Open Journal of Polymer Chemistry
Vol.2 No.2(2012), Article ID:19533,7 pages DOI:10.4236/ojpchem.2012.22008
PEBAXTM-Silanized Al2O3 Composite, Synthesis and Characterization
1Departamento de Química de Radiaciones y Radioquímica, Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, México City, México
2Institut für Kunststoffe und Verbundwerkstoffe, Technische Universität Hamburg-Harburg, Hamburg, Germany
Email: *ebucio@nucleares.unam.mx
Received February 21, 2012; revised March 27, 2012; accepted April 13, 2012
Keywords: Nanocomposites; Alumina; Functionalization; Thermoplastic Elastomers; Thermal Behavior
ABSTRACT
Alumina nanoparticles were dispersed in poly(amide 12-b-tetramethylene oxide) copolymer through extrusion. The alumina particles were functionalized with 3-(2 trimethoxysilylethyl) cyclohexene oxide. The following PEBAXTM/ Al2O3 proportions were prepared: 0.1, 1.0, 5.0, and 10.0% w/w. The thermal stability of the nanocomposites was evaluated by thermograviemtric analysis under N2 and was comparable to the neat PEBAXTM polymer. The thermo-oxidative degradation of the polymeric matrix by oxygen was strongly hindered by the functionalized alumina. The rule of mixture would predict that the thermal degradation should be strongly dominated by PEBAXTM matrix. Therefore, the physical mixture of PEBAXTM and silanized alumina should be almost as stable as pure PEBAXTM. However, the experimental results suggest that the nanocomposites are more stable than the mixture of their components. This stabilization effect is evident in the temperature range between 300˚C and 400˚C, in which the degradation of the PA12 block takes place.
1. Introduction
In the past decades the development of organic-inorganic composites containing polymeric phases as the organic component [1-5] and further on polymer nanocomposites [6-10] has been receiving a great deal of attention due to the unique combination of light weight, processability of polymers with the outstanding chemical and thermal stabilities of the inorganic components. This technology was extended to different fields, which includes, reinforced polymeric materials, polymeric membranes for several application as for instance: gas-separation [11], fuel cell applications [9,12], pervaporation [13,14]; protecting coatings [1,15] to name a few. One of the key issues is to assure a good dispersion and distribution of the inorganic phase in the polymeric matrix [16]. When dealing with organic-inorganic hybrids prepared by the sol-gel method, the controlled hydrolysis and condensation of metal alkoxides in polymeric solutions are paramount to achieve the desired properties (enhanced mechanical stability, higher resistance against liquid and vapors) [17]. Even though this method enables the control of the size of inorganic domains; it requires the use of solvents and drying steps, which are drawbacks for the development of polymer-based materials for industrial applications. On the other hand, the surface of inorganic and carbon-based nanoparticles can be modified in order to enhance the compatibility between the polymeric and the inorganic phases and ensure an optimal improvement of the mechanical, chemical and thermal properties of the final functional materials [18-20]. Gas separation using polymeric membranes has drawn a great deal of interest from researchers because it offers advantages such as low energy costs and environmental benignity. This is especially true for hydrocarbon separations performed in the petrochemical industry, which generally incur heavy operating costs. The membrane-based method is particularly attractive for the removal of heavier species present in dilute concentrations, such as CO2 and H2S from the lighter but major constituent, CH4, in natural gas [21-23]. In the last decades, increasing interest has been turned to design new functional polymeric membranes in order to control interfacial phenomena, traditionally affecting separation and purification processes. It is well known, the first step of each membrane separation process involves chemical interactions at the membrane-feed interface, directing positively or negatively the productivity and efficiency of the overall transport [24]. Specifically, the behavior of CO2 in relation to water vapor and other non-polar gases (CH4, O2, and N2) was evaluated, after introducing different chemical moieties in an elastomeric block co-polyamide (Pebaxw2533: 80PTMO/PA). Many studies, devoted to gas separations based on membrane processes, elucidated the importance to correlate the characteristics of polymers, as free volume, polymer chain packing and intersegment distance, with their transport properties, emphasizing the role of the microstructure of the polymers in gas/vapor permeability and selectivity [25-30]. PEBAX polyether block amides are a new family of engineering thermoplastic elastomer, their general structure is (A-B)n; they consist of linear and regular chains of hard polyamide segments having molecular weight between 600 and 4000, and soft polyether segments of molecular weight between 600 and 2000, the soft and hard segments are relatively short blocks. In the temperature region of exploitation, the soft segment component is viscous or rubbery while the hard segment is glassy or semi-crystalline. The ratio of the polyamide and polyether blocks controls the hardness. The length of the polyamide block influences the melting point of the polymer. Since polyamide and polyether segments are not miscible, the polyether block amides have a morphology characterized by phase separation (bi-phase separation) [31-33]. PEBAX is a group of copolymers including hard polyamide segments and soft polyether segments. These copolymers have extremely high polar/non polar (e.g. H2S/CH4, or CO2/N2) gas selectivity coefficients, making them interesting candidates for the removal of CO2 from synthesis gas, natural gas, and flue gas. Crystalline amides block in PEBAXTM works as impermeable phase, whereas soft ether block in PEBAXTM acts as a permeable phase due to its high chain mobility. The permeability of membranes can be also tailored through the dispersion of inorganic particles [34]. During the past decades, different inorganic phases (e.g. SiO2, TiO2, and ZrO2) were in situ generated during the casting of PEBAXTM membranes from solutions using alkoxides as precursors [35,36]. The incorporation of inorganic phase in a polymer matrix leads also to improved solvent resistance and thermal stability of PEBAXTM polymer [37-41]. In the present work we report the preparation of PEBAXTM/Alumina nanocomposites obtained by extrusion process. The properties of the nanocomposites are discussed in terms of thermal stability, crystallinity degree and interactions between the filler and the soft and hard segments of the PEBAXTM matrix.
