition properties) based on whether there are an even or odd number of unit in the LC polymer spacers [8-13]. Our previous papers described how thertropic liquid crystal homoand copoly(arylidene-ether)s containing cycloalkanone moieties were synthesized [14-17] and the relationship between structure and liquid crystallinity was evaluated. It was found that the copolymers showed stable mesophases over the entire range of composition. In this paper, attention was addressed to 4-teriary-butyl-cyclohexanone moiety to prepare new thermotropic liquid crystal homoand copoly(arylidene-ther)s. A major purpose of this work was to study the effect of inclusion of the 4-teriary butyl cyclohexanone moiety in the polymer main chain on the LC properties of these polymers. In addition, other characterization of these polymers such as thermostability, solubility, morphology, and crystallinity were discussed.
2. Experimental
2.1. Measurements
Elemental analyses were carried out using an Elemental Analyses system GmbH, VARIOEL, V2.3 July 1998 CHNS Mode. Infrared Spectra from 4000 - 600 cm−1 of solid samples of the synthesized monomers and polymers were obtained by the KBr method using a Shimadzu 2110 PC Scanning Spectrophotometer. The 1H-NMR spectra were recorded on a GNM-LA 400-MHz NMR spectrophotometer at room temperature in DMSO or CDCl3 using TMS as the internal reference. Mass spectra were recorded on a Jeol JMS600 mass spectrometer. The inherent viscosity was measured with an Ubbelhode Viscometer in DMSO at 25˚C (0.5 g/L). The solubility of polymers was examined using 0.02 g of polymer in 3 - 5 ml of solvent at room temperature. The X-ray diffractographs of the polymers were obtained with a Philips X-ray pw 1710 diffractometer, and Ni-filtered CuKα radiations. Thermogravimetric analysis (TGA) and differential thermalgravimetric (DTG) were carried out in air with TA 2000 thermal analyzer at heating rate of 10˚C/min. in air. The maximum position of the melting endotherms was taken to be the m.p.s. The isotropization temperatures were determined by observing polymer melts with a polarizing microscope, GARL-ZEISS (JENA) equipped with a hot-stage Chaixmeca (Nancy, France). The temperature at which initial formation of isotropic phases occurred was taken as the isotropization temperature, Ti. At the same time, optical textures of the polymer melts were very closely followed to determine the nature of their mesophase. The morphologies of polymers were examined by scanning electron microscopy (SEM) using a Jeol JSM-5400 LV instrument.
2.2. Reagents and Solvents
4-Teriary butyl cyclohexanone (Merk) was used without purification. p-Hydroxy-benzaldehyde (Aldrich) was used without crystallization. 4-Hydroxy-3-methoxybenzaldehyde (vanillin) from EL-Nassr Chemical Company (Egypt) was used as it is. Dihaloalkanes (Aldrich) were used without purification. All solvents and other reagents were of high purity and were further purified by standard methods [18].2.3. Monomers Syntheses
4,4’-Diformyl-α,ω-diphenoxyalkanes Ia-d and 4,4’-diformyl-2,2’-dimethoxy-α,ω-diphenoxyalkane IIa-d. These monomers were prepared as described in our previous papers [16, 19].
2.4. Polymerization
The polycondensation of the dialdehydes and 4-teriary butyl cyclohexanone were carried out using the hightemperature solution method under the following conditions: the concentration of the monomers was 0.2 mol/L in ethanol (95%) in the presence of KOH (few drops) as a catalyst. The reaction temperature was 75˚C - 80˚C. Polymers were precipitated during the reflux, filtered off, washed with hot ethanol, acetone, and then dried under reduced pressure (1 mm/Hg) at 60˚C for 24 hours. All the poly(arylidene-ether)s were synthesized by an analogous procedure. Their yields, inherent viscosities, and elemental analyses are listed in Table 1.
