Open Journal of Obstetrics and Gynecology
Vol.4 No.6(2014), Article ID:44781,13 pages DOI:10.4236/ojog.2014.46043
Predicting Birth-Related Levator Ani Tear Severity in Primiparous Women: Evaluating Maternal Recovery from Labor and Delivery (EMRLD Study)
Lisa Kane Low1,2,3, Ruth Zielinski1, Yebin Tao4, Andrzej Galecki4,5, Catherine J. Brandon6, Janis M. Miller1,3*
1School of Nursing, University of Michigan, Ann Arbor, Michigan, USA
2Department of Women’s Studies, University of Michigan, Ann Arbor, Michigan, USA
3Department of Obstetrics and Gynecology, School of Medicine, University of Michigan, Ann Arbor, Michigan, USA
4School of Public Health, University of Michigan, Ann Arbor, Michigan, USA
5Institute of Gerontology, School of Medicine, University of Michigan, Ann Arbor, Michigan, USA
6Department of Radiology, School of Medicine, University of Michigan, Ann Arbor, Michigan, USA
Email: *janismm@umich.edu
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 28 February 2014; revised 24 March 2014; accepted 31 March 2014
ABSTRACT
Objective: To determine which maternal characteristics or birth events independently predict severity of levator ani muscle (LA) tears at first vaginal birth in a longitudinal/observational investigation in a tertiary care hospital. Sample: Ninety primiparas with at least one at risk for LA tear inclusion factor at vaginal birth: maternal age ≥ 33 years, second stage ≥ 150 minutes, macrosomia, instrumented delivery, and/or anal sphincter laceration were studied. Methods: Magnetic Resonance Imaging (MRI) was obtained early postpartum (mean ± sd 48.9 ± 21.6 days) to identify LA tear. Severity of LA muscle fiber loss was graded on an ordinal scale of: “0” as no loss, “1” as <50% unilateral loss, “2” as ≥50% unilateral or <50% bilateral loss, and “3” as ≥50% bilateral loss. Data were analyzed using proportional odds modeling. Inclusion factors were explored as predictors of LA tear severity and at analysis episiotomy, time spent actively pushing, epidural, and oxytocin were also considered. The main outcome measures of interest included grading of severity of LA muscle fiber loss on an ordinal scale. Results: Respective counts/percentages of women within each 0 thru 3 ordered category of LA tear severity were: “0” = 58(64%), “1” = 9(10%), “2” = 15(17%), and “3” = 8(9%). Estimates and 95% CI for significant demographic or obstetric univariate predictors of LA tear severity level were age, OR 1.093 (CI 1.012 - 1.180), p = 0.023; and time spent in active pushing, OR 1.089 (CI 1.005 - 1.180), p = 0.038. The other factors considered were not significant. There were too few women with forceps deliveries to analyze. Conclusion: In our enriched sample of primiparous women, 26% showed a significant LA tear. Maternal age and time spent actively pushing independently predict LA tear severity.
Keywords:Reproductive Physiological Phenomena; Birth Injuries; Parturition; Labor; Pelvic Floor Disorders; Soft Tissue Injuries of the Pelvis

1. Introduction
Vaginal delivery increases risk of pelvic floor disorders (PFDs); including prolapse, incontinence, and pain [1] - [3] . Purportedly, one reason is birth-related tear of the levator ani muscle (LA). We do not know definitively how birth events and demographic factors link to LA tear. Multiple investigative challenges are noted including: many potentially confounding maternal and obstetric birth factors, length of time between the delivery event and onset of PFDs, and expense/availability of technologies necessary for evaluating LA status. Currently, magnetic resonance imaging (MRI) is the most accurate method for identifying and classifying severity of LA tear and structural injuries. Use of MRI early post-birth potentially bridges the time lapse between exposure to injury at birth and the manifestation, often over many years, of PFD symptoms that are associated with the more severe LA injuries [3] . However, simple strategies for case finding the women at highest risk for pronounced LA tear are necessary as MRI is not appropriate for routine postpartum screening.
Prior studies examining women post-childbirth reported rates of any LA tear as high as 36% [4] , with the majority reporting rates from 18% - 22% [5] -[8] . These studies explored broadly for maternal characteristics and childbirth events that might identify women with even a slight LA tear. Factors shown to be significant in at least one study include: higher maternal age, forceps or vacuum delivery, anal sphincter laceration, prolonged second stage, episiotomy, greater infant size [4] , and infant head circumference, [7] -[11] (Table 1). However, conflicting results remained since the factors are numerous and interrelated. Few studies have focused on the best factors for predicting the tears of greatest magnitude, despite that evidence tear severity contributes to clinical symptoms [3] . The objective of this investigation was to identify key maternal demographic or obstetric factors that contribute independently to rank order severity level of LA tear, thus enhancing our ability to identify women who are more likely to experience clinically relevant LA tear. A secondary goal was to explore the feasibility and heuristic value of considering many potential risk factors―termed “complex birth factors” that may cascade or cluster. To approach that goal, we use the results of this initial work to offer power analysis information from simulation techniques. This information will assist clinicians in risk identification and assist researchers in developing future study designs to explore the potential cascade of factors and nuances of childbirth offering possible opportunities for injury prevention.
