Advances in Materials Physics and Chemistry
Vol.05 No.05(2015), Article ID:56645,5 pages
10.4236/ampc.2015.55019
Comparative Study of the Effect of Temperature on the Corrosion Behaviour of 2205 Duplex Stainless Steel and 316 Austenitic Stainless Steel in Acidic Chloride Environment
O. A. Olaseinde
Advanced Materials and Electrochemical Research Group, Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria
Email: adenikeseinde@yahoo.com
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 January 2014; accepted 22 May 2015; published 26 May 2015
ABSTRACT
The 2205 duplex stainless steel and 316 austenitic stainless steels were studied in 1 M sulphuric acid and 1% NaCl solution. The microstructures of the specimens were investigated with scanning electron microscopy with energy dispersive X-ray analysis. X-ray Diffraction analysis was used for phase analysis. The electrochemical behaviour was evaluated using potentiodynamic method. The results show that the critical current density is higher for 316 austenitic stainless steels than 2205. The passive range was longer for 316 than 2205 at all the temperatures understudy. 2205 was found to have better corrosion resistance than 316.
Keywords:
Stainless Steels, Potentiodynamic, Acidic Chloride

1. Introduction
Stainless steels are steel with high percentages of chromium. The brightness of stainless steel is retained for long periods. It has different types which could be austenitic, ferritic, martensitic or duplex. The stainless steels possess an especially useful characteristic in resisting corrosion as they perform best under the oxidising conditions which are most harmful to ordinary steels and many of the non-ferrous alloys. Cold forming and cold rolling do not decrease the corrosion resistance. Duplex stainless steels (DSS) are family of steels having a two-phase microstructure with roughly the same volume fractions of ferrite and austenite. The advantageous properties of duplex stainless steels are based on the equilibrium of the microstructure [1] - [5] . The combination improved the mechanical properties and corrosion resistance of these steels [2] . The best performance of duplex stainless steel is obtained when the ferrite-austenite ratio is close to 50:50 [1] - [5] . Duplex stainless steel is used in petrochemical, desulphurization, pulp and papers, chemical tankers and architecture.
Austenitic stainless steels are Fe-based alloys with a chromium content of at least 10.5%, its microstructure comprised primarily of austenite phase. It has good corrosion resistance and high toughness but they have high sensitivity to local corrosion in chloride environments. The 316 austenitic stainless steels contain between 16 - 18 wt percent Cr. It has been reported to have a very high corrosion resistance than grade 304 as a result of its high molybdenum content. This alloy is suitable for welding because it has carbon content lower than 301 to 303 series alloys [6] [7] . 316 are widely used in many industrial components, food preparation equipment particularly in chloride environments; it is used in architectural paneling, railings and trim, boat fittings, chemical containers and heat exchangers.
The 2205 have better corrosion resistance than low nickel duplex stainless steels [5] while the 316 had better corrosion resistance than most austenitic stainless steels.
This paper investigates the response of these two stainless steels in acidic chloride environments and also compares the effect of temperature on their corrosion behaviour.
2. Experimental Methods
The studied materials are EN.14462 (2205) duplex stainless steel and 316 austenitic stainless steels. They were supplied as plates with thickness of 6mm. the microstructural characterization was done by means of optical microscopy, scanning electron microscopy. Electrolytic etching in 40 g NaOH in 100 ml of distilled water was used for the 2205 to reveal its duplex microstructures. This treatment coloured the ferrite dark [8] . While for the 316 grade electrolytic etching with 10% oxalic acid was used.
Potentiodynamic polarization studies were carried out in a three electrode cell with graphite as counter electrode and Ag/AgCl reference electrode; the samples were studied in 1 M sulphuric acid and 1% NaCl solution at 25˚C, 40˚C, 60˚C, and 80˚C. The temperatures were maintained with the aid of water baths.
The surfaces of the samples were prepared by grinding up to 800 grit. Potentials were scanned from −250 to 1200 mV at a scan rate of 1 mV/sec with the aid of potentiostat with GPES 4.19 software, each test was repeated and good reproducibility was observed.