3. Results and Discussion
3.1. Synthesis of Dialdehyde Monomers Ia-d and IIa-d
Two series of dialdehydes, namely of 4,4-diformyl-α,ω-diphenoxyalkanes Ia-d and 4,4-diformyl-2,2-dimethoxy-α,ω- diphenoxyalkanes IIa-d were prepared by interaction of one mole of dihaloalkanes with two moles of the sodium salt of 4-hydroxybenzaldehyde or sodium salt of 4-hydroxy-3-methoxy benzaldehyde respectively, in DMF in the presence of anhydrous potassium carbonate, as mentioned previously in our previous papers [13-16] as shown in Scheme 1.
The structures of these monomers were confirmed by elemental and spectral analyses as shown in the experimental part.
3.2. Synthesis of Model Compound 2, 6-Dibenzylidene-4-Tert-ButylcycloHexanone IV
Before attempting the polymerization process, model compound IV for the desired poly(arylidene-ether)s was synthesized by the interaction of 1 mole of the monomer named 4-tert-butyl-cyclohexanone with 2 moles of benzaldehyde in absolute ethanol and in the presence of few drops of (30%) alcoholic KOH as basic catalyst according to Scheme 2.
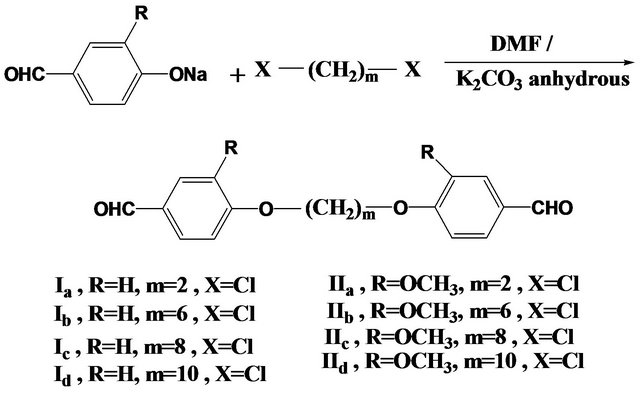
Scheme 1. Synthesis of dialdehydic monomers Ia-d, IIa-d.
Table 1. Solubility characteristic and inherent viscosity of copoly(arylidene-ether)s containing 4-tertiary butyl cyclohexanone moiety in the main chain Va-d and VIa-d.
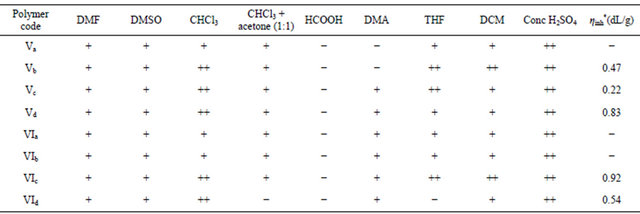
++: Soluble at room temperature (RT); +: Partially soluble at (RT); −: Insoluble; *Inherent viscosity was measured in CHCl3 at 23˚C.
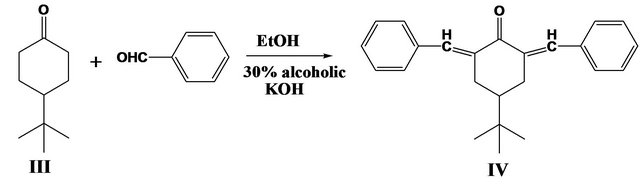
Scheme 2. Synthesis of model compound IV.