2. Methods
The study was part of an NIH-supported longitudinal observational study Evaluating Maternal Recovery from Labor and Delivery (EMRLD), for which participants were recruited from January 14, 2004 to April 1, 2012. Institutional review board approval was obtained prior to initiation of the study (University of Michigan MED IRB-HUM00051193).
2.1. Sample
This report provides results from ninety primiparous women who were recruited from a university-based, tertiary care teaching hospital using enriched sampling techniques to maximize the probability of women in the study with some degree of any LA tear (Figure 1 portrays the enriched sampling strategy used for a projected enrollment of 100 women (one per month)).
The enriched sampling relied on inclusion criteria of “complex birth factors” heuristically suggestive of LA
Table 1. Summary of the literature exploring demographic and obstetric risk factors for levator ani muscle tear early post vaginal birth.
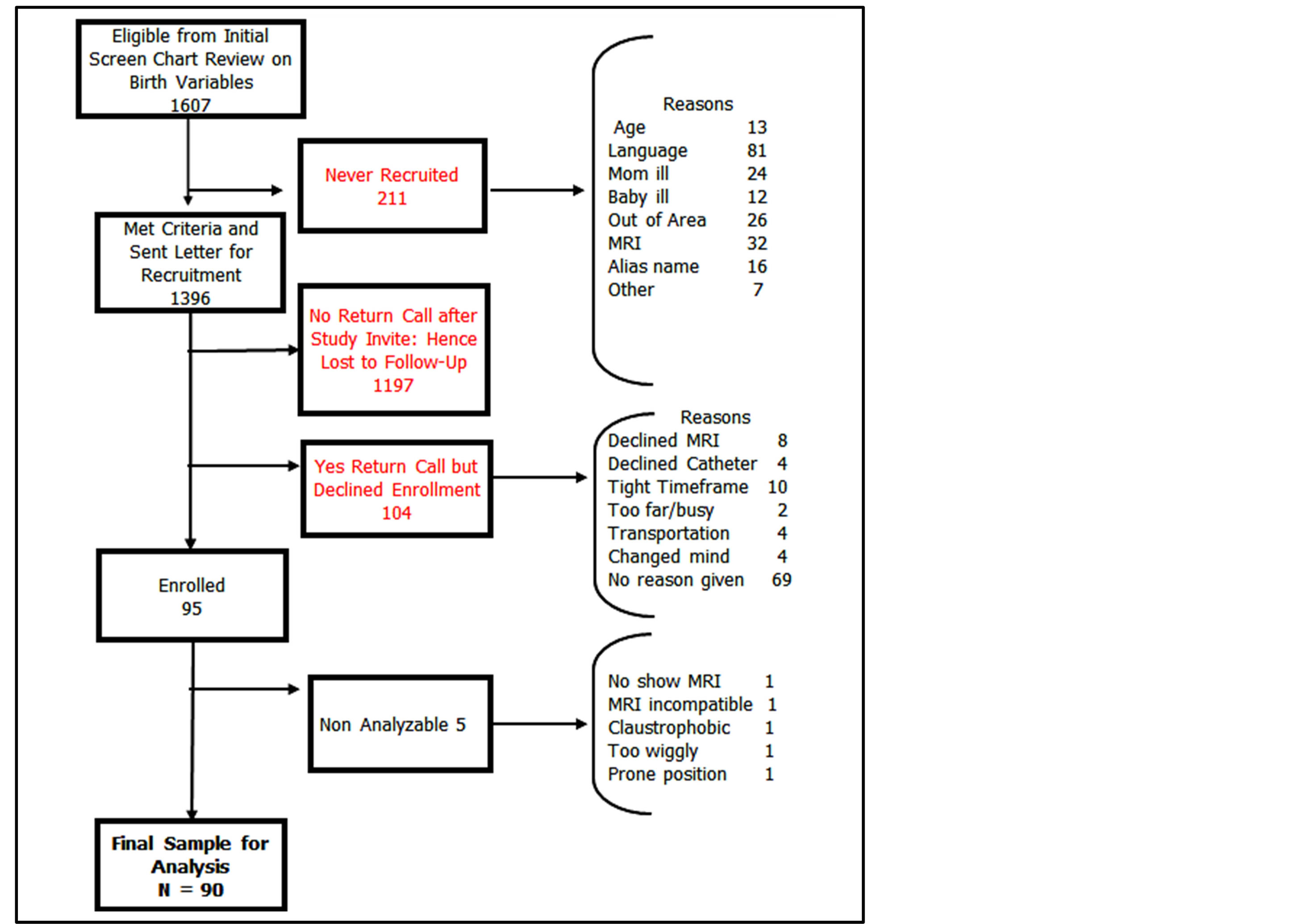
Figure 1. Enriched sampling strategy used for a projected enrollment of 100.
tear. Because the study began in 2004, we relied on our earlier pilot work [9] to select women who experienced what we refer to as a “complex vaginal birth.” That is, at least one of the following in their first vaginal delivery: 1) maternal age greater than 33 years, 2) second stage labor greater than 150 minutes, 3) infant weight greater than 4000 gm, 4) forceps, 5) vacuum, 6) third or fourth degree anal sphincter laceration. At the point of data analysis, additional studies in the field had been published so we considered other factors identified as potential complex birth events: episiotomy, larger infant head circumference, length of passive pushing time and use of epidural and oxytocin. Women were excluded from participating if age was less than 18 years, the primary healthcare language was not English, birth occurred before 36 weeks gestation, twin gestation, or an infant admitted to the neonatal intensive care unit.