2. Experimental
2.1. Materials
Alumina: Al2O3 supplied by Sasol South Africa. Silane coupling agent: 3-(2 trimethoxysilylethyl) cyclohexene oxide (Figure 1) from Aldrich Germany. PEBAX™ (Figure 1) supplied by Arkema (France) is a polyamide-12, poly(tetramethylene oxide) multi-block copolymer (Figure 1). The polymer was supplied as a black powder, due to the presence of pigments.
2.2. Silanization Process
A suspension containing 15 g of alumina (Sasol, South Africa) 15 mL hexane and 5 g alkoxysilane were stirred during 24 h at room temperature. After 8 h, the formation of spherical yellowish agglomerates of silanized alumina could be observed. The precipitate was recovered by filtration and dried overnight at 100˚C.
2.3. Manufacturing of the Nanocomposite
Neat PEBAXTM powder and mixtures of 0.1, 1.0, 5.0, and 10.0% w/w PEBAX™ /modified alumina were extruded in a Haake Poly lab system twin-screw extruder using the conditions summarized in Table 1. The extruded nanocomposites were polluted at room temperature and milled at –120˚C in a cryo-milling machine (Table 1).
2.4. Measurements
Differential Scanning Calorimetry (DSC) of each composite were carry out in a DSC calorimeter under nitrogen at 10˚C/min temperature ramp from –100˚C to 200˚C
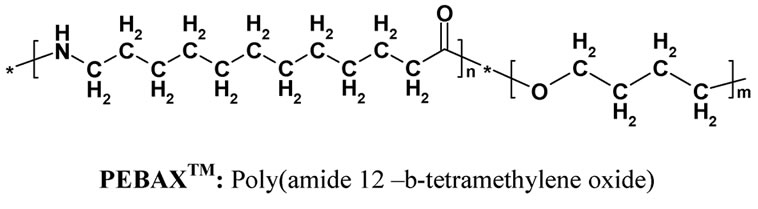
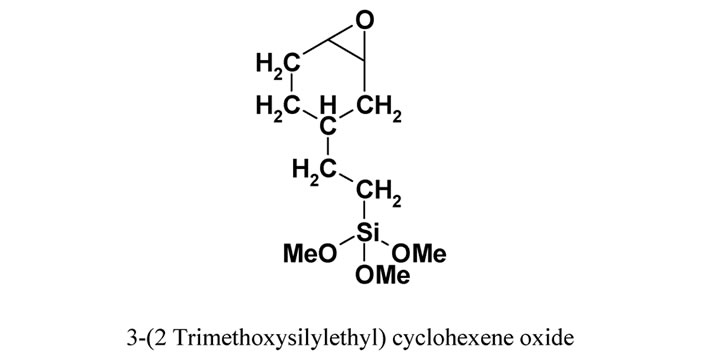
Figure 1. Chemical structure of PEBAXTM and 3-(2 Trimethoxysilylethyl) cyclohexene oxide.
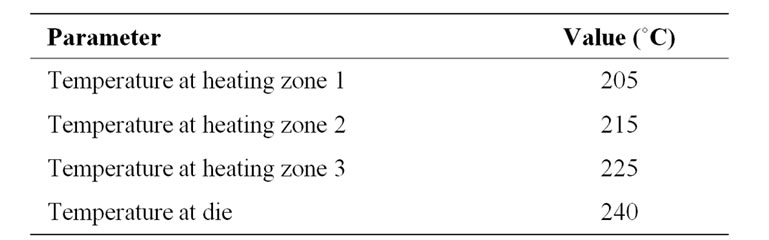
Table 1. Conditions for the extrusion of PEBAXTM and composites thereof.
(SEIKO: Japan). The decomposition temperatures in nitrogen and air were determined from the thermogravimetric analysis carried out in a TGA Q500 (TA Instruments, New Castle, DE).
3. Results and Discussion
Alumina Functionalization
The fictionalization of alumina took place through condensation reaction involving the species produced from the hydrolysis of 3-(2 trimethoxysilylethyl) cyclohexene oxide. This process was accelerated by the moisture adsorbed at the surface of the alumina nanoparticles. The hydrolysis of the methoxy groups attached to the silicon atom yields a silanol groups (as described by Equation (1)) [42,43], which in turn can react with the Al-OH groups at the surface of alumina nanoparticles, as exemplified by the Equations (2) and (3).