The structure of model compound IV was confirmed by elemental and spectral analyses. The FT-IR spectrum of [2,6-dibenzylidene-4-tertbutylcyclohexanone] IV showed characteristic absorption bands at 2995 cm−1 for (CH) stretching vibration of aromatic ring, at 1680 cm−1 for (C =O) of cyclohexanone and at 1595 cm−1 for (C=C) group of phenyl rings, beside other characteristics peaks were observed. 1H-NMR spectrum of IV in CDCl3 showed singlet S at δ0.9 (9H of CH3), multiple m at δ1.5 (1CH2 cyclohexanone), multiple m at δ2.3 - 3.1 (2CH2 cyclohexanone), multiple m at δ7.3 - 7.6 (2CH=C and 10 aromatic). The mass spectrum showed molecular ion peak at m/z = 330.45 (3.2%) which in agreement with its molecular formula (C24H26O), other peaks appeared at m/z = 273.52(M+-C20H17O, 2%), at m/z = 57.91(M+-C4H9 2.1%), and at m/z = 90.48 (M+-C7H6 5.4%).
3.3. Synthesis of Copoly(Arylidene-Ether)s Va-d & VIa-d
The present work had as its aim the synthesis of new thermotropic liquid crystal copoly(arylidene-ether)s Va-d and VIa-d by the solution of polycondensation of the two series of diformyl-α,ω-diphenoxyalkane (Ia-d) and diformyl-2,5-dimethoxy-α,ω-diphenoxyalkane (IIa-d) with 4- teriary butyl-cyclohexanone III and cyclopentanone and as shown in Scheme 3.
The structure of the resulting polymers was established from elemental and spectral analyses including (FT-IR and 1H-NMR). The results of elemental analyses for the
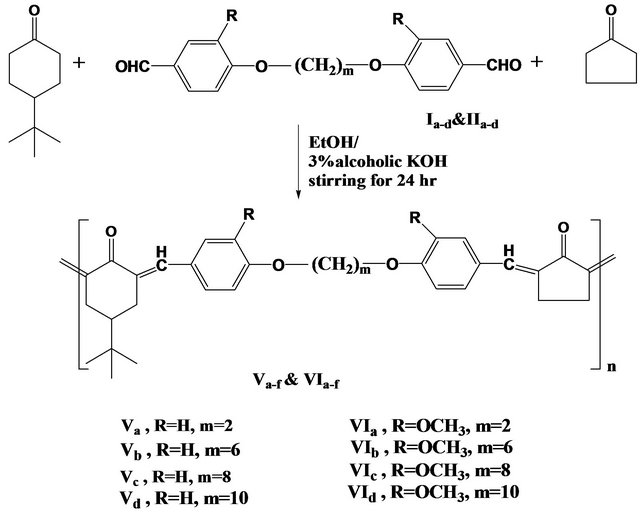
Scheme 3. Synthesis of copoly (arylidene-ether)s Va-f and VIa-f.
copoly(arylidene-ether)s Va-f and VIa-f prepared agree with calculated values (see experimental part). It should be noted that the elemental analysis results from the polymer deviated by 2% - 5% for the proposed structures. These differences can be accounted for by: 1) poor solubility and hence poor reactivity in the initial stage of polymerization; 2) difference in the reactivity of monomers during the polymerization, which lead to polymers with different compositions, the so called “composition drift” [20-23].
The FT-IR spectra of the poly(arylidene-ether)s Va-d and VIa-d as examples, showed characteristic absorption bands at 3035 - 2926 cm−1 for (CH) stretching vibration of aromatic ring, at 2869 - 2852 cm−1 for (CH) stretching of (CH2) groups, at 1691 - 1678 cm−1 for (C=O) of cyclohexanone, at 1600 - 1594 cm−1 for (C=C) group, at 1511 - 1465 cm−1 for phenyl rings and at 1266 - 1240 cm−1 for (C-O-C) bond (ether linkages). In addition, other characteristic bands, due to specific groups present in various polymers were also shown.
1H-NMR of polymers in CDCl3 such as Va showed singlet S at δ0.94 (9H of CH3), weak multiple m at δ1.4 - 1.7 (H of CH and 2H of 2CH2 in cyclohexanone), multiple m at δ2.4 (H of CH2 β to C=CH ph), weak multiple m at δ3 (2H of CH2 near C=O bond), multiple m at δ4.3 (4H of CH2 linked to oxygen in ether linkage) and multiple m at δ7 - 7.8 (8H of aromatic and H of CH=C).
