Open Journal of Obstetrics and Gynecology
Vol. 3 No. 4 (2013) , Article ID: 31615 , 5 pages DOI:10.4236/ojog.2013.34073
A controlled, randomized, open label study in postmenopausal women to assess the safety and the efficacy of a vaginal moisturizer: An instrumental approach
![]()
1DermIng, Clinical Research and Bioengineering Institute, Monza, Italy
2Scientific Department, Polichem S.A., Lugano, Switzerland
Email: francesco.scarci@polichem.com
Copyright © 2013 A. Sparavigna et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 9 April 2013; revised 10 May 2013; accepted 18 May 2013
Keywords: Vaginal Atrophy; Vaginal Dryness; Menopause; Hygrometry
ABSTRACT
Background: Vulvovaginal atrophy is an inflammatory condition linked with estrogenic deficiency, as well as decreased lubrication. The study aimed at the objective measurements of vaginal moisture (RH%), in fertile women and in postmenopausal women before and during treatment with a non-estrogenic moisturizing gel. Methods: The study followed a stepwise design. Acute: 5 women with postmenopausal vaginal dryness were measured their RH% at baseline and 30 minutes after the application of a moisturizer. Fertile Controls: 20 women were measured in the follicular phase and 20 in the luteal phase. Chronic: Forty postmenopausal women with mild/moderate vaginal dryness were randomly assigned to treatment (once/day for the first week and twice/week for the following 11 weeks) or untreated control group. Primary parameter was RH%, done by means of a vaginal hygrometer manufactured and validated for the specific purpose. Secondary parameters were the evaluation of erythema and oedema, scored for severity. Results: Fertile women showed an average 93/95 RH%, independently on the cycle phase. Postmenopausal women had 49/69 RH% in absence of any treatment. A 37% increased RH% was observed 30 min after a single application. During chronic treatment, RH% measured not earlier than 2 hours after the application of the product, resulted 7.8% to 10.4% higher than baseline (p < 0.05). Erythema improved accordingly. Conclusions: The vaginal moisturizer proved to be safe and to increase vaginal moisture short after treatment initiation; moreover, its effect proved to be long lasting.
1. INTRODUCTION
Vulvovaginal atrophy (VVA) is a common condition associated with estrogenic deficiency whose most characteristic symptoms include vaginal dryness, irritation, soreness and dyspareunia with urinary frequency, urgency and incontinence [1]. Beyond the physiologic estrogen depletion associated with menopause, several other risk factors have been associated with VVA: decreased ovarian function due to radiotherapy or chemotherapy, immune disorders, oophorectomy, postpartum and lactation, non-vaginal birth, oral contraceptives or drug with antiestrogenic properties, cigarette smoking and non-fluctuating estrogen levels [2].
Fifteen per cent of pre-menopausal and 40% - 57% of post-menopausal women report on symptoms resulting from VVA [3].
The clinical findings include the presence of pale and dry vulvovaginal mucosa with petechiae, the loss of vaginal rugae and a raise in vaginal pH which increases the risk for vaginal infections [4]. These atrophic changes of the vulva, vagina and lower urinary tract can have a large impact on the quality of life of women [5]. Moreover, these symptoms are unlikely to improve spontaneously, therefore a prompt diagnosis and the treatment of VVA are recommended.
Estrogen therapy, administered either locally or systemically, provides significant relief from symptoms related to VVA and has been considered the therapy of choice up to the ‘90s. Nevertheless, the hormonal replacement therapy (HRT) has been recently re-considered due to its side-effects [6].
A new vaginal moisturizer [Gynomunal® (Taurus Pharma, Germany), also marketed as Rosaltrof® (Spain) and Me AgainTM (United States)], based on hyaluronic acid, liposomes, Vitamin E and hops (pH = 6), revealed in a previous paper [7] a valid alternative for shortand long-term relief of vaginal dryness.