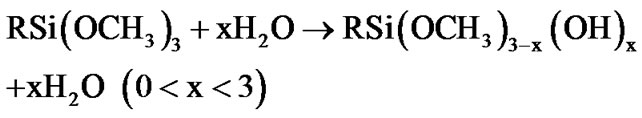 (1)
(1)
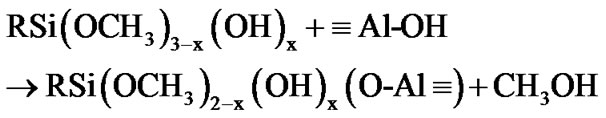 (2)
(2)
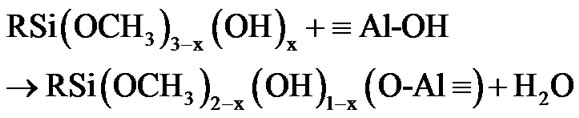 (3)
(3)
where:
Evaluation of the thermal properties of the nanocomposites.
Table 2 contains the DSC data of the neat PEBAXTM and their nanocomposites. The mobility of the soft PTMO segment is strongly hindered by the presence of the silanized nanoparticles, since the glass transition temperature of a nanocomposite containing only 0.10 wt% of nanoparticle exhibits much higher Tg values for the PTMO block than the neat PEBAXTM.
The hard semi-crystalline PA-12 blocks were also influenced by the presence of the nanoparticles. The overall crystallinity degree (˚C) of the nanocomposites was estimated using the Equation (4), which correlates the crystallinity degree, melting enthalpy 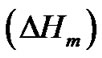 with the equilibrium melting enthalpy
with the equilibrium melting enthalpy 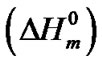 [44]. The value of
[44]. The value of 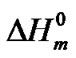 used was 246 J/g [45].
used was 246 J/g [45].
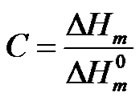 (4)
(4)
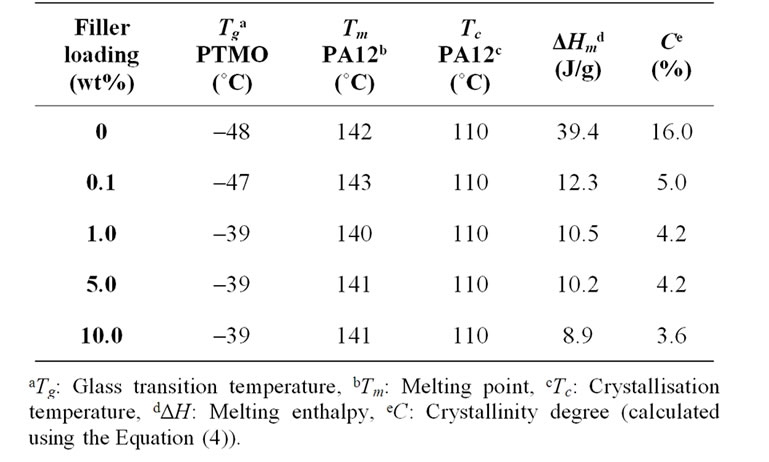
Table 2. Thermal properties of polymers.
Although Equation (4) provides an approximation of the crystallinity degree of the PA12 phase of the PEBAXTM used in the present work, because it does not take the weight percentage of PA12 in the copolymer, it can be used to evaluate the potential of the filler to enhance or decrease the crystallinity of the PA12 segments.
The values for crystallinity degree of the nanocomposites were significantly lower than of the neat PEBAXTM. A 66% reduction of the crystallinity degree was reached at very low filler loadings (0.10 wt% of silanized alumina). This fact can be explained by the partial crosslinking of the PA-12 block by the functionalized alumina nanoparticles.
The crystallisation temperatures (Tc), determined during the second cooling cycle, did not change for the nanocomposites. This reflects the little interaction of PA12 with nanoparticles and other fillers, as described in several papers [46-50].