1H-NMR of polymers in CDCl3 such as VIb showed singlet S at δ0.94 (9H of CH3), multiple m at δ1.4 - 1.7 (8H of middle methylene group, H of CH and 2H of 2 CH2 in cyclohexanone), multiple m at δ2.3 (H of CH2 β to C=CH ph), multiple m at δ3.1(2H of CH2 near C=O bond), multiple m at δ3.9 (4H of CH2 linked to oxygen in ether linkage) and multiple m at δ6.9 - 7.8 (8H of aromatic and H of CH=C).
4. Polymer Characterization
The various characteristics of the resulting polymers including solubility, viscometry, X-ray diffraction analysis, DSC, TGA, OPM and SEM were also determined and the data are discussed below.
4.1. Solubility
Room temperature solubility characterization of poly(arylidene-ether)s Va-d and VIa-d were tested using various solvents including: DMF, DMSO, CHCl3, CHCl3-acetone mixture, HCOOH, DMA, THF, DCM and conc. H2SO4. A 0.5% (w/v) solution was taken as a criterion for solubility. It can be clarified from Table 1 that poly(arylidene-ether)s Va-d and VIa-d are soluble in protonic acids, e.g. conc. H2SO4 (concentrated H2SO4 gave reddishviolet color).In polar aprotic solvents, such as DMF and DMSO all polymers dissolved partially. In DMA all polymers dissolve partially except Va,b, which are not soluble. In HCOOH all polymers are not soluble except Ve, which are dissolved partially. In CHCl3 all polymers dissolved partially except Vd not soluble and Vb-d and VIc,d are completely soluble. In CHCl3-acetone mixture all polymers dissolved partially except VId not soluble. In THF all polymers are partially soluble except VId not soluble but Vb and VIc are completely soluble. In DCM all polymers are partially soluble except Vb,e and VIc,d are completely soluble. They have good resistance to most Solvents. The difference in solubility can be attributed to the crystallinity associated with various LCPs. The presence of methoxyl group as substitute in the phenyl ring with tertiary butyl group in cyclohexanone moiety caused some hindering between the repeating units so decrease chain packing distances and increasing inter chain interactions such as hydrogen bonding so that making salvation of polymers containing methoxyl group more difficult than those which contain hydrogen atom instead, while increase length of the chain increase this solubility even for those which contain methoxyl group.
4.2. Inherent Viscosity
The inherent viscosity (ηinh) of some poly (arylideneether)s Vb-d and VIc,d were determined in CHCl3 at 23˚C with Ubbelohde Suspended Level Viscometer. The inherent viscosity value is defined as:
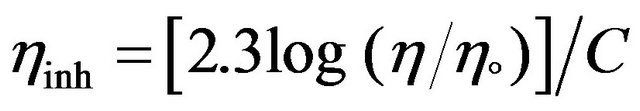
The solution concentration C is 0.5 g/100 ml, η/η° = relative viscosity (or viscosity ratio). The data are listed in Table 2. It can be clarified from this table that polymer VIc,d have high viscosity value (0.92, 0.22 dL/g ) and this may be attributed to high molecular weight of these polymers. On the other hand, polymers Vb-d have low viscosity (0.049, 0.26, 0.08, 0.18 dL/g) respectively, and this may be attributed to low molecular weight of these polymers.
4.3. U.V. Visible Spectra
The electronic spectra of selected examples of poly(arylidene-ether)s Vb, Vd and Vf were obtained in chloroform CHCl3 at a concentration 2 × 10−5 (w/v). The electronic spectra of Vb,d showed absorption band at 269 - 270 nm due to π-π* transition within the benzenoid system for all polymers. For polymers Vb,d λmax near 270 nm and at λmax near 269 nm for polymer. All of these polymers showed absorption band at λmax near 363 - 375 nm which was due to the π-π* and n-π* excitation of C=C and C=O groups. For polymer Vb λmax near 364 nm, and at λmax near 363 nm for polymer Vd.