Aim of the present study was to measure vaginal moisture by an original validated instrumental tool and to objectively evaluate vaginal moisture changes, following topical treatment with the aforementioned vaginal moisturizer.
2. MATERIALS AND METHODS
2.1. Trial Design
A randomized, controlled open label, parallel groups study: a total of 40 postmenopausal women (mean age 58 years; range 49 - 65 years) with mild to moderate symptoms and signs of vaginal atrophy were included in the study and randomly 1:1 allocated to either the treatment (n = 20) or the untreated postmenopausal control (n = 20) group. The study was performed on an ambulatory basis at the DermIng Institute, Monza (Italy).
Eligibility criteria included age range between 45 to 65 years old, healthy status, in post-menopause since at least 2 years. Women with active illnesses of genitals or under hormonal replacement therapy were not included. No changes in the eligibility criteria occurred. Women belonging to the treatment group were instructed to apply the product deeply in the vagina by an applicator, once a day for the first week and twice a week for the following 11 weeks.
Five visits were scheduled during the study, at baseline (T0) and after 1 week (T1), 4 weeks (T4), 8 weeks (T8) and 12 weeks (T12). At each visit, performed not earlier than 2 hours after the application of the product, women underwent both the instrumental determination of vaginal moisture and the clinical score of vaginal erythema and edema severity (0 = absent; 0.5 = mild; 1 = moderate; 2 = severe). The safety profile of the product was monitored throughout the whole study period.
No restriction was used for randomization. The randomization was done in DermIng, directly by the Investigator, on the basis of the subjects’ inclusion order. The statistical analysis of clinical and instrumental data was performed on the intention to treat (ITT) population. The statistical analysis of the clinical evaluation and of instrumental data at the different time points versus baseline was performed by Dunnett test; Wilcoxon or Student t test were used to compare the two groups (treated vs control) at each time point. No changes on the trial outcomes were made after the trial was commenced.
Due to the preliminary nature, the study was not dimensioned. Instrument set up: A thermometer-hygrometer equipped with a disposable sterile probe supplied with a sensor (Tecnolab del Lago Maggiore S.r.l.—Italy) (Figure 1) was set up to measure vaginal moisture, in terms of percent relative humidity (RH%). The calibration of the instrument was performed in a climatic room (Angelantoni, Italy), using as reference instrument a digital thermo-hygrometer (Hygroclip Probe, Rotronic). Values of percentage relative humidity ranging from 10% to 95% were created in the climatic room at 36˚C and detected in parallel with the reference and the thermometer-hygrometer under calibration. The disposable probe was designed to be positioned in vagina at a deepness of 2 cm. The result of the measurement is readable on the display after 5 - 10 seconds.
2.2. Instrumental Application to Monitor Vaginal Moisture Variations
The studies were conducted according to the GCP (Good Clinical Practice) and the principles deriving from the Helsinki declaration.
2.3. Acute Study
A pilot study was performed on 5 women (mean age 63 years; range 56 - 69) with menopause started not earlier than 2 years, to validate the instrumental method described below. Vaginal moisture was measured before and 30 minutes after the application of the test product. For each woman, 5 replicates of the measurements were performed at each time point.
2.4. Fertile Controls
A group of 40 women (mean age 41 years; range 21 - 52 years) of childbearing potential, 20 in follicular phase (days 8th to 12th) and 20 in luteinic phase (days 16th to 28th) were included in the study and their vaginal moisture was measured once by the same instrumental method.

Figure 1. Thermo-hygrometer equipped with a vaginal sterile probe (Tecnolab del Lago maggiore S.r.l., Italy).
3. RESULTS
3.1. Acute Study
The pilot study demonstrated that the method was reliable, reproducible (mean Standard Deviation (SD) < 2% and mean Standard Error of the Mean (SEM) < 1% both before and after treatment) and sensitive to the moisture changes induced by the vaginal moisturizer: a statistically significant mean percentage moisture increase by 37% versus baseline (p < 0.001), indicative of an immediate moisturizer effect, was observed 30 minutes after application (mean relative humidity RH% T0 + SD = 49.3 + 1.31 vs mean RH% T30 min 67.58 + 1.71).
