Open Journal of Animal Sciences
Vol.05 No.01(2015), Article ID:53088,5 pages
10.4236/ojas.2015.51004
Liver Lead Levels in Snow Goose (Chen caerulescens) in a Wetland near the City of Durango, Mexico
Martín Emilio Pereda-Solís1, Alicia Zulema Cárdenas González1, José Hugo Martínez Guerrero1*, Luis Francisco Sánchez Anguiano2, Federico Rosales Alférez1
1Facultad de Medicina Veterinaria y Zootecnia, Cuerpo Académico de Fauna Silvestre, Universidad Juárez del Estado de Durango, Durango, México
2Instituto de Investigaciones Científicas, Universidad Juárez del Estado de Durango, Durango, México
Email: *conplandg@hotmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 November 2014; revised 16 December 2014; accepted 26 December 2014
ABSTRACT
The use of lead in ammunition for hunting exposes waterfowl to lead poisoning (plumbism) by accidental consumption of shotgun pellets. To test this hypothesis we sampled 18 liver tissue samples of Snow Goose (Chen caerulescens) collected during the 2012-2013 hunting season in a wetland near the city of Durango, Mexico. We quantified liver lead levels using an atomic absorption spectrophotometer and portions of liver were fixed and stained for their histological study. Average lead concentration (in dry weight) were under the normal range (mean = 0.73 ± 0.2, standard error) which do not represent any risk of poisoning. Liver tissue injuries were not observed in the histopathological analysis, suggesting no reaction to a xenobiotic agent such as lead. Gastrointestinal content analysis showed lead pellet in the gizzard of one individual, but we could not find a relationship between pellet ingestion and lead concentration in the liver. Although the results did not provide evidences of lethal or sublethal effects caused by lead poisoning, they show a possible risk due to the presence of lead pellets in the digestive tract.
Keywords:
Snow Goose, Chen caerulescens, Lead Poisoning, Lead Pellets Ingestion, Lead Concentration, Liver

1. Introduction
Birds have been commonly used as valuable environmental indicators in eco-toxicological studies and are crucial to properly document the qualitative and quantitative environmental changes of certain environmental pollutants owing to both natural and anthropogenic causes [1] . Among all potentially toxic substances that can affect birds, heavy metals are especially interesting as they constitute a serious risk to migratory and resident water bird species [2] . These polluting elements are present in ecosystems [3] but usually at low concentrations under natural conditions. However, human activities have actively released these agents into the environment where the specific properties of these metal substances are of great importance in the uptake, accumulation and toxicity that may occur in aquatic organisms [3] .
In Mexico, there are not many wildlife studies about heavy metals and metalloids contents in waterfowl. Recent studies [4] have evaluated the concentrations of Pb, Cd, Zn and As in hepatic tissue of three groups of water birds (ducks, geese and shorebirds), and found no evidence of potential lethal or sub lethal effects based on liver concentration of this metals and metalloids. Other studies [5] have been focused on whether or not Hg and Pb affect some duck species health such as Anas clypeata and Anas acuta. These studies were oriented to set base lines on the presence of these pollutants in waterfowl.
Among heavy metals, lead has a very special interest because of its use in the manufacture of ammunition for shotguns and the Mexican government does not apply any restrictions on its use. Unfortunately, hunting is a common activity in wetlands where many species of resident and migratory water birds live. Lead is a heavy metal that has no physiological significance and is highly toxic. The absorption of minimum concentrations produces various sublethal effects in birds and in high concentrations can cause death [6] . Waterfowl and other birds are exposed to large amounts of lead in a very particular way, through the ingestion of lead pellets that spread over during hunting. These pellets are ingested accidentally with food, which has led to problems of waterfowl mortality in the United States, Europe and many other places where shotgun shells with lead pellets are still used [7] .
The objective of this study was to determine the concentration of lead in liver tissue of Snow Goose (Chen caerulescens) and relate it to lethal or sublethal effects on health.