2.2. Procedures
Women who met the initial inclusion criteria for complex birth factors based on chart review received an introductory letter approximately two weeks post-birth that described the study’s purpose and methods and provided instructions for contacting the study coordinator if interested in participating. If a woman expressed interest she received an informed consent document that she reviewed and signed prior to formal enrollment. A summary of screening results, number of signed consent forms, and reasons known for non-participation is shown in Figure 1.
A certified nurse-midwife (author RZ) conducted a chart review to extract data on birth variables and birth events for each participant. All vaginal examinations in this institution were recorded in an electronic medical record format that was completed at the time of the examination at the bedside by the RN who was required to be present for such examinations. The timing of complete dilation was indicated by the first vaginal examination that noted the woman was completely dilated to 10 centimeters.
MRI was performed postpartum using a 3 Tesla Philips Achieva scanner (Philips Medical Systems, Eindhoven, and The Netherlands) and included multiple tailored planes and sequences incorporating fluid sensitive sequences with an 8-channel cardiac coil positioned over the pelvis. Additional imaging details have been previously reported [12] [13] . Two board-certified musculoskeletal radiologists (author CB as one), both blinded to the detailed birth data, independently reviewed the MRIs. Each side of the LA muscle was graded according to severity of muscle volume loss: none, subtle, <50%, or ≥50% muscle volume loss (example provided in Figure 2).
2.3. Data Management and Statistics
Data management procedures included reducing the muscle grading data into a composite score characterizing LA status for each woman. This score was created by combining the raw data for each side of the LA according to an a priori rank order (a range of 4 levels) as follows: “0” was none or subtle, “1” was <50% loss unilateral, “2” was ≥50% unilateral or <50% bilateral and “3” was ≥50% bilateral muscle fiber loss.
The raw data obtained from the chart review were used to calculate birth variables including length of second stage labor, time spent in active pushing, and time spent in passive descent (sometimes called “laboring down”). For example, passive descent was derived from the point of complete dilation until active pushing was initiated. Active pushing, as measured in 15-minute intervals, was calculated from the time of initiation of active pushing to the point of delivery. Total time of second stage labor represented the time from complete dilation until delivery.
Statistical analysis was conducted using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA). Levels of significance were set at .05 (two tailed). Simple counts/percentages were used to descriptively portray distribution of MRI composite scores across the sample. Univariate analysis was performed separately for each of the literature-based demographic or birth factors to test for association with the outcome of LA status. Since the outcome variable (LA status) was measured on an ordinal scale, we fitted proportional odds models to our data [14] [15] . As part of the analysis, the proportional odds assumption was tested. The regression coefficient indicates how the log-odds of having a more severe LA tear is associated with the one-unit increased values of one of the demographic or birth factors [15] . Results are expressed in terms of cumulative odds ratios and respective 95% confidence intervals. Exploratory Pearson correlation was used to investigate whether variables might be interrelated. Finally, we considered sample size calculations to allow for multiple rather than only univariate models in future studies concerning additive relationships between various factors associated with increased risk of and severity of LA tears [16] .
3. Results
Ninety-five primiparous women signed consent forms to participate, and of these, we obtained complete MRIs from 90 women (Figure 1). Our final analysis was based on the 90 women with complete MRI data. MRIs were obtained on average seven weeks postpartum (mean = 48.9, SD = 21.6 days).
