Open Journal of Immunology
Vol.3 No.3(2013), Article ID:37018,5 pages DOI:10.4236/oji.2013.33014
Blastomyces dermatitidis: Stability studies on different yeast lysate antigens
![]()
Department of Biological Sciences, Idaho State University, Pocatello, USA; *Corresponding Author: allitiff@isu.edu
Copyright © 2013 Tiffany R. Allison et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 8 June 2013; revised 7 July 2013; accepted 21 July 2013
Keywords: Blastomyces dermatitidis; Lysate Antigens; Antibody Detection; Stability; ELISA
ABSTRACT
In Trial 1, 19 lots of Blastomyces dermatitidis (T-58; Tennessee dog isolate) were assayed to determine the stability of the reagents following storage. The reactivity of the antigens, produced from 1989 to 2012 and stored at 4˚C, was determined by comparing antibody detection (enzyme-linked immunosorbent assay; ELISA) in 12 serum specimens from immunized rabbits. All of the 19 reagents produced during this 23-year period exhibited a high degree of stability and were able to detect antibody in the sera. Mean absorbance values ranged from 0.798 (1989) to 0.827 (2012) and a mean value for all 19 antigens of 0.728. In a related evaluation, Trial 2, B. dermatitidis lysate antigens prepared from 8 isolates (dog, human, soil) at two different time periods were assayed as above to determine reactivity. The time of storage between the first and second reagents varied from 4 to 17 years. The results indicated that all 16 of the lysate antigens detected antibody in the 15 rabbit serum specimens with mean absorbance values ranging from 0.346 to 0.682, but variations in reactivity were observed depending on the lysate and the serum specimen assayed. This comparative study provided evidence that the antigenic reagents do exhibit some lot-to-lot variation in reactivity, but they did not lose any appreciable potency during prolonged storage.
1. INTRODUCTION
Blastomyces dermatitidis, a thermally dimorphic fungal organism, is the etiologic agent that causes blastomycosis. The disease of humans and animals is found mainly in North America as a soil saprophyte associated with sandy slightly acidic soils which are often found near a water source. In the United States blastomycosis has been associated with southeastern and south-central states that border the Ohio and Mississippi rivers and is also prevalent in Minnesota and Wisconsin and in areas of southern Canada that border these states. The disease also occurs in other locations including certain regions of Africa and India [1-3]. Blastomycosis is acquired by inhaling mycelial spores into the lung, where they are converted to a single-budding yeast cell. A primary pulmonary infection may result with no progression of the disease or the organisms may disseminate into other organs of the body including the central nervous system with potentially fatal meningeal involvement. In addition cutaneous lesions may also develop in a high percentage of the cases as the disease disseminates from the lung [4-8].
Current laboratory diagnostic methods include direct visualization, histologic methods or culturing the organism, but these methods may be time consuming or may not provide the desired diagnostic results or possibly a misdiagnosis. Thus researchers have been concerned with the development and use of immunodiagnostic assays in attempts to provide a more rapid diagnosis, but problems are still evidenced with regard to sensitivity and specificity of the laboratory assays [9-11]. Another concern of investigators associated with immunodiagnostic assays for antibody detection in blastomycosis is the development and production of antigens that are not only sensitive and specific, but also reagents that retain their stability for prolonged periods of storage.
There are only a limited number of data in the literature concerned with stability studies on antigenic reagents for use in immunodiagnostic assays. Stability studies in recent years have been primarily concerned with developing methods to extend the stability of antigens in vaccines. This is specifically important in developing countries where access to refrigeration may not always be accessible. Research scientists have evaluated variables from storage at different temperatures to coating of micro needles, and the addition of aluminum-containing adjuvants to lengthen the stability of vaccines [12-14]. Currently, medical personnel are working with scientists to develop new ways to prolong the shelf life and stability of vaccines.