2. Materials and Methods
2.1. Site of Study
We conducted this study in 96.0 ha of the UMA Ejido “El Arenal” (SEMARNAT-UMA-EX0336-DGO) Durango, Mexico (Figure 1). The area represents a small portion of the 5264 ha of natural vegetation of UMA. This is a site where from 8000 to 10,000 snow geese winter each year.
The climate is BS1kw (w), semidry and semiarid [8] , with a low mean temperature of −4.4˚C and high of 36.1˚C, low mean rainfall of 420 mm and high of 525 mm, relative humidity of 40%, rainy season ranges from July to August, with few winter rains in January. Soil origin is in situ, derived from igneous rock with abundant rocky outcrops, dominance of brown, reddish alluvium of sandy loam texture [9] .
Shrub vegetation is characterized by Opuntia leucotricha, O. streptacantha, O. megacantha, Acacia tortuosa, Prosopis juliflora, Mimosa biuncifera, O. imbricata, Yucca spp., and some Agave ssp. The herb stratum is dominated by grasses such as Bouteloa gracilis, B. curtipendula, B. hirsuta, Setaria macrostachya, Leptochloa dubia, Stipa eminens, Botriochloe barbinoidis, Aristida ternipes, A. glauca, A. divaricata, Heteropogon contortus and Melinis repens [10] .
2.2. Bird Specimens
During the hunting season (December 2012 to March 2013) 18 snow geese were collected. Sampling depended on geese hunted during the season. Each goose was weighed on a TorRey scale (Model L-EQ 10/20) with a 10 kg capacity and 0.02 kg accuracy. At the same time livers were removed and weighed on an OHAUS balance (Model CS 2000) with a 2000 g capacity and 1 g accuracy.
The hepatosomatic index (HSI) was calculated as:
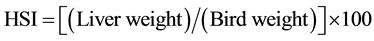 (1)
(1)
Figure 1. Location of study site, UMA of Ejido “El Arenal”, Durango, México.
Livers were placed in sterile plastic bottles and stored in coolers and sent to the FMVZ laboratory and kept at a temperature of −20˚C (Friend and Franson [11] ). In addition to the liver, the digestive tract was recovered to determine its content, and thus to detect the possible presence of lead pellets. The proventricle and gizzard of each goose was explored in detail to detect probable entry holes caused by shots.
2.3. Laboratory Analyses
A fraction (1 g) of the liver was sent to the laboratory of CIIDIR-Durango where the concentration of Pb was determined with an atomic absorption spectrophotometer (Perkin Elmer AAnalyst 700), in agreement to NOM- 010-ZOO-1994. Lead concentrations were obtained in both dry and wet weight, to facilitate comparison with values reported in other studies.
The histological analysis was performed by slicing a portion of each liver and fixing in 10% formalin to perform a microscopic examination. Samples were cut to 4 microns with a microtome and stained with a hematoxylin- eosin solution. This microscopic examination allowed us to observe the characteristics of cells and possible changes to support the diagnosis. We used a Leica ICC50HD microscope with an integrated digital photography system connected to a computer.
2.4. Statistical Analysis
Statistical analysis of data was performed using the Number Cruncher Statistical Software (NCSS) 2000 program [12] . Average values and standard error (se) were calculated. Assumption of data normality was verified using a Shapiro-Wilk’s test. We used the Spearman’s coefficient of ranks to explore correlations between the hepatosomatic index and lead concentrations (dry weight and fresh weight), due to the lack of normality of the data.
3. Results and Discussion
3.1. Lead Concentration in Geese Livers
Mean lead concentration (wet weight) was 0.17 ppm ± 0.04 error standard (n = 18). Lead concentration in liver tissue (Table 1) ranged around <2 ppm in wet weight which does not imply a risk of poisoning, according to a similar study of Pain [13] . Locke and Thomas [14] also suggested that lead concentrations (wet weight) in the
Table 1. Body weight, liver weight, hepatosomatic index (HSI) and lead concentrations in liver tissue of Snow Goose (Chen caerulescens) in Durango, Mexico.
liver of waterfowl express the likelihood of intoxication as 2 - 6 ppm subclinical intoxication, 6 - 15 ppm clinic intoxication, and over 15 ppm severe intoxication. The maximum acceptable lead levels in wildlife are based on experiments on wild birds with no previous exposure to lead, and with the administration of different doses of lead in which sublethal effects and death occurred. Waterfowl with no history of lead exposure generally have concentrations in liver tissue of <2 ppm in wet weight and more frequently <1 ppm [15] . Livers from dead birds are the most likely analyzed tissue when there is a suspected case of lead exposure.