4.4. X-Ray Analysis
The X-ray diffractograms of selected examples of the poly(arylidene-ether)s Vd, VIb and are shown in Figures 1 and 2 as example. The polymers show few reflection peaks that are ranging between crystalline and amorphous lying in region 2θ = 5° - 60°. This indicates that there is a large class of structures that are intermediate in the ordered states between crystalline and amorphous phases (with pronounced long-range order) in the arrangement of their atoms and molecules. Moreover, the presence of C=O as polar group in addition to high content of C=C bonds provides some order between the two extents of crystallinity(128). More particularly, the diffractogram of polymer Ve which contains short spacer 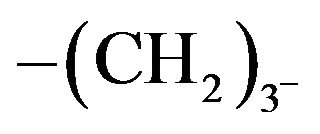 in Figure 1, Shows some reflection sharpness peaks, when the length of spacer increase as in polymer Vd which contains ten methylene groups
in Figure 1, Shows some reflection sharpness peaks, when the length of spacer increase as in polymer Vd which contains ten methylene groups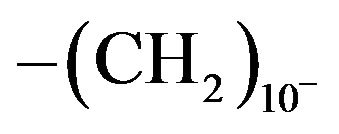 , in Figure 2, the reflection sharpness increased and the polymer became semicrystalline. This is explained by the fact that increasing the number of methylene groups in the spacers results in increasing polymer chain flexibility, and hence
, in Figure 2, the reflection sharpness increased and the polymer became semicrystalline. This is explained by the fact that increasing the number of methylene groups in the spacers results in increasing polymer chain flexibility, and hence
Table 2. Transition behavior of selected copoly(arylidene-ether)s containing 4-tertiary butyl cyclohexanone moiety in the main chain Va-d and VIa-d.


Figure 1. X-ray diffraction pattern of polymer IVd.

Figure 2. X-ray diffraction pattern of polymer IVd.
increases crystallinity. This can be explained by the trans molecular conformation of polymer when the number of methylene units is even, molecules can be fitted easily into the crystal lattice and the rate of crystalization is faster as it cools down from the liquid crystal states.
4.5. Scanning Electron Microscope (SEM) Measurement
The morphology of selected examples of copoly(arylidene-ether)s Vd, VId were examined as example by SEM using a low dose technique. Figure 3(a) (X = 1000) shows that polymer Vd has aggregates of layer structure. The higher magnification (X = 3500, 1500) in Figure 3(b) show that the aggregates show cavity shape. After dissolving of Vd in (DCM) and evaporation of solvent. Figures 4(a) and (b) (X = 500, 1000) show that polymer Vd has sponge structure.
4.6. Thermotropic Liquid Crystalline Properties of Poly(Arylidene-Ether)s Va-d and VIa-d
The thermal properties of the selected poly(arylideneether)s Vb,d and VIb,d were characterized by TGA and optical polarized microscope (OPM) with heating stage. The copoly(arylidene-ether)s Vb,d and VIb,d did not exhibit liquid crystalline properties while observed under optical polarized microscope with heating rate (6˚/min). The mesophase could be observed when the microscopic observation was made by placing the sample on a preheated hot stage. The liquid crystalline states of polymers Vb,d, and, VIb,d measured on a preheated hot stage of polarizing microscope at temperature 109˚C, 89˚C, 102˚C, 170˚C, 197˚C, 80˚C, 160˚C and 110˚C for the eight respective polymers; upon continued heating or heating at higher temperatures the liquid crystalline properties appeared at 112˚C, 92˚C, 114˚C, 182˚C, 210˚C, 87˚C, 162.7˚C and 119˚C for these polymers (Figures 5 and 6).