3.2. Fertile Controls
Mean RH% was 95.45% ± 5.58 in luteinic phase and 92.96% ± 8.41 in follicular phase group (Figure 2).
3.3. Core Study
Overall, 20 women were randomised to be treated and 20 to control group. All of them received the allocated intervention and were analysed for the primary outcome. One woman in the treated group prematurely discontinued due to vaginal discomfort (feeling moist).
Recruitment started on May 4th 2009 and the study ended on July 31st 2009.
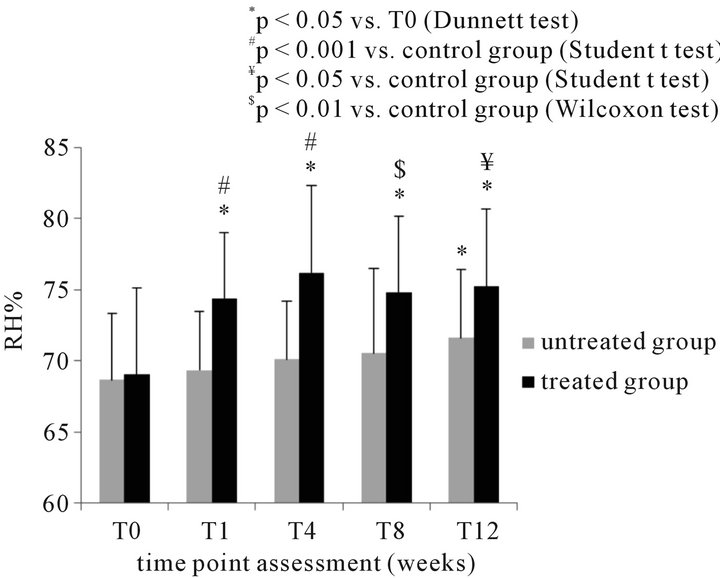
The mean RH% in the treated and control postmenopausal women were comparable at baseline (69.02 + 6.13 and 68.7 + 4.61, respectively). RH% significantly increased by 7.8%, 10.4%, 8.4% and 9% at T1, T4, T8 and T12 in treated group (p < 0.05 T1, T4, T8, T12 versus T0; Dunnett test). In the control group, a significant increase by 4.3% in vaginal moisture was recorded only at T12
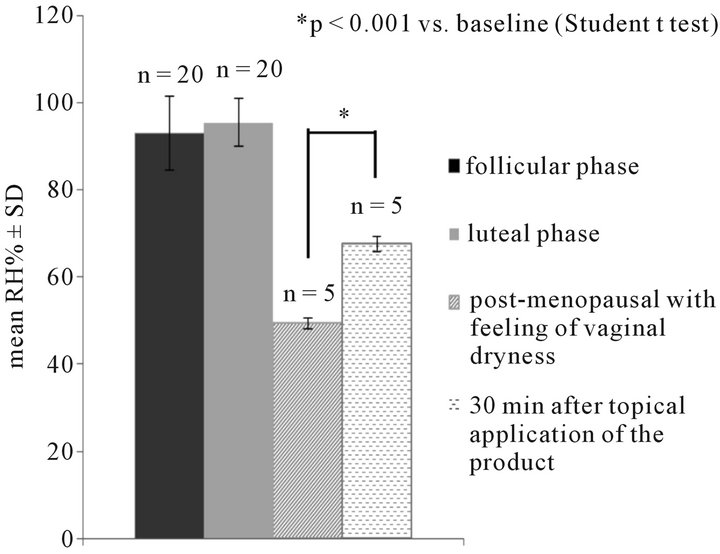
Figure 2. Instrumental determination of vaginal moisture (Relative Humidity RH% mean + SD) in 40 fertile women grouped according to the menstrual cycle phase and in 5 post-menopausal women before and 30 minutes after product application.
versus baseline (p < 0.05; Dunnett test). Statistically significant differences were observed between treated and postmenopausal control group at all-time points (Figure 3(a)).