3.2. Hepatosomatic Index
The hepatosomatic index (HSI) was 1.57% ± 0.11%, (se) which is lower than one reported by Ross [5] for some species of ducks such as A. clypeata (mean 3.85, max = 4.78, min = 2.81) and A. acuta (mean = 2.89, max = 4.55, min = 1.59). Spearman’s correlations showed no relationship between HSI and Pb concentration (Figure 2) both on dry (CS = 0.01) and wet basis (CS = −0.21). Similar studies on waterfowl indicate that it is possible an increase in liver size when birds are exposed to certain pollutants [5] .
3.3. Gastrointestinal Tract Contents
We found that only one individual contained lead pellets in the gizzard representing an incidence of 5.5%, and we did not observe any proventricle or gizzard lesions related to shooting. It was possible that pellets were ingested just before the time of collection, maybe 2 or 3 days earlier. This assumption is based on the fact that geese were collected during the first visit by hunters and based on the state of little dissolution of lead particles found and we confirmed it whit relevant results from histopathological analysis or hepatosomatic index.
Acid conditions (pH 2.5) in geese stomachs may facilitate lead dissolution, which produces toxic lead salts that are absorbed into the bloodstream. Lead traces can remain in the gizzard for approximately 6 weeks after ingestion, with an average retention of 18 to 21 days. When lead particles are discarded immediately after intake, only a small amount of lead is absorbed [16] . Lead circulating in the bloodstream is rapidly deposited in soft tissues, mainly in liver and kidney, and later in bones. This is the reason why concentration of lead in bones is of little use in detecting recent exposures to lead [17] .
It is important to remark that the presence of shotgun pellets in geese gizzards does not necessarily indicate lead poisoning. The absorption and tolerance to lead depends primarily on two factors, namely concentration and exposure time. These factors are determined by the size and number of pellets ingested, age and sex of the bird, as well as composition and volume of food ingested [18] .
3.4. Histological Analysis of Liver Tissue
Histological lesions in liver tissue when lead poisoning has occurred consist of blood cells infiltrated in the parenchyma, generalized hemosiderosis and acidophilic bodies at cytoplasmic level [19] . In our liver histological analysis none of these lesions were observed. The most characteristic finding was the accumulation of lymphocytes, suggesting a slight liver inflammation (Figure 3), possibly caused by storage time of samples before processing.
Figure 2. Lead concentration in hepatic tissue and hepatosomatic index in Snow Goose.
Figure 3. Hepatic tissue of Snow Goose showing lymphocyte accumulation (100×).
4. Conclusion
Results obtained in this study did not showed evidence of lethal or sublethal effects caused by lead poisoning. However, the possibility of this risk due to the presence of lead shots in the digestive tract of some birds can not ruled out.
Acknowledgements
We thank Dr. J. Herrera Corral for his support in lead contents analysis, and we also thank MVZ J. Breton for his help and support in providing the geese. This manuscript has benefited by comments from Dr. H. Weir.
References
- Winker, K. (2004) Seabird Samples as Resources for Marine Environmental Assessment. University of Alaska Museum, Fairbanks.
- Carbonell, M., Bravo Yague, J.C., Fernández Torija, C., López Beceiro, A., Fidalgo Álvarez, L.E., Hernández Moreno, D., Soler Rodríguez, F. and Pérez López, M. (2007) Contenido Hepático de Mercurio y Plomo en Cormorán Moñudo (Phalacrocorax aristotelis) y Alcatraz Atlántico (Morus bassanus) Procedentes de las Costas de Galicia (España). Revista de Toxicología, 24, 31-35.