4.6.1. TGA Studies
The thermal behavior of the selected copoly(arylideneether)s Vb-f and VIb-f was evaluated by TGA and DTG under nitrogen atmosphere at heating rate of 10˚C·min−1. The thermographs of these polymers samples are given in Figures 5 and 6 while Table 3 gives the temperature for various% weight loss. TGA curves show initial decomposition of these polymers (10% loss) is considered to be the polymer decomposition temperature (PDT), which occurred in the range 503˚C to 694˚C for the samples.
 (a)
(a) (b)
(b)
Figure 3. SEM images of polymer Ve surface at different magnification; (a): X = 3500; (b): X = 1500.
 (a)
(a) (b)
(b)
Figure 4. SEM images of polymer Ve surface at different magnification; (a): X = 500; (b): X = 1000.

Figure 5. The TGA and Dr TGA traces of polymer IVb in nitrogen at a heating rate of 10˚C/m.
In Figure 5, for polymer Vb the mass loss is seen to be rapid loss between ~282.02˚C - 557.46˚C (−81.95%). For polymer Vd in Figure 6, the mass loss was rapid between ~310.22˚C - 574.38˚C (−85.38%). From these data, it appears that the polymers containing methoxyl groups instead of hydrogen atoms with tertiary butyl group tend to exist in separated rather than more packed chains, appeared to be readily susceptible to easier thermal decomposition [24,25].

Figure 6. The TGA and Dr TGA traces of polymer IVb in nitrogen at a heating rate of 10˚C/m.
4.6.2. Texture Observations
The phase behavior of the selected copoly(arylideneether)s Va as example according to optical polarizing microscope is summarized in Table 3. The examples ofpoly(arylidene-ether)s Va is shown in Figure 7. Observation of polymer IVa under polarizing microscope revealed that this polymer exhibited a good spheroid structure Figure 7(a) (before melting), in polarized light show that polymer has yellow color Figure 7(b). The meso-
 (a)
(a) (b)
(b)
Figure 7. Photomicrographs of polymer 119f in the heating cycle at (a) < 81˚C (Magnification ×500), (Magnification ×200); (b) > 87˚C, in polarized light (Magnification ×200).
Table 3. Thermal properties of selected copoly(arylideneether)s containing 4-tertiary butyl cyclohexanone moiety in the main chain Vb,d and VIb,d.
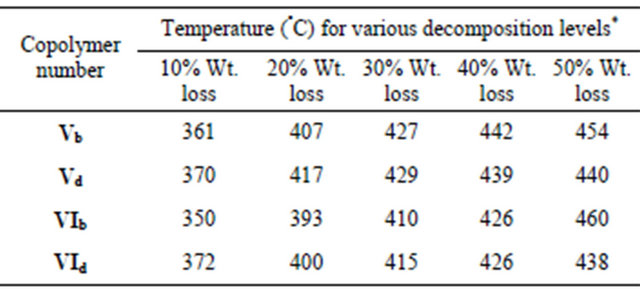
*Heating rate: 10˚C·min−1.
phase extended up to the isotropic temperature at > 81˚C (Ti). After cooling to room temperature a highly spheroid structure with coalescence appeared as shown in Figure 7(b) in polarized light. Observation of polymer Va under polarizing microscope revealed that this polymer exhibited good spheroid structure with thick dark rim Figure 7(a) (before melting), and the mesophase to the isotropic temperature at 87˚C (Ti). After cooling to room temperature a spheroid structure appeared as shown in Figure 7(b).
5. Conclusion
A novel series of liquid crystalline copoly(arylideneether)s containing 4-teriary butyl cyclohexanone moiety have been synthesized. A solution polycondensation technique at ~80˚C was used. All the copoly(arylidene-ether)s were insoluble in common organic solvents but dissolved completely in concentrated H2SO4 and formic acid. The majority of the polymers are insoluble in common organic solvents and halogenated hydrocarbons. Most of them exhibited melt birefringence and stirred opalscence during polarized microscope observation. Both (Tm) and (Ti) values increased as the length of the flexible aliphatic spacers increased and decreased with introduction of the methoxy group as a substituent in the polymers main chain.