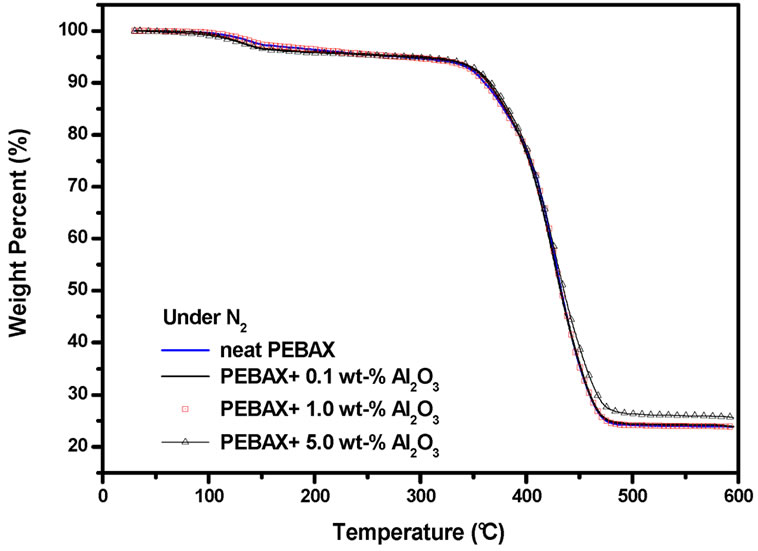
The thermal stability of the PEBAXTM/Alumina nanocomposites was evaluated by TGA under N2. Under these constrains, the degradation of polyamides takes place via depolymerisation reaction, i.e., formation of lactams (in the case of polyamide 12, lauryl lactam [51]) or via elimination of alkenes and nitriles [51]. Whereas the de-polymerization involves the end-groups of the polymer, the g-elimination can take place at any part of the chain Hence, it is relatively independent of the molecular weight, while the de-polymerization is more extensively observed for polymers having lower molecular weight.
Epoxies and alcohols are prone to react with nitriles at higher temperatures [52]. In this case, after the chain scission into an amide (followed by its dehydration towards a nitrile), the resulting nitrile could be trapped by the fillers bearing epoxy or –OH groups. Therefore, an improvement of the thermal stability should be observed for the PEBAXTM nanocomposites containing epoxyfunctionalized alumina.
The thermal stability of the nanocomposites was marginally higher than neat PEBAXTM. Therefore the filler does not seem to interfere in the degradation process of this polymer under inert atmosphere.
Figures 2(a) and 2(b) depict the TGA curves of the nanocomposites under N2 and synthetic air, respectively. It can be seen that the incorporation of the silanized alumina nanoparticles with excellent thermal stability under oxidation conditions. It could be observed that the chair yield at 600˚C increases proportionally to the alumina content. This fact can be explained in terms of higher inertness of alumina at higher temperatures.
In order to understand and better evaluate this stabilization effect, some calculations were done to estimate the thermal stability of a mixture of PEBAXTM and silanized alumina. For example, the TGA curve of PEBAXTM nanocomposite containing 10.0 wt% silanized alumina was compared to a calculated TGA curve, which was obtained by adding the TGA curve of pure PEBAXTM to the TGA curve of the silanized alumina. It was taken into consideration that there 90 wt% of PEBAX and 10 wt% of
 (a)
(a)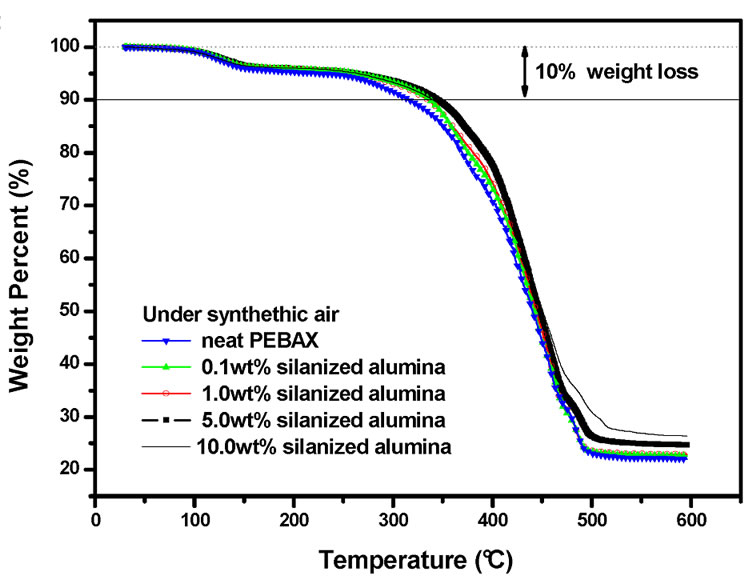 (b)
(b)
Figure 2. TGA traces of the neat PEBAXTM and PEBAXTM/Al2O3 nancomposites under N2 (a); and under synthetic air (b).
silanized alumina in the nanocomposite. Therefore the TGA curves of PEBAXTM and the alumina were multiplied by factor of 0.90 and 0.10, respectively. The same procedure was taken for the PEBAXTM nanocomposite containing 5 wt% of silanized alumina. These curves as depicted in the Figures 3 and 4, respectively.
The rule of mixtures would predict that the thermal degradation should be strongly dominated by the PEBAXTM matrix. Therefore, the physical mixture of PEBAXTM and silanized alumina should be almost as stable as pure PEBAXTM. However, the experimental results suggest that the nanocomposites are more stable than the mixture of their components. This stabilization effect is evident in the temperature range between 300˚C and 400˚C, in which the degradation of the PA12 blocks takes place. Hence, the alumina nanoparticles did indeed improve the thermo-oxidative stability of the PEBAXTM matrix.