- Kahle, S. and Becker, P.H. (1999) Bird Blood as Indicator for Mercury in the Environment. Chemosphere, 39, 2451- 2457. http://dx.doi.org/10.1016/S0045-6535(99)00154-X
- Pereda-Solís, M.E., Martínez-Guerrero, J.H. and Toca-Ramírez, J.A. (2012) Estimation of Hepatic Levels of Heavy Metals and Metalloids in Aquatic Birds from a Wetland Irrigated with Residual Water in the City of Durango, Mexico. Journal of Animal and Veterinary Advances, 11, 826-830. http://dx.doi.org/10.3923/javaa.2012.826.830
- Ross Muñoz, S. (2011) Utilización de biomarcadores para evaluar los efectos de Hg y Pb en aves migratorias del nor- oeste de México. Master’s Thesis, Centro de Investigación en Alimentación y Desarrollo A.C., Mazatlán.
- Demayo, A., Taylor, M.C., Taylor, K.W. and Hodson, P.V. (1982) Toxic Effects of Lead and Lead Compounds on Human Health, Aquatic Life, Wildlife, Plants, and Livestock. Critical Reviews in Environmental Control, 12, 257-305. http://dx.doi.org/10.1080/10643388209381698
- Pain, D.J. (1992) Lead Poisoning in Waterfowl. In: Pain, D.J., Ed., Proceedings IWRB Workshop, International Waterfowl and Wetlands Research Bureau, Special Publication, Slimbridge, 7-13.
- García, E. (1988) Modificaciones al Sistema de Clasificación Climática de Köppen 3ª ed. (para adaptarlo a las con- diciones de la República Mexicana). Offset Larios, México D.F.
- COTECOCA-SARH (1979) Comisión Técnica Consultiva para la Determinación Regional de los Coeficientes de Agos- tadero. Durango. Ed. Calypso, S.A., México D.F.
- González-Elizondo, M.S., González-Elizondo, M. and Márquez-Linares, M.A. (2007) Vegetación y Ecorregiones de Durango. Plaza Valdés S.A. de C.V., México D.F.
- Friend, M. and Franson, J.C. (1990) Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds. USGS-National Wildlife Health Center, Madison, 440 p.
- Hintze, J. (2001) NCSS and PASS. Number Cruncher Statistical Systems. Kaysville, Utah. www.ncss.com
- Pain, D.J. (1996) Lead in Waterfowl. In: Beyer, W.N., Heinz, G.H. and Redmon-Norwood, A.W., Eds. Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations, Lewis Publishers, Boca Raton, 251-264.
- Locke, L.N. and Thomas, N.J. (1996) Lead Poisoning of Waterfowl and Raptors. In: Fairbrother, A., Locke, L.N. and Hoff, G.L., Eds., Noninfectious Diseases of Wildlife, 2nd Edition, Iowa State University Press, Ames, 108-117.
- Kingsford, R.T., Flanjak, J. and Black, S. (1989) Lead Shot and Ducks on Lake Cowal. Australian Wildlife Research, 16, 167-172. http://dx.doi.org/10.1071/WR9890167
- Jordan, J.S. and Bellrose, F.C. (1951) Lead Poisoning in Wild Waterfowl. III Natural History Survey Biological Notes, 26-27.
- Clausen, B., Elvestad, K. and Karlog, O. (1982) Lead Burden in Mute Swans from Denmark. Nordisk Veterinaermedicin, 34, 83-91.
- Danell, K., Anderson, A. and Marcstrom, V. (1977) Lead Shot Dispersed by Hunters Ingested by Ducks. Ambio, 6, 235- 237.
- Romero, D., Martínez López, E., Navas, I., María Mojica, P., Peñalver, J. and García Fernández, A.J. (2007) Alteraciones anatomo-patológicas en un flamenco común (Phoenicopterus roseus) por intoxicación aguda por plomo. Revista de Toxicología, 24, 52-55.
NOTES
*Corresponding author.