In recent years, investigators have made substantial contributions with regard to the development and evaluation of immunodiagnostic assays for blastomycosis [9, 10,15-21]. Our laboratory has been involved in the production and comparative studies of B. dermatitidis yeast lysate antigens, prepared from various isolates of the fungus, for the detection of antibodies in sera from immunized and infected animals [22-30]. Encouraging results have been obtained with yeast lysate antigenic preparations. As a result, studies in our laboratory have been directed to evaluating antigens from a number of different isolates of the fungus from human, animal and environmental sources. This has been done in an effort to produce an efficient and reliable reagent for the immunodiagnosis of blastomycosis in humans and animals.
Therefore, in an effort to continue evaluations of B. dermatitidis lysate antigens for use as immunodiagnostic reagents and since we desired to learn more concerning the stability of our antigens, the objective of this present study was to evaluate the stability of different yeast phase lysate antigens that had been stored for various periods of time. The ability of the stored antigenic reagents to detect antibodies in serum specimens from immunized rabbits was determined using the indirect enzymelinked immunosorbent assay (ELISA) of 19 lots of yeast lysate antigen produced from B. dermatitidis T-58. This study evaluated the stability of these lysate antigens over time by detecting antibodies in rabbit sera using the indirect ELISA.
2. MATERIALS AND METHODS
2.1. Lysate Antigen Preparation
Nineteen lots of B. dermatitidis yeast phase lysate reagents (T-58, dog Tennessee, from the years:1989, 1990, 1991, 1992, 1993, 1994, 1995, 1996, 1997, 2002, 2003, 2004, 2005, 2006, 2007, 2008, 2009, 2011, and 2012) were prepared by a method similar to one that was previously used for the production of yeast lysate antigen from Histoplasma capsulatum [31,32] and modified in our laboratory for B. dermatitidis lysate antigen production [21]. The yeast phase cells were grown for 7 days at 37˚C in a chemically defined medium in an incubator shaker, harvested by centrifugation (700 ×g; 5 min), followed by washing with distilled water, re-suspended in distilled water and then allowed to lyse for 7 days at 37˚C in water with shaking. The preparations were centrifuged, filter sterilized, merthiolate added (1:10,000) and stored at 4˚C for up to 23 years. Protein determinations were performed on the lysates using the BCA Protein Assay Kit (Pierce Chemical Company, Rockford, IL) and dilutions of the antigenic reagents used in the ELISA assays were based on protein concentration.
Trial 2: 8 different lysate antigens were prepared at two different times and of differing origins (Table 1). These antigens were also injected into rabbits and assayed as described above. The methods for lysate preparation utilized methods as stated above in Trial 1.
2.2. Serum Specimens
New Zealand White rabbits were immunized (intramuscularly; 2 ml and subcutaneously; 1 ml) 100 ug/ml (protein concentration) of B. dermatitidis lysate antigen. The rabbits were bled at various intervals following immunization, but this study used antibodies collected on day 7 following a booster injection (as above) on day 56.
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
The ability of each yeast lysate reagent to detect antibodies in the above serum specimens was determined using the indirect enzyme-linked immunosorbent assay (ELISA). Each lysate antigen was diluted (2000 ng/ml of protein) in a carbonate-bicarbonate coating buffer (pH 9.6, see below) and then added to triplicate wells (100 ul) of a Costar 96-well microplate (Fisher-Thermo). The plates were then incubated overnight at 4˚C in a humid chamber followed by washing three times with phosphate buffered saline containing 0.15% Tween 20 (PBS-T). The serum specimens (1:2500 dilution; 100 ul) were added to the microplate wells and incubated for 30 min at 37˚C in a humid chamber. Following this incubation
Table 1. Trial 2 of the B. dermatitidis lysates produced in past and recent time intervals are shown above with their corresponding absorbance. We did not see a significant difference between strains and their stability over time.
the wells were washed as above and 100 ul of goat antirabbit (H & L) peroxidase conjugate (Kirkegaard and Perry, Gaithersburg, MD) was added to each well and incubated for 30 min at 37˚C. The plates were again washed as above and 100 ul of TMB peroxidase substrate (Pierce/Fisher-Thermo) was added to each well and incubated for approximately 2 min at room temperature. The reaction was stopped by the addition of sulfuric acid and the absorbance read at 450 nm using a BIO-RAD 2550 EIA reader.