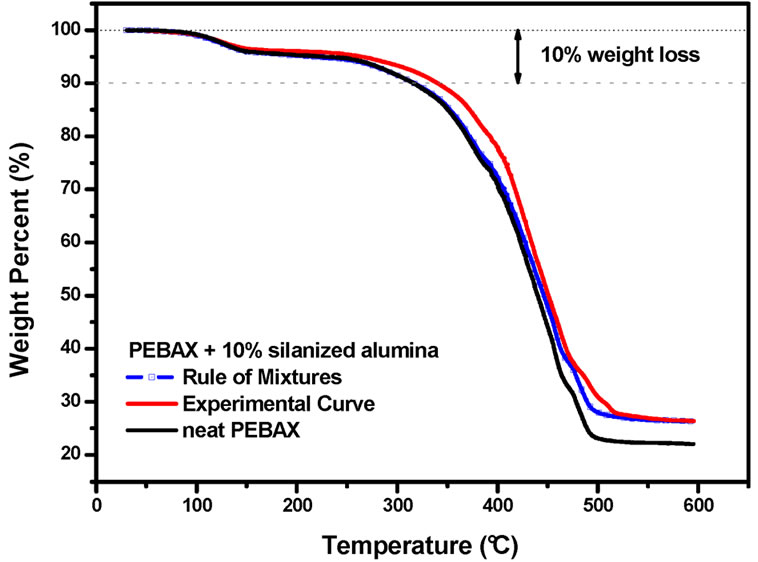
Figure 3. Experimental and calculated (following a rule of mixtures) TGA curves of PEBAXTM/silanized alumina 90/10 w/w. The TGA curve of neat PEBAXTM is shown for the sake of comparison.
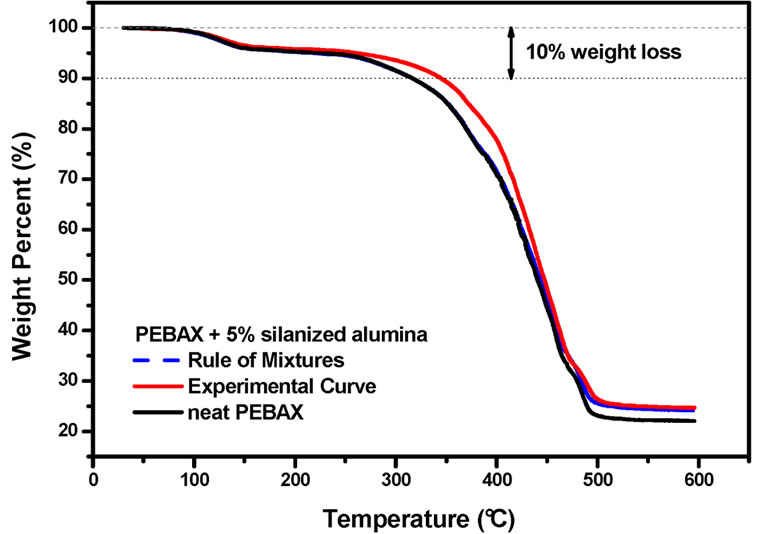
Figure 4. Experimental and calculated (following a rule of mixtures) TGA curves of PEBAXTM/silanized alumina 95/5 w/w The TGA curve of neat PEBAXTM is shown for the sake of comparison.
4. Conclusion
The dispersion of nanoparticles constituted of silanized alumina in PEBAXTM matrix using extrusion process could produce nanocomposites with improved thermooxidative stability. The chemical coupling between the PA12 blocks and the functional groups at the surface of the alumina nanoparticles caused a decrease of the crystallinity of the hard PA-12 segments, as well as, constrained the mobility of the soft PTMO segment, as evidenced by the increase in Tg of this component.
5. Acknowledgements
The authors are grateful to E. Palacios and M. Cruz from ICN-UNAM for technical assistance. This work was supported by DGAPA-UNAM Grant IN202311 and CONACYT-CNPq Project 174378.
REFERENCES
- E. Amerio, P. Fabbri, G. Malucelli, M. Messori, M. Sangermano and R. Taurino, “Scratch Resistance of Nano-Silica Reinforced Acrylic Coatings,” Progress in Organic Coatings, Vol. 62, No. 2, 2008, pp. 129-133. doi:10.1016/j.porgcoat.2007.10.003
- C. Sanchez, B. Julian, P. Belleville and M. Popall, “Applications of Hybrid Organic-Inorganic Nanocomposites,” Journal of Materials Chemistry, Vol. 15, No. 35-36, 2005, pp. 3559-3592. doi:10.1039/b509097k
- F. Mammeri, E. Le Bourhis, L. Rozes and C. Sanchez, “Mechanical Properties of Hybrid Organic-Inorganic Materials,” Journal of Materials Chemistry, Vol. 15, No. 35-36, 2005, pp. 3787-3811. doi:10.1039/b507309j
- N. M. Jose and L. A. S. A. Prado, “Hybrid OrganicInorganic Materials: Preparation and Some Applications,” Quimica Nova, Vol. 28, No. 2, 2005, pp. 281-288.
- T. Ogoshi and Y. Chujo, “Organic-Inorganic Polymer Hybrids Prepared by the Sol-Gel Method,” Composite Interfaces, Vol. 11, No. 8-9, 2005, pp. 539-566.