2.1. Results and Discussion
2.1.1. Metallography
The observations of the surfaces of the specimens were carried out using scanning electron microscopy. The SEM image of 316 austenitic stainless steel shows austenite structure and no other phases was observed as shown in Figure 1. The SEM image of 2205 duplex stainless steel is presented in Figure 2, showing austenite particles randomly dispersed in matrix of ferrite and are elongated along the rolling direction. Porosity was observed in the micrograph. Inter-metallic phases were not observed. The XRD analysis showed that the 2205 contains austenite and ferrites while the 316 contains only the austenite phase. A very wide band in the 2 Theta ranges 15 - 35 is usually observed with crystalline peaks. The wide band does not affect the peaks position or intensity.
2.1.2. Potentiodynamic Curves
Figure 3 show the potentiodynamic curves of 2205 and 316 stainless steels in 1 M sulphuric acid and 1% NaCl solutions at 25˚C. The corrosion potential of 2205 was 348 mV which was nobler than −255 mV for 316 and this implies that 316 have more tendencies for corrosion than 2205. The passive current density was 4.1 × 10−6 A/cm2 in 2205 and was lower than 1.08 × 10−5 A/cm2 in 316. The passive region is longer for 316 than 2205. The icrit value for 316 was 2.8 × 10−5 A/cm2 and is higher than 2.9 × 10−6 A/cm2 for 2205 duplex stainless steel. The length of the passive region is longer in 316 than for 2205. The critical current density of 2205 was lower than 316 at 25˚C.
The critical current density of 316 was 3.4 × 10−4A/cm2 and is higher than 6.8 × 10−5A/cm2 which is for 2205;
Figure 1. SEM-BSE image and X-ray Diffraction pattern of 316 austenitic stainless steel.
Figure 2. SEM-BSE image and X-ray Diffraction pattern of 2205 duplex stainless steel.
Figure 3. Polarization curves of 2205 and 316 stainless steel in 1 M sulphuric acid + 1% NaCl solutions at 25˚C.
the corrosion potential of 316 was −225 mV while for 2205 was 82 mV. The passive current density is lower in 316 than 2205, an active to passive transition was observed in the 316 at 40˚C while displayed passivity (Figure 4).
The corrosion potential is nobler in 2205 than 316 at 80˚C as shown in Figure 5; the critical current density in 316 was 1.6 × 10−3A/cm2. The 2205 alloy exhibited passive behaviour. The high critical current density in 316
Figure 4. Polarization curves of 2205 and 316 stainless steel in 1 M sulphuric acid + 1% NaCl solutions at 40˚C.
Figure 5. Polarization curves of 2205 and 316 stainless steel in 1 M sulphuric acid + 1% NaCl solutions at 80˚C.
could cause an increase in the corrosion rate of the sample than 2205. The ipass is lower in 316 than in 2205 and the length of the passive region is lower than in 2205.
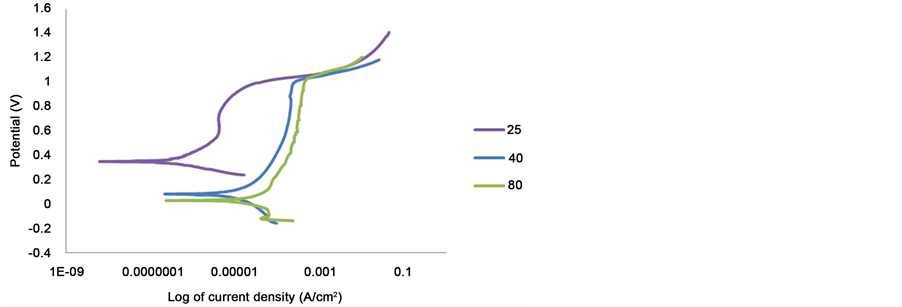
The increased in temperature increases the corrosion rates, the current density of 2205 duplex stainless steel in 1 M sulphuric acid contaminated with 1% NaCl. Figure 6 showed that the corrosion of 2205 at 25˚C was better than at higher temperature.
Type 316 gives useful service at room temperature in sulphuric acid, at elevated temperatures the corrosion rate increases. The increased in corrosion rates of type 316 in sulphuric acid is a function of concentration and temperature. Figure 7 showed that increasing the temperature shifted increases the critical current density and also increased the passive current density. The passivity ranges also decreases from 1064 at 25˚C to 1044 at 80˚C. 316 showed active to passive transition at all temperatures.