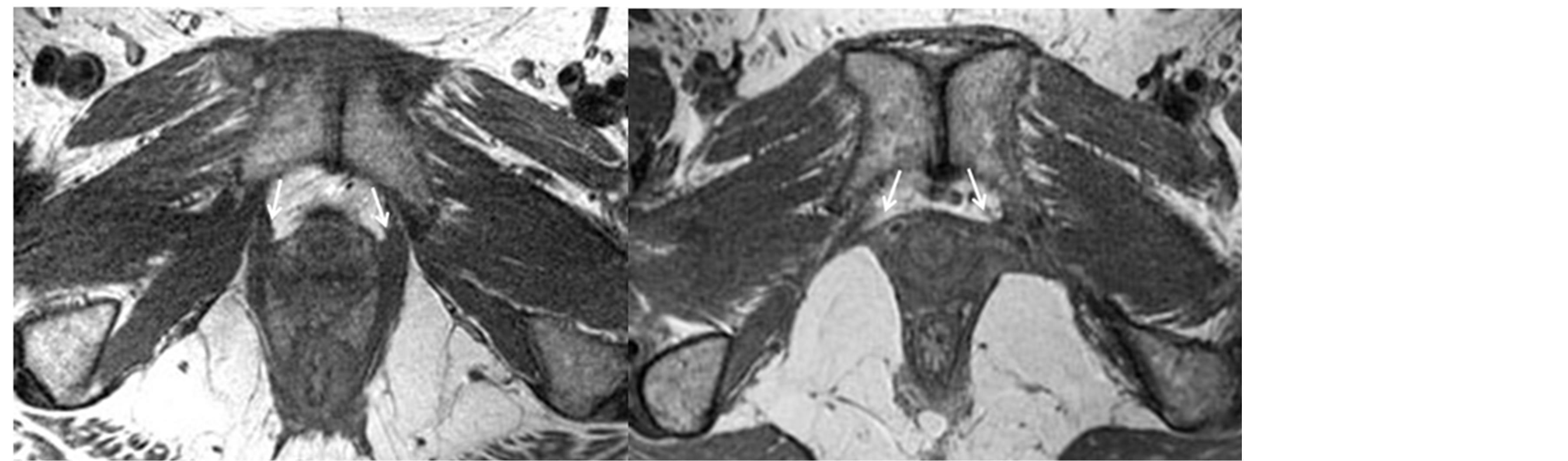
Figure 2. MRI images of levator ani muscles with and without evidence of tears at post vaginal birth in two primiparous women post complex vaginal birth. (a) 30 year old woman 6 months after normal first vaginal delivery. MRI in the axial plane demonstrates normal anterior attachment of the levator ani muscles onto the public bones (arrows); (b) 39 year old woman 6 months after complex first vaginal delivery. MRI in the axial plane demonstrates complete tears of the anterior attachment of the levator ani muscles onto the pubic bones (arrows).
Table 2 shows detailed characteristics of the participants, including age, labor and delivery factors, as well as LA status at the (on average) seven-week time point. In this sample of 90 women, all of whom had at least one “complex birth factor” the majority (64.4%) showed no visible loss of LA muscle volume at seven weeks postpartum, 10% showed only a minor degree of loss, and the remaining 25.6% of the women were distributed across the two more severe muscle loss categories. In all cases of muscle loss occurrence, the site of loss was where the muscle attaches to the pubic bone [13] .
Results of the analyses exploring each independent “complex birth factor” for its predictive value of LA tear severity, indicated by the odds ratio (95% CI) for each model, are presented in Table 3. Since only two women had forceps deliveries, we were unable to adequately analyze the contribution of forceps use to LA status. Maternal age was a significant predictor (p = 0.023). The estimated odds (CI) of being in a more severe LA tear category were 9.3% (1.2% to 18.0%) greater per year of increase in maternal age (Table 3). Time engaged in active pushing was also significant (p = 0.038). For active pushing, there was a higher severity level of LA tear where the estimated odds increased by 8.9% per 15 minute increase in time of active pushing.
None of the other variables tested reached significance, though episiotomy was p = 0.053. The estimated odds of being in a higher injury category were 2.708 times greater for women who had an episiotomy compared to women who did not. The rate of episiotomy for the sample was 19.4%. Other non-significant variables included total length of second stage (p = 0.129), infant weight (p = 0.906), head circumference (p = 0.910), vacuum delivery (p = 0.986), oxytocin use during labor (p = 0.677), anal tear (p = 0.703), time spent in passive descent (p = 0.561), and epidural use (p = 0.293) (Table 3).
Since maternal age and active pushing were the key significant predictors amongst the larger host of potential risk factors, we also explored the relationship of these independent variables with each other and the multiple additional complex birth factors using a correlation (Table 4). Age was weakly correlated with length of active pushing (r = 0.27, p = 0.018). Age also weakly correlated with length of total second stage, baby weight, and use of episiotomy, ranging from r = −0.216 for baby weight to r = 0.255 for total length of second stage. Length of active pushing, along with its correlation with age, also significantly correlated with baby weight (r = 0.333), total length of second stage (r = 0.837), length of passive stage (r = 0.293), and use of episiotomy (r = 0.296).
In order to provide estimates of sample size calculation for future studies using multivariate analysis (for instance age and active pushing in the same model), we simulated variables and set parameters using the results of our current study. Details are shown in Appendix S1. The simulation model includes predictors of age and active pushing time under two situations: single site and multiple sites. We applied multivariate regression to
Table 2. Sample demographic and obstetric care characteristics.
*The distinction of passive vs. active phases of second stage labor was inconsistently available in the chart review.
Table 3. Cumulative odds ratios of levator ani tear severity level for different risk factors using univariate proportional odds models. Bolded indicates statistically significant, evaluated as p ≤ 0.05. Interpretation example: For every year increase in a particular woman’s age, the likelihood of a more severe level of LA tear increases by 9.3%.
Table 4. Correlation matrix of variables of interest.
assess power using the simulated data. We found the results for the single-site analysis were the same as those for the multi-site analysis after adjustment for site effect by using an indicator variable for each site as a fixed effect. For multivariate regression analysis, we found that a sample size of 400 would be sufficient for both age and active pushing time to be powered. However, this is based on the assumption that the effects of age and active pushing time are exactly the same across sites. So in practice, more participants may be necessary to ensure the appropriate power.