Erythema was present, at baseline, in 14 subjects of either group. Clinical scores at baseline were comparable in the treated and control groups: 0.53 + 0.51 and 0.45 + 0.36, respectively.
In the treated group, erythema was found significantly improved after 8 weeks (0.08 + 0.19), and 12 weeks (0.11 + 0.2) (p < 0.05, T8 and T12 vs T0; Dunnett test).
Statistically significant differences (Wilcoxon test) were observed between treated and postmenopausal control group at the following time points: 0.24 + 0.26 vs 0.53 + 0.34 at T4 (p < 0.01), 0.08 + 0.19 vs 0.33 + 0.29 at T8 (p < 0.01), 0.11 + 0.21 vs 0.45 + 0.36 at T12 (p < 0.01) (Figure 3(b)).
At the end, a significant reduction in visual score was observed in 63% of the treated subjects.
Oedema, which was present in few patients at baseline, did not change significantly in either group.
One subject in the treatment group prematurely interrupted the trial because of an unpleasant wet sensation, persisting for several hours after application. Eleven out of the 19 women referred a mild to moderate burning sensation, lasting 5 - 10 minutes after product application, mainly during the first week of treatment.
No adverse event or reaction related or unrelated to the study product occurred during the trial.
4. DISCUSSION
Comprehensive therapies that target several aspects of menopause are currently hormone based. However, hormone therapy is not recommended in those women at high risk for cardiovascular disease, breast cancer and venous thromboembolic events [8].
Alternative approaches, targeting the relief of specific symptoms have been recently developed. In this context, vaginal lubricants and moisturizers seem to be beneficial in the treatment of vaginal dryness [9].
Up to now, diagnosis of vaginal dryness has been based on clinical signs and symptoms. In this study, in addition to the clinical signs, an instrumental tool, able to record the vaginal moisture, has been set up, checked for reliability, reproducibility and sensitivity and then used to specifically and objectively monitor the vaginal moisture in both control and treated groups.
Results obtained demonstrate that the vaginal moisturizer under investigation is able to increase vaginal moisture short after treatment initiation and that this humectant effect is long lasting. The increase in vaginal moisture 30 minutes after product application demonstrates a direct humectant effect due to the presence of the product in the vagina, whereas increased vaginal
 (a)
(a)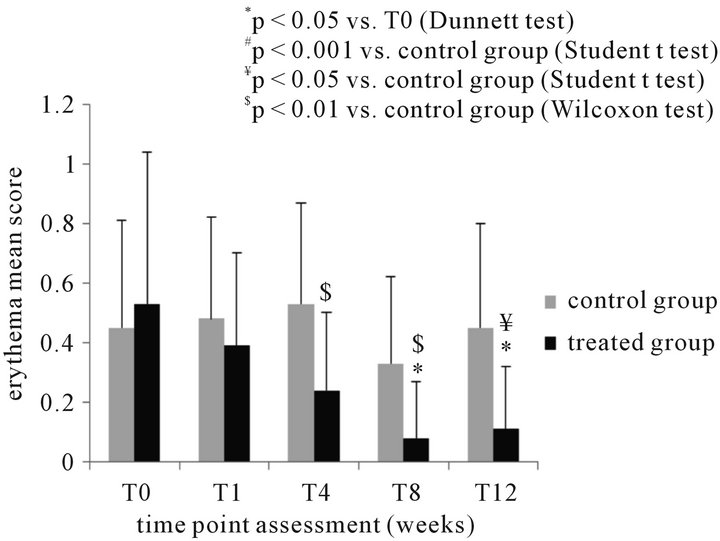 (b)
(b)
Figure 3. (a) Instrumental determination of vaginal moisture (RH% mean + SD) in 20 postmenopausal women before and during treatment with the test product and comparison with the control (untreated – N = 20) group at the different time points; (b) clinical evaluation of erythema (mean score + SD) (b) in 20 postmenopausal women before and during treatment with the test product and comparison with the control (untreated – N = 20) group at the different time points.
moisture levels observed after both daily and twice a week applications could suggest a role of the product in the induction of the “physiologic” vaginal moisturizer process.