3. RESULTS
The results (Trial 1) indicated that all 19 lysate samples remained stable over time and were able to detect an appreciable amount of antibody in the serum specimens. Additionally, when we evaluated their stability over time by graphing the sera values for the immunized rabbit, we found a slightly negative slope −0.0024 respectively (Figure 1). The statistical analysis (t-test) for immunized rabbit sera yielded p-values > 0.05. This finding leads to the rejection of the null hypothesis; the null states that there is a difference in stability between lysates over time. This leads to the conclusion that there is no significant difference in absorbance values, therefore no difference in antibody-antigen reactivity, between the lysates from 1989 to those from 2012.
In Trial 2, we found no significant differences between the different antigens of different time periods. Across all 8 antigens, the lysates remained stable over time, demonstrating similar reactivity. The mean absorbance of all antigens ranged from 0.346 - 0.648 for the older specimens and 0.410 - 0.682 for the new specimens (Table 2). The mean overall absorbance was 0.508 and 0.546 for the old and new respectively. The reactivity values are demonstrated in Table 1 and in Figure 2.
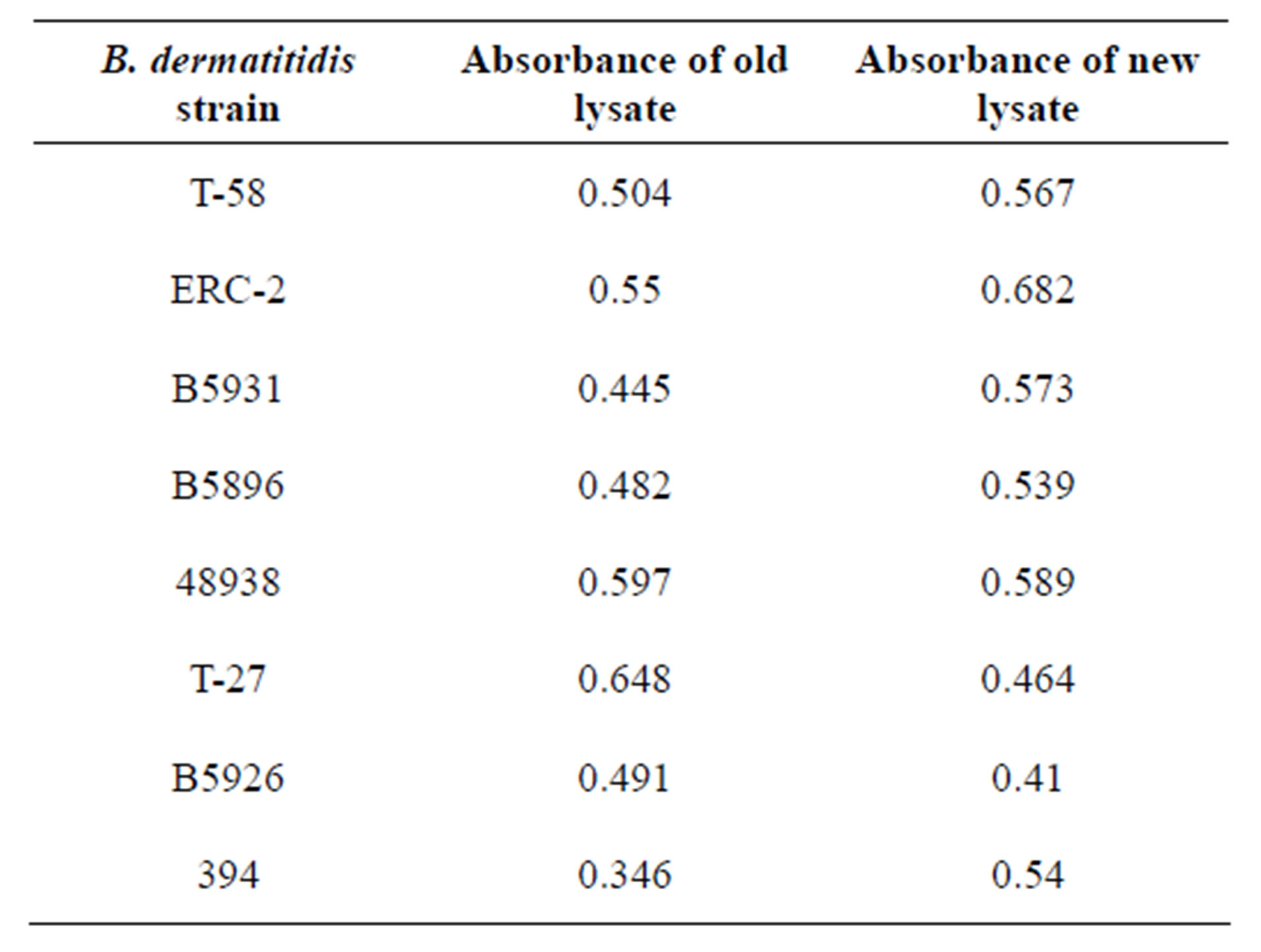
Table 2. In Trail 2 of this stability study 8 different antigens were evaluated. The strain identifier, date of lysate preparation and origin are shown above.
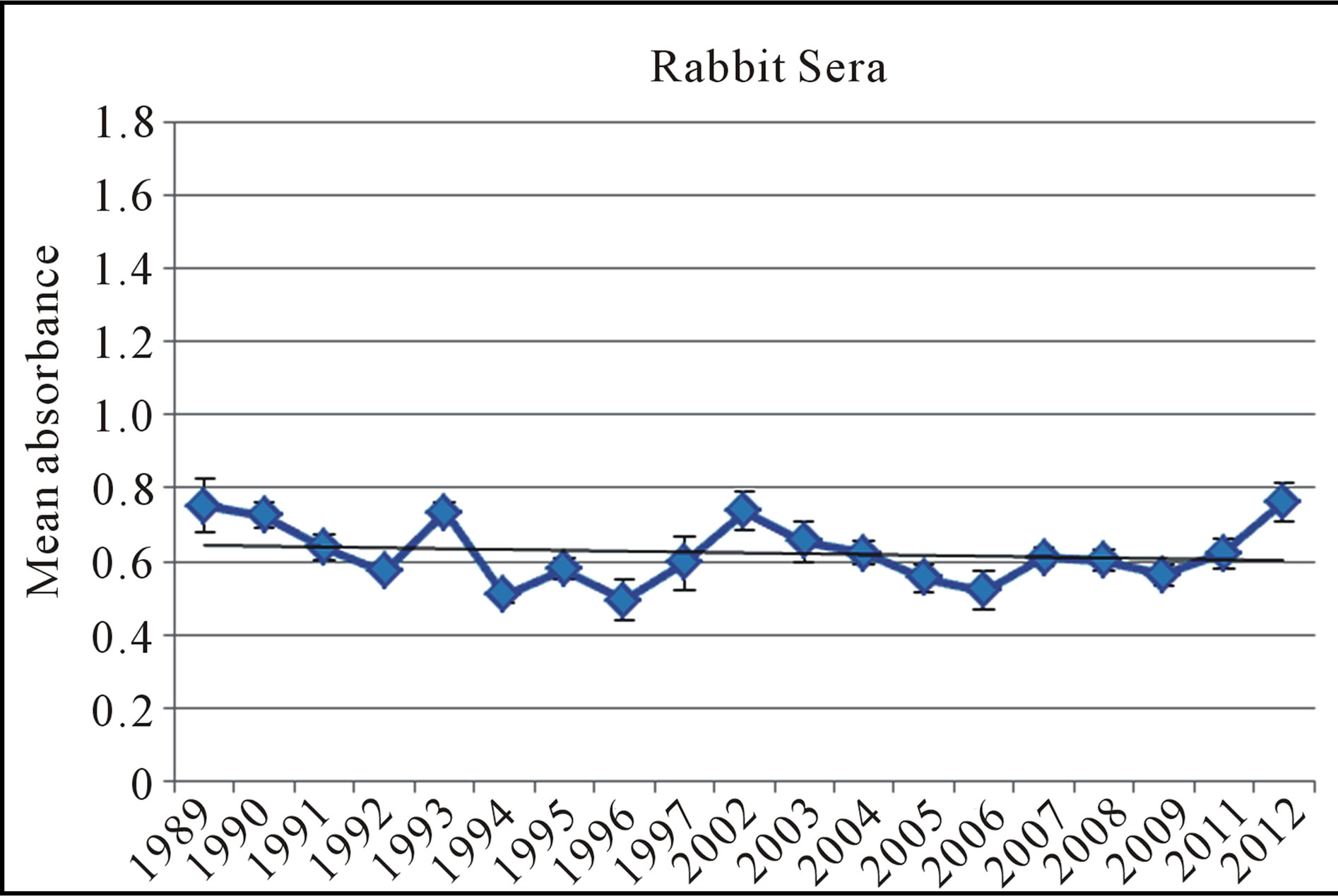
Figure 1. The mean absorbance values of antibody detection with an indirect ELISA in from 19 B. dermatitidis lysate antigens against the rabbit serum. A linear regression was utilized to give the p-values for this test, demonstrating no significance difference in the antigen’s reactivity over time. Error bars represent standard error.
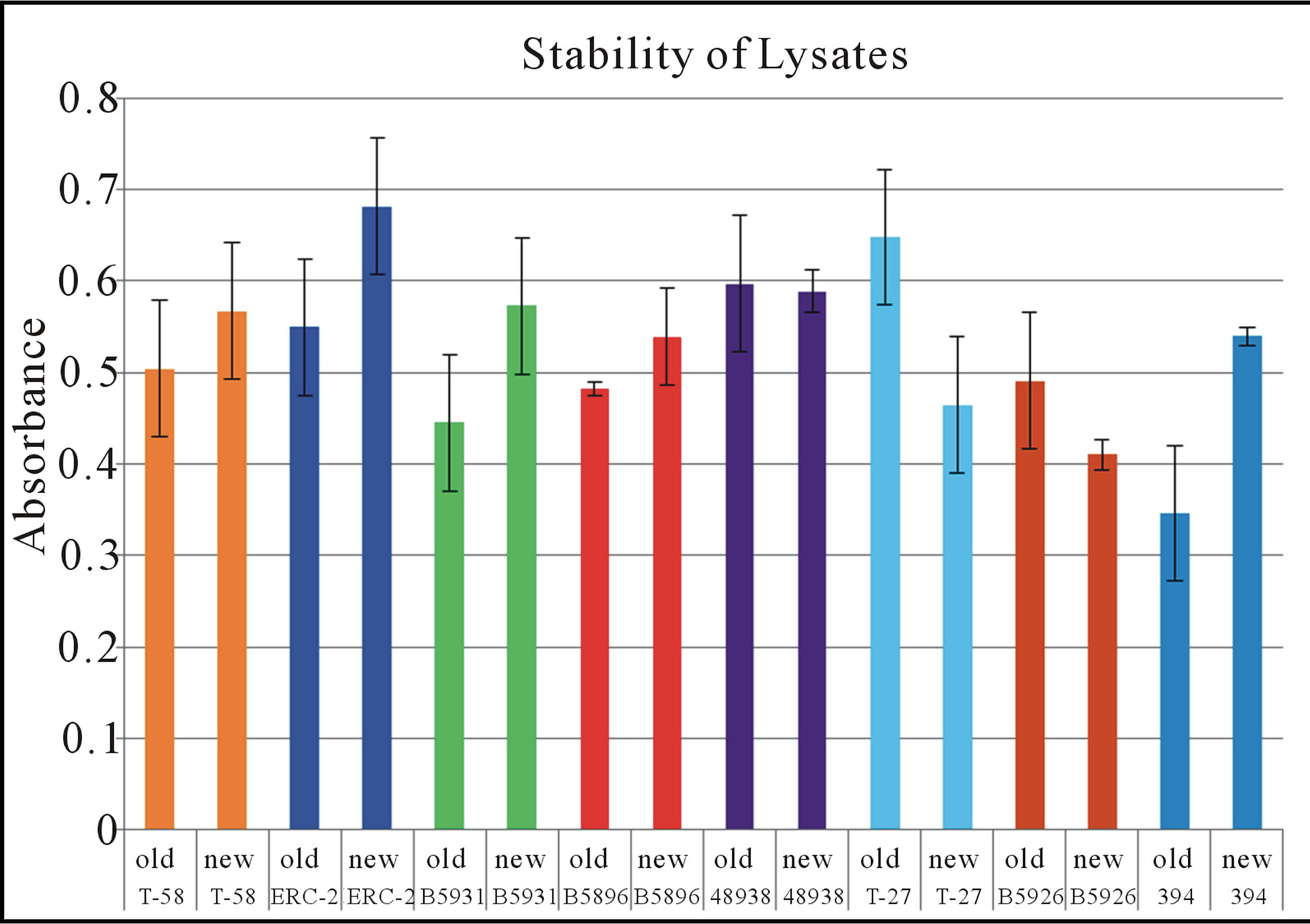
Figure 2. In trial 2 of this stability study 8 different antigens were evaluated. This graph demonstrates the old and the new antigens from each time designation. In comparing the old and new values of each antigen no significant differences were found in their reactivity. Error bars represent standard error.
4. DISCUSSION AND CONCLUSIONS