4. Discussion
4.1. Main Findings
Despite recruiting participants who had complex birth factors associated with LA tear, 64.4% of the participants were without any evidence of LA tear on an early post partum MRI. Those who did show evidence of tear demonstrated varying levels of severity from the most minor partial tear to full tear of the muscle away from its origins bilaterally at the pubic bone. Significant demographic or obstetric univariate predictors of LA tear severity level were age, and time spent in active pushing. The other demographic or obstetric factors studied were not significant. With regards to forceps, there were too few women to analyze (only two with forceps deliveries).
4.2. Strengths and Limitations
More recently, investigators have used larger sample sizes and statistical analysis techniques that are more sophisticated to explore the question of predicting LA injury [8] [11] . However, even findings from these studies have not provided a consistent profile. Our study, like many others, had the limitation of relatively small sample numbers and hence multi-variate analysis strategies were not used. To address this limitation we have provided sample size calculations for future investigations that would allow for multi-variate regression analysis (Appendix S1). However, our study is unique in sampling for women with complex birth factors rather than “all comers,” and by analyzing according to the severity level of the tear. By sampling for participants having at least one complex birth factor, we were able to reduce the long list of potential predictors for the worst LA tear down to a few key factors, specifically maternal age and active pushing along with the previously accepted significant risk factor of forceps.
Although our sample size was not large, our approach of purposely recruiting a sample of women with higher likelihood of having had any LA tear did permit women with LA tears to be well represented (35.4% of the sample) with 25.6% having potentially clinically important severity levels of tear. However, our study is limited by the fact that results are not generalizable to the broader population of women who give birth vaginally without complex birth factors. An additional study of women without complex birth factors is ongoing by our team and may help determine factors that may be protective in a lower-risk group.
Another limitation of our study is the relative lack of racial and economic diversity. Although the study participants were representative of the local region, findings might not be generalizable, for instance, to women of color with complex birth factors since our sample was primarily non-Hispanic Caucasian. Finally, our study is limited by the observational design and retrospective chart review for obstetric data.
4.3. Interpretation
The significant decrease in forceps use at the recruiting facility in recent years likely influenced the lower than expected percentage of participants with complex birth factors but without LA tear. Previous research demonstrated a 17-fold risk in LA tear with forceps use [9] . As noted, we were only able to recruit two participants who had undergone vaginal delivery with forceps.
Greater maternal age significantly increased the likelihood (odds) of any and/or more severe LA tear. The significance of age in our findings concurs with the results of some researchers [4] -[9] ; however, other investigators did not find a significant difference associated with age in other studies focused on women who were primigravidas [7] [8] [11] . As shown in Table 1, this may be due to sampling differences. Our sample was older with a mean age of 29.1 ± 5.7 years versus 25.8 ± 4.3 years in the Valsky [7] study located in Jerusalem. Chan [8] studying women in Hong Kong, reported mean age of 30.6 ± 3.9 years, which was similar to our sample, however they did not identify an association with LA injury in the bivariate analysis. In the Cassadó Garriga [11] study located in Spain, age was similar to our study, but the sampling reflected a case/control study, with cases selected on the basis of forceps delivery [11] . Their findings concur with others that forceps is an overwhelming factor in LA tear. Advancing maternal age has been shown to be a risk factor for third and fourth degree lacerations during delivery [17] . Considering that the risk of injury to soft tissues is greater with advancing age, [18] our study’s finding of increased risk for greater severity of LA tear with greater maternal age seems conceptually sound.
Time spent in active pushing, but not the total duration of second stage labor, significantly increased risk of greater LA tear severity in this investigation. Increasing use of epidural anesthesia has led to changing management strategies for second stage labor [19] . Providers may encourage a process of passive descent, allowing women to delay active pushing until there is a spontaneous urge to push. This results in a period of time where a woman is not engaged in active pushing. Active pushing begins when the woman either experiences an urge to push or begins pushing because she is instructed to do so. Together these two phases constitute the total duration in time of second stage labor. The effect of this practice, which generally increases the total length of second stage labor but decreases time spent in active pushing [20] and how this reduced active pushing time may protect the pelvic floor remains to be seen. Generally, the practice of laboring down or passive descent has been shown to have a positive effect on other obstetric outcomes (e.g. reduction of forceps or vacuum assisted delivery rates) [20] [21] which then may have a positive effect on reducing LA tear severity.
Prior research has shown episiotomy to be associated with LA tear [6] [9] and perineal trauma [22] . Our results indicate a trend that did not reach statistical significance. Because the rate of episiotomy in our study population was only 19.4% and all of the episiotomies were midline, we were not able to compare LA tear severity level by midline versus mediolateral episiotomy. It was noted that length of active pushing was correlated with larger infant size and use of episiotomy. Not surprisingly, the longer active pushing phase may provide indication for use of episiotomy and larger size infant may cause an increased duration of pushing time, and hence a cascade of events may be occurring when duration of active pushing time is explored in detail. This detailed exploration was beyond the scope of our study.