REFERENCES
- L. L. Chapoy, “Recent Advances in Liquid Crystalline Polymers,” Elsevier, London, 1986.
- A. Blumstein, “Polymeric Liquid Crystals,” Plenum Press, New York, 1985.
- A. G. Grin and J. F. Johnson, “Liquid Crystals and Ordered Fluids,” Plenum Press, New York, 1984. doi:10.1007/978-1-4613-2661-8
- W. J. Jackson Jr., “Liquid Crystal Polymers. XI. Liquid Crystal Aromatic Polyesters: Early History and Future Trends,” Molecular Crystals and Liquid Crystals, Vol. 169, 1989, pp. 23-49.
- A. C. Grien and S. J. Haren, Journal of Polymer Science Part B-Polymer Physics, Vol. 19, 1986, p. 951.
- W. J. Jackson Jr., Polymer Journal, Vol. 12, 1980, p. 154.
- D. Acierno, F. P. La Mantina, G. Polizzotti, A. Giferri, W. R. Krigbaum and R. Koiek, “Thermotropic Homopolyesters. IV. Study of Fiber Formation,” Journal of Polymer Science Part B-Polymer Physics, Vol. 21, No. 10, 1983, pp. 2027-2036.
- J. J. Ge, M. Guo, Z. Zhang, P. S. Honigfort, S.-Y. Wang, F. W. Harris, et al., “Phase Structures, Transition Behavior, and Surface Alignment in Polymers Containing Rigid-Rod Backbones with Flexible Side Chains. 4. Solid-State 13C NMR Studies of Molecular Motions in PEFBPs(n) (n = 10 and 11),” Macromolecules, Vol. 33, No. 11, 2000, pp. 3983-3992. doi:10.1021/ma981970y
- J. Ruan, J. J. Ge, A. Zhang, S. Jin, S.-Y. Wang, F. W. Harris, et al., “Polymorphous Structures and Their Phase Relationships in a Main-Chain/Side-Chain Liquid Crystalline Polyester,” Macromolecules, Vol. 35, No. 3, 2002, pp. 736-745. doi:10.1021/ma0115702
- Y. S. Hu, R. Y. F. Liu, D. A. Schiraldi, A. Hiltner and E. Baer, “Solid-State Structure of Copolyesters Containing a Mesogenic Monomer,” Macromolecules, Vol. 37, No. 6, 2004, pp. 2128-2135. doi:10.1021/ma030439m
- J. Watanabe and M. Hayashi, “Thermotropic Liquid Crystals of Polyesters Having a Mesogenic P,P’-Bibenzoate Unit. 2. X-Ray Study on Smectic Mesophase Structures of BB-5 and BB-6,” Macromolecules, Vol. 22, No. 10, 1989, pp. 4083-4088. doi:10.1021/ma00200a046
- J. Watanabe, Y. Nakata and K. Simizu, “Frustrated Bilayer Smectic Phase in Main-Chain Polymers with Two Different Spacers,” Journal de Physique Ⅱ, Vol. 4, 1994, pp. 581-588
- J. Watanabe, M. Hayashi, A. Morita and M. Tokita, “Thermotropic Liquid Crystals of Main-Chain Polyesters Having a Mesogenic 4,4’-Biphenyldicarboxylate Unit. 6. Chiral Mesophases of Polyesters with a (S)-2-Methylbutylene Spacer,” Macromolecules, Vol. 28, No. 24, 1995, pp. 8073-8079. doi:10.1021/ma00128a015
- K. I. Aly, “Liquid Crystalline Polymers: 2. Synthesis and Thermotropic Studies of Poly(Arylidene-Ether)s Containing a Cyclopentanone Moiety in the Main Chain,” High Performance Polymers, Vol. 11, No. 4, 1999, pp. 437-452. doi:10.1088/0954-0083/11/4/308