- S. Bredeau, S. Peeterbroeck, D. Bonduel, M. Alexandre and P Dubois, “From Carbon Nanotube Coatings to High-Performance Polymer Nanocomposites,” Polymer International, Vol. 57, No. 4, 2008, pp. 547-667. doi:10.1002/pi.2375
- B. Chen, J. R. G. Evans, H. C. Greenwell, P. Boulet, P. V. Coveney, A. A. Bowden and A. Whiting, “A Critical Appraisal of Polymer-Clay Nanocomposites,” Chemical Society Reviews, Vol. 37, No. 3, 2008, pp. 568-594. doi:10.1039/b702653f
- A. J. Crosby and J. Y. Lee, “Polymer Nanocomposites: The ‘Nano’ Effect on Mechanical Properties,” Polymer Reviews, Vol. 47, No. 2, 2007, pp. 217-229. doi:10.1080/15583720701271278
- A. M. Herring, “Inorganic-Polymer Composite Membranes for Proton Exchange Membrane Fuel Cells,” Polymer Reviews, Vol. 46, No. 3, 2006, pp. 245-296.
- A. C. C. Esteves, A. Barros-Timmons and T. Trindade, “Polymer Based Nanocomposites: Synthetic Strategies for Hybrid Materials,” Química Nova, Vol. 27, No. 5, 2004, pp. 798-806. doi:10.1590/S0100-40422004000500020
- H. L. Cong, M. Radosz, B. F. Towler and Y. Q. Shen, “Polymer-Inorganic Nanocomposite Membranes for Gas Separation,” Separation and Purification Technology, Vol. 55, No. 3, 2007, pp. 281-291. doi:10.1016/j.seppur.2006.12.017
- N. W. DeLucca and Y. A. Elabd, “Polymer Electrolyte Membranes for the Direct Methanol Fuel Cell: A Review,” Journal of Polymer Science and Physics, Vol. 44, No. 16, 2006, pp. 2201-2225. doi:10.1002/polb.20861
- S. Anilkumar, M. G. Kumaran and S. Thomas, “Characterization of EVA/Clay Nanocomposite Membranes and Its Pervaporation Performance,” Journal of Physical Chemistry B, Vol. 112, No. 13, 2008, pp. 4009-4015. doi:10.1021/jp7096444
- M. L. Sforca, I. V. P. Yoshida, C. P. Borges and S. P. Nunes, “Hybrid Membranes Based on SiO2/Polyetherb-polyamide: Morphology and Applications,” Journal of Applied Polymer Science, Vol. 82, No. 3, 2001, pp. 178- 185. doi:10.1002/app.1837
- C. F. Canto, E. Radovanovic, L. A. S. A. Prado and I. V. P. Yoshida, “Organic-Inorganic Hybrid Materials Derived from Epoxy Resin and Polysiloxanes: Synthesis and Characterization,” Polymer Engineering Science, Vol. 48, No. 1, 2008, pp. 141-148. doi:10.1002/pen.20931
- B. Fiedler, F. H. Gojny, M. H. G. Wichmann, M. C. M. Nolte and K. Schulte, “Fundamental Aspects of NanoReinforced Composites,” Composites Science and Technology, Vol. 66, No. 16, 2006, pp. 3115-3125. doi:10.1016/j.compscitech.2005.01.014
- N. M. Jose, L. A. S. A. Prado and I. V. P. Yoshida, “Synthesis, Characterization, and Permeability Evaluation of Hybrid Organic-Inorganic Films,” Journal of Polymer Science and Physics, Vol. 42, No. 23, 2004, pp. 4281- 4292. doi:10.1002/polb.20292
- A. de la Vega Oyervides, J. B. Rios, L. F. R. de Valle, L. A. S. A. Prado and K. Schulte, “Peroxide Assisted Coupling and Characterization of Carbon-Nanofiber-Reinforced Poly(Propylene) Composites,” Macromolecular Materials and Engineering, Vol. 292, No. 10-11, 2007, pp. 1095- 1102. doi:10.1002/mame.200700201
- C. S. Karthikyean, L. A. S. A. Prado, S. P. Nunes and K. Schulte, “Impact of Functionalization of Nanoparticles on the Barrier Properties of Ionomernanocomposite Membranes for DMFC,” Journal of the Electrochemical Society, Vol. 3, No. 1, 2006, pp. 1297-1301.
- M. H. G. Wichmann, M. Cascione, B. Fiedler, M. Quaresimin and K. Schulte, “Influence of Surface Treatment on Mechanical Behaviour of Fumed Silica/Epoxy Resin Nanocomposites,” Composite Interfaces, Vol. 13, No. 8-9, 2006, pp. 699-715. doi:10.1163/156855406779366723
- S. Sridhar, R. Suryamurali, B. Smitha and T. M. Aminabhavi, “Development of Crosslinked Poly(Etherblock-amide) Membrane for CO2/CH4 Separation,” Colloids and Surfaces A, Vol. 297, No. 1-3, 2007, pp. 267-274. doi:10.1016/j.colsurfa.2006.10.054
- J. C. Chen, X. Feng and A. Penlidis, “Gas Permeation through Poly(Ether-b-amide) (PEBAX 2533) Block Copolymer Membranes,” Separation Science and Technology, Vol. 39, No. 1, 2005, pp. 149-164.