The corrosion resistance exhibited by 2205 could be attributed to its dual structure. The high corrosion resistance is a major advantage of duplex stainless steels. Many researchers have concluded that duplex stainless steels have high corrosion resistance due to its dual phase [9] . The corrosion resistances of stainless steels have been correlated with their chemical composition [3] [10] .
The pitting resistance equivalent (PREN) is calculated by PREN = %Cr + 3.3 × %Mo + 30 × %N − 1 × %Mn
Therefore the PREN values for 2205 and 316 are 37.4 and 28.9 respectively. The PREN of 2205 is 8.5 higher than the PREN of 316. These indicate that 2205 have a higher pitting corrosion resistance than 316.
3. Conclusion
The electrochemical behaviours of 316 and 2205 stainless steel were studied in 1 M sulphuric acid contaminated by sodium chloride at different temperatures. The corrosion potential measurements show that the corrosion potential of 2205 was nobler than 316 at 25˚C, 40˚C and 80˚C. The potentiodynamic measurements indicated that at 25˚C the passive current density of 2205 was lower than 316. The passivity range in 316 was longer than for 2205 at the understudy temperatures. The passive current density was lower for 2205 at room temperature while at elevated temperature, it was lower for 316. The critical current density is higher for all samples of 316. The
Figure 6. Potentiodynamic curves of 2205 duplex stainless steel in 1 M sulphuric and 1% NaCl solutions at different temperatures.
Figure 7. Potentiodynamic curves of 316 austenitic stainless steel in 1 M sulphuric and 1% NaCl solutions at different temperature.
increase in temperature exhibited an increase in corrosion rates for the 2205 and 316 stainless steels. The 2205 duplex stainless steel showed a better corrosion resistance at 25˚C.
Acknowledgements
Science Initiative Group, Institute for Advanced Study, USA.
References
- Solomon, H.D. and Devine, T.M. (1983) Duplex Stainless Steels. American Society of Metals, Metals Park, Ohio, USA, 693-756.
- Desestret, A. and Charles, J. (2006) Stainless Steels. In: Lacombe, P., Baroux, B. and Beranger, G., Eds. Les Editions de Physique, Paris, 2006.
- Zhang, W., Jiang, L., Hu, J. and Song, H. (2008) Study of Mechanical and Corrosion Properties of a Fe-21.4Cr-6Mn- 1.5Ni-0.24N-0.6Mo Duplex Stainless Steel. Materials and Engineering A, 497, 501-504. http://dx.doi.org/10.1016/j.msea.2008.07.062
- Ibrahim, O.H., Ibrahim, I.S. and Khalifa, T.A.F. (2010) Effect of Aging on the Toughness of Austenitic and Duplex Stainless Steel Weldments. Journal of Materials Science & Technology, 26, 810-816. http://dx.doi.org/10.1016/S1005-0302(10)60129-6
- Olaseinde, O.A., Van der Merwe, J. and Cornish, L. (2014) Characterization and Corrosion Behaviour of Selected Duplex Stainless Steels in Acidic and Acidic-Chloride Solution. Advances in Chemical Engineering and Science, 4, 89-93. http://dx.doi.org/10.4236/aces.2014.41012
- Trillo, E.A. and Murr, L.E. (1999) Effects of Carbon Content, Deformation, and interfacial Energetics on Carbide Precipitation and Corrosion Sensitization in 304 Stainless Steel. Acta Materialia, 47, 235-245. http://dx.doi.org/10.1016/S1359-6454(98)00322-X
- Amudarasan, N.V., Palanikumar, K. and Shanmugam, K. (2013) Impact Behaviour and Micro Structural Analysis of AISI 316L Stainless steel Weldments. International Journal of Application or Innovation in Engineering & Management, 2, 269-272.
- ASTM Designation: A923-06, Standard Test Methods for detecting Detrimental Intermetallic Phases in Duplex Austenitic/Ferritic Stainless Steel.
- Olsson, C.O.A. and Landolt, D. (2003) Passive Films on Stainless Steels: Chemistry, Structure and Growth. Electrochimica Acta, 48, 1093-1104. http://dx.doi.org/10.1016/S0013-4686(02)00841-1
- Solomon, H.D. and Devine Jr., T.M. (1982) Duplex Stainless Steels: A Tale of Two Phases. Conference of the Duplex Stainless Steels, ASM, Ohio, 693-756.