5. Conclusion
In summary, our investigation provides further evidence of birth-related LA tears and suggests that maternal age may be an important and non-modifiable contributor. Time spent in active pushing is another significant contributor, while total duration of second stage labor is not. This is consistent with recent recommendations to assess duration of second stage labor by the time spent in active pushing instead of the total time of second stage, which may include both a passive phase and the active phase [23] . The potential cascade of events that may increase the risk for any individual woman, given her age, still remains unclear and requires larger samples and likely multi-site trials to address the question of cumulative risk factors and their effects. When considering best practices related to risk for childbirth-associated pelvic floor changes on a first vaginal delivery, maternity care providers should consider age and duration of active pushing along with the already accepted risk factor of forceps. Our data suggest the need in future investigations to differentiate between time in active pushing and “laboring down” or passive descent compared to analysis using only the total time in the second stage. It remains to be confirmed if the use of passive descent in combination with active pushing time is potentially protective against pelvic floor changes.
Acknowledgements
We gratefully acknowledge John DeLancey and James Ashton-Miller as Principle Investigator/Core A Director and Core B Director respectively of the University of Michigan SCOR on Sex and Gender Factors Affecting Women’s Health.
We thank the EMRLD study staff Ruta Misiunas, Lee Park, Caroline Garcia, Meg Tolbert, and all the women who participated in EMRLD.
Disclosure
The authors report no conflict of interest or relevant financial relationships.
Details of Ethics Approval
University of Michigan MED IRB-HUM00051193.
Funding
The Evaluating Maternal Recovery from Labor and Delivery (EMRLD) study has grant support from the National Institutes of Health (NIH) through the National Institute on Child and Human Development (NICHD) (Grant # R21 HD049818) and through the Office for Research on Women’s Health (ORWH) with NICHD Specialized Center of Research (SCOR) on Sex and Gender Factors Affecting Women’s Health (Grant #P50 HD044406 002)). Interpretation of findings is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, ORWH, or the NIH.
References
- Lukacz, E.S., Lawrence, J.M., Contreras, R., Nager, C.W. and Luber, K.M. (2006) Parity, Mode of Delivery, and Pelvic Floor Disorders. Obstetrics and Gynecology, 107, 1253-1260. http://dx.doi.org/10.1097/01.AOG.0000218096.54169.34
- Hendrix, S.L., Clark, A., Nygaard, I., Aragaski, A., Barnabei, V. and McTiernan, A. (2002) Pelvic Organ Prolapse in the Women’s Health Initiative: Gravity and Gravidity. American Journal of Obstetrics and Gynecology, 186, 1160- 1166. http://dx.doi.org/10.1067/mob.2002.123819
- DeLancey, J.O., Morgan, D.M., Fenner, D.E., Kearney, R., Guire, K., Miller, J.M., et al. (2007) Comparison of Levator Ani Muscle Defects and Function in Women with and without Pelvic Organ Prolapse. Obstetrics and Gynecology, 109, 295-302. http://dx.doi.org/10.1097/01.AOG.0000250901.57095.ba
- Dietz, H.P. and Lanzarone, V. (2005) Levator Trauma after Vaginal Delivery. Obstetrics and Gynecology, 106, 707- 711. http://dx.doi.org/10.1097/01.AOG.0000178779.62181.01
- Lavy, Y., Sand, P., Kaniel, C. and Hochner-Celnikier, D. (2011) Can Pelvic Floor Injury Secondary to Delivery Be Prevented? International Urogynecology Journal, 6, 2011.
- Shek, K.L. and Dietz, H.P. (2010) Intrapartum Risk Factors for Levator Trauma. BJOG, 117, 1485-1492. http://dx.doi.org/10.1111/j.1471-0528.2010.02704.x
- Valsky, D.V., Lipshuetz, M., Bord, A., Eldar, I., Messing, B., Hochner-Celnikier, D., et al. (2009) Fetal Head Circumference and Length of Second Stage of Labor Are Risk Factors for Levator Ani Muscle Injury, Diagnosed by 3-Dimensional Transperineal Ultrasound in Primiparous Women. American Journal of Obstetrics and Gynecology, 201, e1-e7.
- Chan, S.S., Cheung, R.Y., Yiu, A.K., Lee, L.L., Pang, A.W., Choy, K., et al. (2012) Prevalence of Levator Ani Muscle Injury in Chinese Primiparous Women after First Delivery. Ultrasound in Obstetrics & Gynecology, 39, 704-709. http://dx.doi.org/10.1002/uog.10132
- Kearney, R., Miller, J., Ashton Miller, J. and DeLancey, J.O. (2006) Obstetric Factors Associated with Levator Ani Muscle Injury after Vaginal Birth. Obstetrics and Gynecology, 107, 144-148. http://dx.doi.org/10.1097/01.AOG.0000194063.63206.1c
- Dietz, H.P. and Shek, K.L. (2008) Validity and Reproducibility of the Digital Detection of Levator Trauma. International Urogynecology Journal, 19, 1097-1101. http://dx.doi.org/10.1007/s00192-008-0575-1
- Cassadó Garriga, J., Pessarrodona Isern, A., Espun Pons, M., Duran Retamal, M., Felgueroso Fabrega, A. and Rodriguez Carballeira, M. (2011) Four-Dimensional Sonographic Evaluation of Avulsion of the Levator Ani According to Delivery Mode. Ultrasound in Obstetrics & Gynecology, 38, 701-706. http://dx.doi.org/10.1002/uog.10062
- Miller, J.M., Brandon, C., Jacobson, J., Kane Low, L., Zielinski, R., Ashton-Miller, J., et al. (2010) MRI Findings in Patients Considered High Risk for Pelvic Floor Injury Studied Serially after Vaginal Birth. AJR, 195, 786-790. http://dx.doi.org/10.2214/AJR.09.3508
- Brandon, C., Jacobson, J., Low, L., Park, L., DeLancey, J. and Miller, J. (2012) Pubic Bone Injuries in Primiparous Women: Magnetic Resonance Imaging in Detection and Differential Diagnosis of Structural Injury. Ultrasound in Obstetrics & Gynecology, 39, 444-451. http://dx.doi.org/10.1002/uog.9082
- McCullagh, P. (1980) Regression Models for Ordinal Data (with Discussion). Journal of the Royal Statistical Society, Series B, 42, 109-142.