- R. E. Kesting and A. K. Fritzsche, “Polymeric Gas Separation Membranes,” Wiley, New York, 1993.
- A.Gugliuzza, R. Fabiano, M. G. Garavaglia, A. Spisso and D. Drioli, “Study of the Surface Character as Responsible for Controlling Interfacial Forces at Membrane-Feed Interface,” Journal of Colloid Interface Science, Vol. 303, No. 2, 2006, pp. 388-403. doi:10.1016/j.jcis.2006.07.017
- A. Gugliuzza and E. Drioli, “Evaluation of CO2 Permeation through Functional Assembled Mono-Layers: Relationships between Structure and Transport,” Polymer, Vol. 46, No. 23, 2005, pp. 9994-10003. doi:10.1016/j.polymer.2005.08.011
- A. Gugliuzza and E. Drioli, “Role of Additives in the Water Vapor Transport through Block Co-Poly(Amide/ Ether) Membranes: Effects on Surface and Bulk Polymer Properties,” European Polymer Journal, Vol. 40, No. 10, 2004, pp. 2381-2389. doi:10.1016/j.eurpolymj.2004.06.005
- Z. F. Wang, B. Wang, N. Qia, X. M. Ding and J. L. Hu, “Free Volume and Water Vapor Permeability Properties in Polyurethane Membranes Studied by Positrons,” Materials Chemiatry and Physics, Vol. 88, No. 1, 2004, pp. 212-216. doi:10.1016/j.matchemphys.2004.07.012
- J. P. Sheth, J. Xu and G. L. Wilkes, “Solid State StructureProperty Behavior of Semicrystalline Poly(Ether-blockamide) PEBAX® Thermoplastic Elastomers,” Polymer, Vol. 44, No. 3, 2003, pp. 743-756. doi:10.1016/S0032-3861(02)00798-X
- S. Banerjee, G. Maier, C. Dannenberg and J. Spinger, “Gas Permeabilities of Novel Poly(Arylene Ether)s with Terphenyl Unit in the Main Chain,” Journal of Membrane Science, Vol. 229, No. 1-2, 2004, pp. 63-71. doi:10.1016/j.memsci.2003.09.016
- G. García-Ayuso, R. Salvarezza, J. M. Martínez-Duart, O. Sánchez and L. Vázques, “Relationship between the Microstructure and the Water Permeability of Transparent Gas Barrier Coatings,” Surface and Coatings Technology, Vol. 100-101, No. 1-3, 1998, pp. 459-462. doi:10.1016/S0257-8972(97)00671-3
- G. H. Hatfield, Y. Guo, W. E. Killinger, R. A. Andrejak and P. M. Roubicek, “Characterization of Structure and Morphology in Two Poly(Ether-block-amide) Copolymers,” Macromolecules, Vol. 26, No. 24, 1993, pp. 6350-6353. doi:10.1021/ma00076a008
- M. L. Di Lorenzo, M. Pyda and B. Wunderlich, “Calorimetry of Nanophase-Separated Poly(Oligoamide-alt-oligoether)s,” Journal of Polymer Science Physiics, Vol. 39, No. 14, 2001, pp. 1594-1604. doi:10.1002/polb.1131
- E. V. Konyukhova, A. I. Buzin and Y. K. Godovsky, “Melting of Polyether Block Amide (Pebax): The Effect of Stretching,” Thermochimica Acta, Vol. 391, No. 1-2, 2002, pp.271-277. doi:10.1016/S0040-6031(02)00189-2
- C. S. Karthikeyan, L. A. S. A. Prado, M. L. Ponce, H. Silva, B. Ruffmann, K. Schulte and S. P. Nunes, “Polymer Nanocomposite Membranes for DMFC Application,” Journal of Membrane Science, Vol. 254, No. 1-2, 2005, pp. 139-146.
- R. A. Zoppi and C. G. A. Soares, “Hybrids of Poly (Ethylene Oxide-b-amide-6) and ZrO2 Sol-Gel: Preparation, Characterization, and Application in Processes of Membranes Separation,” Advances in Polymer Technology, Vol. 21, No. 1, 2002, pp. 2-16. doi:10.1002/adv.10011
- R. A. Zoppi, S. Das Neves and S. P. Nunes, “Hybrid Films of Poly(Ethylene Oxide-b-amide-6) Containing Sol-Gel Silicon or Titanium Oxide as Inorganic Fillers: Effect of Morphology and Mechanical Properties on Gas Permeability,” Polymer, Vol. 41, No. 14, 2000, pp. 5461- 5470. doi:10.1016/S0032-3861(99)00751-X
- J. H. Kim and Y. M. Lee, “Gas Permeation Properties of Poly(Amide-6-b-ethylene Oxide)-Silica Hybrid Membranes,” Journal of Membrane Science, Vol. 193, No. 2, 2001, pp. 209-225. doi:10.1016/S0376-7388(01)00514-2
- J. F. Feller, D. Langevin and S. Marais, “Influence of Processing Conditions on Sensitivity of Conductive Polymer Composites to Organic Solvent Vapours,” Synthetic Metals, Vol. 144, No. 1, 2004, pp. 81-88. doi:10.1016/j.synthmet.2004.02.006
- J. F. Feller and Y. Grohens, “Evolution of Electrical Properties of Some Conductive Polymer Composite Textiles with Organic Solvent Vapours Diffusion,” Sensors and Actuators B: Chemical, Vol. 97, No. 2-3, 2004, pp. 231-242.