- K. I. Aly and A. S. Hammam, “Liquid Crystalline Polymers: I. Main Chain Thermotropic Poly(ArylideneEther)s Containing Cyclopentanone Moiety Linked with Polymethylene Spacers,” European Polymer Journal, Vol. 36, No. 9, 1999, pp. 1933-1942. doi:10.1016/S0014-3057(99)00253-0
- K. I. Aly “Liquid Crystalline Polymers 3. Synthesis and Liquid Crystal Properties of Thermotropic Poly(Arylidene-ether)s and Copolymers Containing Cycloalkanones Moiety in the Polymer Backbone,” Journal of Macromolecular Science Chemistry A, Vol. 37, 2000, pp. 93-115
- K. I. Aly, Ahmed, R.A. “Liquid Crystalline Polymers V. Thermotropic Liquid Crystalline Poly(Azomethine-ether)s Containing a Cycloalkanone Moiety in the Polymer Backbone,” Liquid Crystals, Vol. 27, No. 4, 1999, pp. 451-458. doi:10.1080/026782900202633
- K. I. Aly, M. A. Hussein and M. M. Sayed, “Liquid Crystalline Polymers X. Main Chain thermotropic Poly (Ary- lidene-ether)s Containing 4-Teriary Butyl-Cyclohexanone Moiety Linked With Polymethylene Spacers,” Liquid Crystals, under Publication, 2013.
- B.-K. Chen, S.-Y. Tsay and J.-Y. Chen, “Synthesis and Properties of Liquid Crystalline Polymers with Low Tm and Broad Mesophase Temperature Ranges” Polymer, Vol. 46, No. 20, 2005, pp. 8624-8633. doi:10.1016/j.polymer.2005.06.084
- H. Catalgil-Giz and A. T. Giz, “Compensating the Composition Drift in Reactivity Ratio Calculations for Copolymerizations Carried to High Conversions,” Macromolecular Chemistry and Physics, Vol. 195, No. 3, 1994, pp. 855-864. doi:10.1002/macp.1994.021950304
- A. Ravikrishnan, P. Sudhakara and P. Kannan, “Liquid Crystalline and Photoactive Poly[4,4’-stilbeneoxy]alkylbiphenylphosphates,” Polymer Degradation and Stability, Vol. 93, No. 8, 2008, pp. 1564-1570. doi:10.1016/j.polymdegradstab.2008.05.017
- S. Y. Lu and I. Hamerton, “Recent Developments in the Chemistry of Halogen-Free Flame Retardant Polymers,” Progress in Polymer Science, Vol. 27, No. 8, 2002, pp. 1661-1712. doi:10.1016/S0079-6700(02)00018-7
- J. Canadell, A. Mantecón and V. Cádiz “PhosphorusContaining Thermosets Obtained by Cationic Copolymerisation of Glycidyl Compounds with a Spiroorthoester or γ-Butyrolactone,” Polymer Degradation and Stability, Vol. 93, No. 1, 2008, pp. 59-67. doi:10.1016/j.polymdegradstab.2007.10.023
- X. H. Du, Y. Z. Wang, X. T. Chen and X. D. Tang, “Properties of Phosphorus-Containing Thermotropic Liquid Crystal Copolyester/Poly(ethylene terephthalate) Blends,” Polymer Degradation and Stability, Vol. 88, No. 1, 2005, pp. 52-56. doi:10.1016/j.polymdegradstab.2004.02.018
- D. Srividhya, S. Senthil and P. Kannan, “Thermotropic Liquid-Crystalline Polyphosphate Esters Containing Phenolphthalein Moiety,” Journal of Applied Polymer Science, Vol. 92, No. 1, 2004, pp. 194-200. doi:10.1002/app.13431