The purpose of this study (Trial 1) was to compare the stability of T-58 lysate antigens of B. dermatitidis over a 23-year period with regard to antibody detection in serum specimens from immunized rabbits.
The lysates prepared from 1989 to 2012 and stored at 4˚C were stable with regard to being able to be used for the laboratory diagnosis of blastomycosis. Variations in reactivity were observed with 19 lots of lysate reagents which were dependent upon the type of serum specimen that was assayed. In comparison, the reactivity values obtained with the rabbit sera no significant differences were noted in absorbance values or stability over time.
The present study provided some important evidence that the B. dermatitidis lysate antigenic reagents seemed to be very stable over a prolonged period of storage. This certainly is a desirable characteristic when immunodiagnostic products are produced. These studies demonstrate the usefulness of this antigen as a potential tool for the detection of antibodies in blastomycosis.
The purpose of Trial 2 was designed to determine the differences between using antigens stored at 4˚C for varying amounts of time and using lysates produced more recently for our immunological studies.
The results were similar to those obtained in Trial 1 as all of the lysates remained stable over time. The stability of the antigens were not affected significantly by the origin of the lysate (soil, bat, dog, and human). This leads us to conclude that any of the antigens evaluated do not loose reactivity over time and as a result would make promising antigens to be used in immunodiagnostic assays.
These findings allow us to quantifiably use any of the antigens with confidence. Comparative studies are continuing in an effort to explore the potential of lysate reagents in immunodiagnostic assays for antibody detection in specimens from animals and humans infected with blastomycosis.
5. ACKNOWLEDGEMENTS
This research was supported by University Research Committee Grant S119 and by the Department of Biological Sciences at Idaho State University, Pocatello, ID.
REFERENCES
- Pfaller, M.A. and Diekema, D.J. (2010) Epidemiology of invasive mycoses in North America. Critical Reviews in Microbiology, 36, 1-53. doi:10.3109/10408410903241444
- DiSalvo, A.F. (1998) Blastomycosis, in Topley and Wilson’s microbiology and microbial infections. 9th Edition, Arnold Publishers, London, 337-355.