- Agresti, A. (2010) Analysis of Ordinal Categorical Data. 2nd Edition, Wiley, Malden. http://dx.doi.org/10.1002/9780470594001
- Hsieh, F.Y., Block, D.A. and Larsen, M.D. (1998) A Simple Method of Sample Size Calculation for Linear and Logistic Regression. Statistical Methodology, 17, 1623-1634. http://dx.doi.org/10.1002/(SICI)1097-0258(19980730)17:14<1623::AID-SIM871>3.0.CO;2-S
- Hornemann, A., Kamischke, A., Luedders, D., Beyer, D., Deidrich, K. and Bohlmann, M. (2010) Advanced Age Is a Risk Factor for Higher Grade Perineal Lacerations during Delivery in Nulliparous Women. Archives of Gynecology and Obstetrics, 281, 59-64. http://dx.doi.org/10.1007/s00404-009-1063-7
- Gabbe, B.J., Bennell, K.L. and Finch, C.F. (2006) Why Are Older Australian Football Players at Greater Risk of Hamstring Injury? Journal of Science and Medicine in Sport, 9, 327-333. http://dx.doi.org/10.1016/j.jsams.2006.12.037
- Osterman, M. (2009) Birth Stats: Percentage of Mothers Receiving Epidural/Spinal Anesthesia by Age, Race, and Hispanic Origin of Mother: Total of 18 US Reporting Areas, Singletons Only, 2006. Birth, 36, 340-342. http://dx.doi.org/10.1111/j.1523-536X.2009.00363.x
- Brancato, R.M., Church, S. and Stone, P.W. (2008) A Meta-Analysis of Passive Descent versus Immediate Pushing in Nulliparous Women with Epidural Analgesia in the Second Stage of Labor. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 37, 4-12. http://dx.doi.org/10.1111/j.1552-6909.2007.00205.x
- Hansen, S.L., Clark, S.L. and Foster, J.C. (2002) Active Pushing versus Passive Fetal Descent in the Second Stage of Labor: A Randomized Controlled Trial. Obstetrics and Gynecology, 99, 29-34. http://dx.doi.org/10.1016/S0029-7844(01)01642-8
- Viswanathan, M., Hartmann, K., Palmieri, R., Lux, L., Swinson, T., Lohr, K.N., et al. (2005) The Use of Episiotomy in Obstetrical Care: A Systematic Review. Evidence Reports/Technology Assessments, No. 112. Agency for Healthcare Research and Quality, Rockville.
- Spong, C., Berghella, V., Wenstrom, K., Mercer, B. and Saade, G. (2012) Preventing the First Cesarean Delivery Summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstetrics and Gynecology, 120, 1181-1193.
Supplemental Materials
Simulation Study