The vaginal moisturizer was also able to improve vulvar erythema. On the contrary, no significant changes in the vulvar edema visual score were recorded, most probably because, at baseline, this sign was almost absent in the population under study.
The moisturizer activity is due to different components of the device formulation: the hyaluronic acid, able to retain and release water, and liposomes, contained in form of monoand multi-lamellar, as well as multi-vesicular liposomes, able to transport water through the vaginal mucosa, allowing both quick and long lasting release of water, responsible for well-being sensation and lubrication of the vaginal mucosa. Moreover, the vaginal microenvironment is protected by the preservative and antioxidant activities exerted by hops and Vitamin E.
The slight burning sensation experienced at the first few applications by most of the treated women was likely due to the dry and therefore sensitive condition of the vaginal epithelium. The disappearance of the symptoms was in fact associated with the increased local moisture. It is of note that none of the subjects discontinued the trial due to burning sensation.
Results obtained in this study are in agreement and further corroborate previous observations on this product obtained in an open, non-controlled study, performed on 108 Caucasian women, where a recovery was observed in vaginal dryness feeling, measured by means of a visual analogue scale, and clinical signs and symptoms. That study reported also evidences on the safety and the optimal acceptability of the product [7].
5. CONCLUSIONS
In conclusion, results obtained in this study demonstrated that this thermo hygrometer is able to record, in a reproducible and objective manner, the vaginal moisture variations, following the application of a new, vaginal moisturizer. The instrumental assessment of the vaginal dryness in our study likely avoided any kind of subjectivity from patient and investigator, strengthening the results collected, though a controlled study would have given a much more robust evidence of the efficacy of the product.
This vaginal moisturizer could therefore be taken into consideration as a non-oestrogenic alternative for the prevention and symptom relief of vaginal dryness.
REFERENCES
- Santos, I. and Clissold, S. (2010) Urogenital disorders associated with oestrogen deficiency: The role of promestriene as topical oestrogen therapy. Gynecological Endocrinology, 26, 644-651. doi:10.3109/09513591003767948
- Bachmann, G.A. and Nevadunsky, N.S. (2000) Diagnosis and treatment of atrophic vaginitis. American Family Physician, 61, 3090-3096.
- Palacios, S. (2009) Managing urogenital atrophy. Mauritas, 63, 315-318. doi:10.1016/j.maturitas.2009.04.009
- Mac Bride, M.B., Rhodes, D.J. and Shuster, L.T. (2010) Vulvovaginal atrophy. Mayo Clinic Proceedings, 85, 87- 94. doi:10.4065/mcp.2009.0413
- Goldstein, I. (2010) Recognizing and treating urogenital atrophy in postmenopausal women. Journal of Women’s Health, 19, 425-432. doi:10.1089/jwh.2009.1384
- Costantino, D. and Guaraldi, C. (2008) Effectiveness and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: An open, non-controlled clinical trial. European Review for Medical and Pharmacological Sciences, 12, 411-416.
- Morali, G., Polatti, F., Metelitsa, E.N., Mascarucci, P., Magnani, P. and Brunenghi Marrè, G. (2006) Open, noncontrolled clinical studies to assess the efficacy and safety of a medical device in form of gel topically and intravaginally used in postmenopausal women with genital atrophy. Arzneimittel Forschung—Drug Research, 56, 230-238.
- Lewis, V. (2009) Undertreatment of menopausal symptoms and novel options for comprehensive management. Current Medical Research and Opinion, 25, 2689-2698.
- Nachtigall, L.E. (1994) Comparative study: Replens versus local estrogen in menopausal women. Fertility and Sterility, 61, 178-180.