- DeGroote, M.A., Bjerke, R., Smith, H. and Rhodes III, L.V. (2000) Expanding epidemiology of blastomycosis: clinical features and investigation of 2 cases in Colorado. Clinical Infectious Diseases, 30, 582-584. doi:10.1086/313717
- Bradsher, R.W. (1997) Clinical features of blastomycosis. Seminars in Respiratory Infections, 12, 229-234.
- Bradsher, R.W., Chapman, S.W. and Pappas, P.G. (2003) Blastomycosis. Infectious Disease Clinics of North America, 17, 21-40. doi:10.1016/S0891-5520(02)00038-7
- Smith, J.A. and Kauffman, C.A. (2012) Pulmonary fungal infections. Respirology, 17, 913-926. doi:10.1111/j.1440-1843.2012.02150.x
- Bariola, J.R. and Vyas, K.S. (2011) Pulmonary blastomycosis. Seminars in Respiratory Critical Care Medicine, 32, 745-753. doi:10.1055/s-0031-1295722
- McKinnell, J.A. and Pappas, P.G. (2009) Blastomycosis: new insights into diagnosis, prevention, and treatment. Clinical Chest Medicine, 30, 227-239. doi:10.1016/j.ccm.2009.02.003
- Saccente, M. and Woods, G.L. (2010) Clinical and laboratory update on blastomycosis. Clinical Microbiology Reviews, 23, 367-381. doi:10.1128/CMR.00056-09
- Cutler, J.E., Deepe Jr., G.S. and Klein, B.S. (2007) Advances in combating fungal diseases: Vaccines on the threshold. Nature Review of Microbiology, 5, 13-18. doi:10.1038/nrmicro1537
- Vyas, K.S., Bariola, J.R. and Bradsher, R.W. (2008) Advances in the serodiagnosis of blastomycosis. Current Fungal Infection Reports, 2, 227-231. doi:10.1007/s12281-008-0033-z
- Clapp, T., Siebert, P., Chen, D. and Braun, L.J. (2011) Vaccines with aluminum-containing adjuvants: Optimizing vaccine efficacy and thermal stability. Journal of Pharmaceutical Sciences, 100, 388-401. doi:10.1002/jps.22284
- Kim, Y.C., Quan, F.S., Compans, R.W., Kang, S.M. and Prausnitz, M.R. (2010) Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. Journal of Conrolled Release, 142, 187-195. doi:10.1016/j.jconrel.2009.10.013
- Brandau, D.T., Jones, L.S., Wiethoff, C.M., Rexroad, J. and Middaugh, C.R. (2003) Thermal stability of vaccines. Journal of Pharmaceutical Sciences, 92, 218-231. doi:10.1002/jps.10296
- Klein, B.S. and Jones, J.M. (1990) Isolation, purification and radiolabeling of a novel surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. Journal of Clinical Investigation, 85, 152-161. doi:10.1172/JCI114406
- Connolly, P., Hage, C.A., Bariola, J.R., Bensadoun, E., Rodgers, M., Bradsher, R.W. and Wheat, J.J. (2012) Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clinical Vaccine Immunology, 19, 53-56. doi:10.1128/CVI.05248-11
- Hage, C.A., Davis, T.E., Egan, L., Parker, M., Fuller, D., LeMonte, A.M., Durkin, D., Connelly, P., Wheat, L.J., Blue-Hindy, D. and Knox, K.A. (2007) Diagnosis of pulmonary histoplasmosis and blastomycosis by detection of antigen in bronchoalveolar lavage fluid using an improved second-generation enzyme-linked immunoassay, Respiratory Medicine, 101, 43-47. doi:10.1016/j.rmed.2006.04.017
- Klein, B.S., Squires, R.A., Lloyd, J.K., Ruge, D.R. and Legendre, A.M. (2000) Canine antibody response to Blastomyces dermatitidis WI-1 antigen. American Review of Veterinary Research, 61, 554-558. doi:10.2460/ajvr.2000.61.554