- N. R. Legge, G. Holden and H. E. Schröder, “Thermoplastic Elastomers,” 2nd Edition, Hanser Publishers, New York, 1996.
- A. Kondyurin, P. Volodin and J. Weber, “Plasma Immersion Ion Implantation of Pebax Polymer,” Nuclear Instruments and Methods B, Vol. 251, No. 2, 2006, pp. 407- 412. doi:10.1016/j.nimb.2006.06.026
- L. A. S. A. Prado, C. S. Karthikeyan, K. Schulte, S. P. Nunes and I. L. Torriani, “Organic Modification of Layered Silicates: Structural and Thermal Characterizations,” Journal of Non-Crystalline Solids, Vol. 351, No. 12-13, 2005, pp. 970-975. doi:10.1016/j.jnoncrysol.2004.12.007
- L. A. S. A. Prado, E. Radovanovic, H. O. Pastore, I. V. P. Yoshida and I. L. Torriani, “Poly(Phenylsilsesquioxane)s: Structural and Morphological Characterization,” Journal of Polymer Science Polymer Chemistry, Vol. 38, No. 9, 2000, pp. 1580-1589. doi:10.1002/(SICI)1099-0518(20000501)38:9<1580::AID-POLA22>3.0.CO;2-7
- G. W. Ehrenstein, G. Riedel and P. Trawiel, “Praxis der Thermischen Analyse von Kunststoffen,” 1st Edition, Hanser, München, 1998.
- V. I. Bondar, B. D. Freeman and I. Pinnau, “Gas Sorption and Characterization of Poly(Ether-b-amide) Segmented Block Copolymers,” Journal of Polymer Science Polymer Physics, Vol. 37, No. 17, 1999, pp. 2463-2475. doi:10.1002/(SICI)1099-0488(19990901)37:17<2463::AID-POLB18>3.0.CO;2-H
- A. R. Bhattacharyya, S. Bose, A. R. Kulkarni, P. Pötschke, L. Häußler, D. Fischer and D. Jehnichen, “Styrene Maleic Anhydride Copolymer Mediated Dispersion of Single Wall Carbon Nanotubes in Polyamide 12: Crystallization Behavior and Morphology,” Journal of Applied Polymer Science, Vol. 106, No. 1, 2007, pp. 345-353. doi:10.1002/app.26680
- J. Z. Lu, T. W. Doyle and K. Li, “Preparation and Characterization of Wood-(Nylon 12) Composites,” Journal of Applied Polymer Science, Vol. 103, No. 1, 2007, pp. 270-276. doi:10.1002/app.25274
- Y. Zhang, J. H. Yang, T. S. Ellis and J. Shi, “Crystal Structures and Their Effects on the Properties of Polyamide 12/Clay and Polyamide 6-Polyamide 66/Clay Nanocomposites,” Journal of Applied Polymer Science, Vol. 100, No. 6, 2006, pp. 4782-4794. doi:10.1002/app.23378
- T. McNally, W. R. Murphy, C. Y. Lew, R. J. Turner and G. P. Brennan, “Polyamide-12 Layered Silicate Nanocomposites by Melt Blending,” Polymer, Vol. 44, No. 9, 2003, pp. 2761-2772. doi:10.1016/S0032-3861(03)00170-8
- I. Arvanitoyannis and E. Psomiadou, “Composites of Anionic (Co)Polyamides (Nylon 6/Nylon 12) with Short Glass E-Fibers. Preparation and Properties,” Journal of Applied Polymer Science, Vol. 51, No. 11, 1994, pp. 1883-1899. doi:10.1002/app.1994.070511105
- S. V. Levchik, E. D. Weil and M. Lewin, “Thermal Decomposition of Aliphatic Nylons,” Polymer International, Vol. 48, No. 7, 1999, pp. 532-557. doi:10.1002/(SICI)1097-0126(199907)48:7<532::AID-PI214>3.0.CO;2-R
- J. L. Fan, G. M. Wang and W. H. Ku, “Nitrile-Containing Imidazole Cured of Novolac Epoxy Resin,” Journal of Applied Polymer Science, Vol. 43, No. 4, 1991, pp. 829- 833. doi:10.1002/app.1991.070430422
NOTES
*Corresponding author.