We used simulation method to calculate sample size for future studies with information gained from the current one. We set Type-I error rate and power to be 0.05 and 0.80, respectively. Specifically, we used the following proportional odds model for subject i with ordinal outcome Yi and predictor(s) xi,
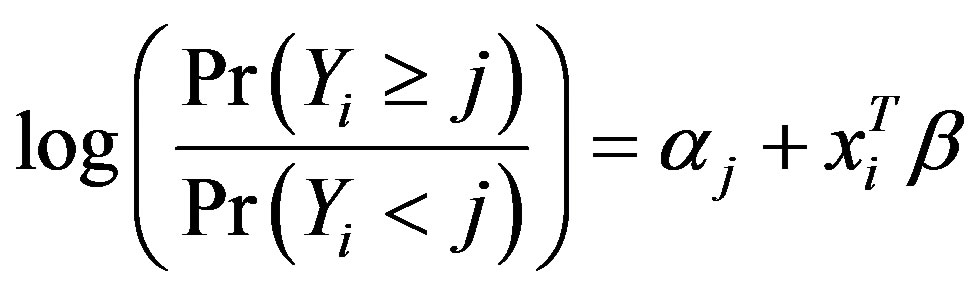 where j = 1, 2 or 3 denoting the categories of the ordinal outcome. From this model we calculated the probability of Yi belonging to each category and we got
where j = 1, 2 or 3 denoting the categories of the ordinal outcome. From this model we calculated the probability of Yi belonging to each category and we got
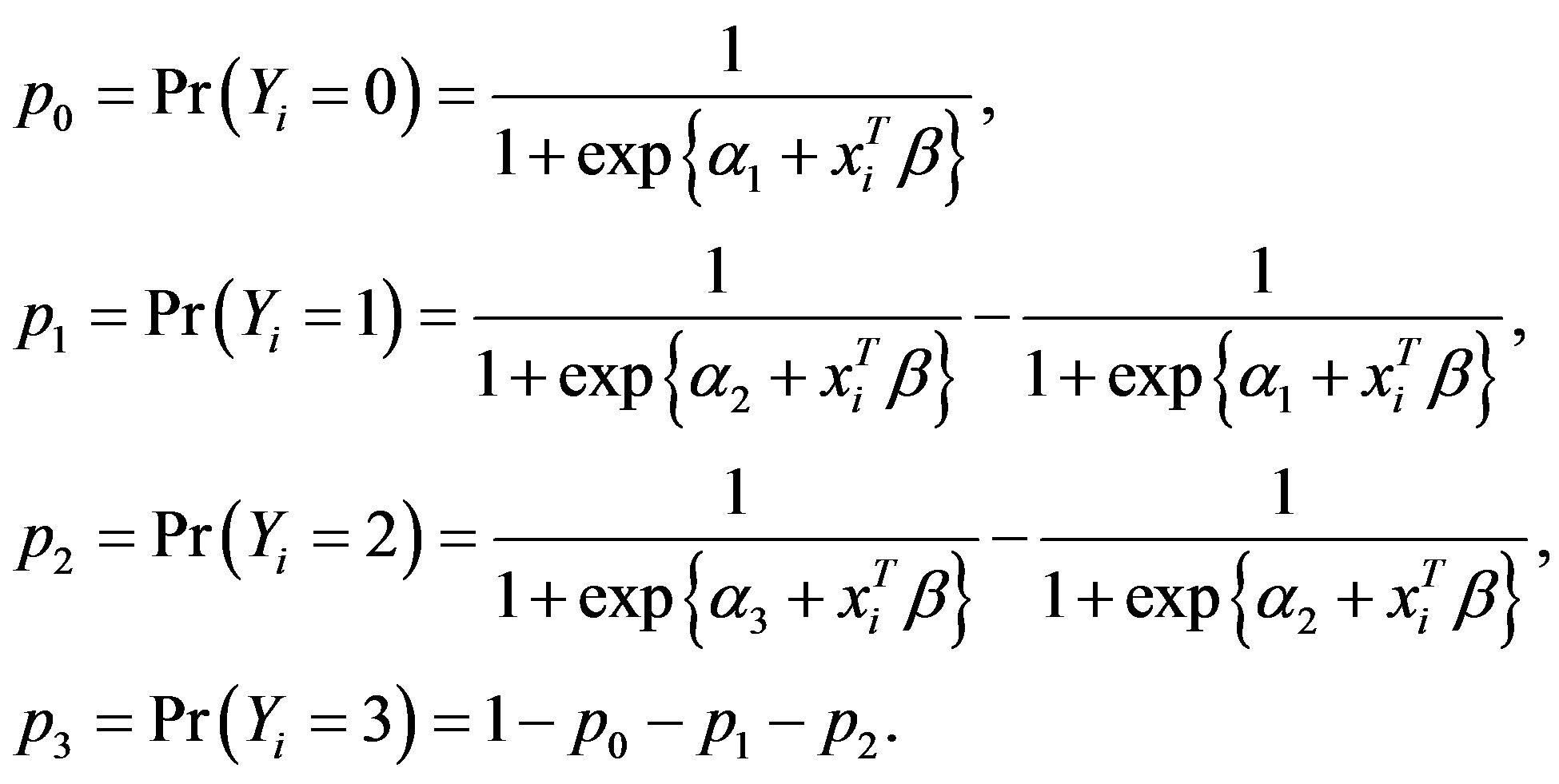
Then we used multinomial sampling to get Yi. Table S1 presents the parameter settings for three different settings: 1) only one continuous (e.g., age) or categorical (e.g., episiotomy) predictor with data collected in one site; 2) one continuous predictor and one categorical predictor (e.g., age and episiotomy), or two continuous predictors (e.g., age and active pushing time) with data collected in one site; 3) two continuous predictors (e.g., age and active pushing time) with data from multiple sites. We sampled age from a normal distribution with mean 30 and variance 25, episiotomy from a binary distribution with frequency 0.4, and active pushing time from absolute values of N(100, 802) to ensure positivity and skewness. All parameters were set by our real data analysis. We simulated the site effect by adding a value sampled from N(0, 0.52) or N(0, 1) for each site and treated it as fixed effect. We assumed that the effects of the predictors were the same across different sites.
Table S2 to Table S5 presents the results of sample size calculation for the three different settings. When we
Table S1. Parameter setting for three situations.
Table S2. Sample size and power calculation for proportional odds model with one predictor.
Table S3. Sample size and power calculation for proportional odds model with two predictors.
Table S4. Sample size and power calculation for proportional odds model with two predictor and site effects (5 sites).
Table S5. Sample size and power calculation for proportional odds model with two predictor and site effects (4 sites).
NOTES

*Corresponding author.