- Spector, D., Legendre, A.M., Wheat, J., Bemis, D., Rohrbach, B., Taboada, J. and Durkin, M. (2008) Antigen and antibody testing for the diagnosis of Blastomycosis in dogs. Journal of Veterinary Internal Medicine, 22, 839- 843. doi:10.1111/j.1939-1676.2008.0107.x
- Bariola, J.R., Hage, C.A., Durkin, M., Bensadoun, E., Gubbins, P.O., Wheat, L.J. and Bradsher, R.W. (2011) Detection of Blastomyces dermatitidis antigen in patients with newly diagnosed blastomycosis. Diagnostic Microbiology and Infectious Diseases, 69, 187-191. doi:10.1016/j.diagmicrobio.2010.09.015
- Johnson, S.M. and Scalarone, G.M. (1989) Preparation and ELISA evaluation of Blastomyces dermatitidis yeast phase lysate antigens. Diagnostic Microbiology and Infectious Diseases, 11, 81-86. doi:10.1016/0732-8893(88)90076-4
- Bono, J.L., Legendre, A.M. and Scalarone, G.M. (1995) Detection of antibodies and delayed hypersensitivity with Rotofor preparative IEF fractions of Blastomyces dermatitidis yeast phase lysate antigens. Journal of Medical and Veterinary Mycology, 33, 209-214. doi:10.1080/02681219580000441
- Fisher, M.A., Bono, J.L., Abuodeh, R.O., Legendre, A.M. and Scalarone, G.M. (1995) Sensitivity and specificity of an isoelectric focusing fraction of Blastomyces dermatitidis yeast lysate antigen for the detection of canine blastomycosis.Mycoses, 38, 177-182. doi:10.1111/j.1439-0507.1995.tb00046.x
- Axtell, R.C. and Scalarone, G.M. (2002) Serological differences in three Blastomyces dermatitidis strains. Mycoses, 45, 1-6.
- Shurley, J.F., Legendre, A.M. and Scalarone, G.M. (2005) Blastomyes dermatitidis antigen detectioni in urine specimens from dogs with blastomycosis using a competitive binding inhibition ELISA. Mycopathologia, 160, 137-142. doi:10.1007/s11046-005-3153-9
- Shurley, J.F. and Scalarone, G.M. (2007) Isoelectric focusing and ELISA evaluation of a Blastomyces dermatitidis human isolate. Mycopathologia, 164, 73-76. doi:10.1007/s11046-007-9033-8
- Sestero, C.M. and Scalarone, G.M. (2006) Detection of IgG and IgM in Sera from canines with blastomycosis using eight Blastomyces dermatitidis yeast phase lysate antigens. Mycopathologia, 162, 33-37. doi:10.1007/s11046-006-0028-7
- Sestero, C.M. and Scalarone, G.M. (2006) Detection of the surface antigens BAD-1 and alpha (1-3) glucan in six different strains of Blastomyces dermatitidis using monoclonal antibodies. Journal of Medical and Biological Science, 1, 1-7.
- Hatch, W.O. and Scalarone, G.M. (2009) Comparison of colorimetric and chemiluminescent ELISAs for the detection of antibodies to Blastomyces dermatitidis. Journal of Medical and Biological Sciences, 3, 1-6.
- Klein, B.S., Vergeront, J.M., Weeks, R.J., Kumar, U.N., Mathai, G., Varkey, B., Kaufman, L., Bradsher, R.W., Stoebig, J.R. and Davis, J.P. (1986) Isolation of Blastomyces dermatitidis in soil associated with a larger outbreak of blastomycosis in Wisconsin. Journal of Infectious Diseases, 155, 262-268. doi:10.1093/infdis/155.2.262
- Levine, H.B., Scalarone. G.M. and Chaparas, S.D. (1977) Preparation of fungal antigens and vaccines: Studies on Coccidioides immitis and Histoplasma capsulatum. Contributions to Microbiology and Immunology, 3, 106-125.
- Scalarone, G.M., Levine, H.B. and Chaparas, S.D. (1978) Delayed hypersensitivity responses of experimental animals to histoplasmin from the yeast and mycelial phases of Histoplasma capsulatum. Infection and Immunity, 21, 705-713.

