World Journal of Neuroscience
Vol.3 No.1(2013), Article ID:27903,7 pages DOI:10.4236/wjns.2013.31004
Evaluation of the neuropharmacological properties of nerol in mice
![]()
1Northeast Biotechnology Network, Federal University of Piauí (UFPI), Teresina, Brazil
2Pharmacology, Universidade Federal do Piauí (UFPI), Teresina, Brazil
3Pharmaceutics Sciences, Chemistry Department, Federal University of Piauí (UFPI), Teresina, Brazil
4Department of Pharmacy, Federal University of Sergipe (UFS), São Cristóvão, Brazil
5Department of Biochemistry and Pharmacology, Campus Ministro Petrônio Portela, Federal University of Piauí (UFPI), Teresina, Brazil
Email: *rivelilson@pq.cnpq.br
Received 10 December 2012; revised 11 January 2013; accepted 21 January 2013
Keywords: Anxiety; Behaviors; Mice; Nerol; Essential Oils
ABSTRACT
The search for therapeutic agents that will provide the ground for man and an improvement in their quality of life is ceaseless. The nerol (cis-2,6-dimethyl- 2,6-octadien-8-ol) is a monoterpene which can be found in various medicinal plants as Lippia spp and Melissa officinalis L. The objective of this study was to analyze the acute effect of nerol in the central nervous system (CNS) by performing behavioral tests in mice (open field, elevated plus-maze, light/dark and rota rod tests). We used male albino mice (Mus musculus), Swiss variety, adult with 2 month-old. The animals were divided into five groups (n = 8) for each experimental protocol, and they were administered intraperitoneally (i.p.), respectively, Tween 80 0.05% dissolved in saline solution 0.9%, nerol (30, 60 or 90 mg/kg) or diazepam (2 mg/kg). In the open field test, all groups treated with nerol showed a significant decrease in motor activity (number of crossings, rearings and groomings) when compared with vehicle group. In the elevated plus-maze test, nerol groups significantly increased the number of entries and time of permanence in the open arms when compared with vehicle group. In the light-dark test, nerol groups showed a significant increase the time of permanence in the room clear when compared with vehicle group. In the rota rod test, the groups treated with nerol didn’t show modification in time spent and number of falls in the revolving bar when compared with vehicle group. These results indicate a possible anxiolytic effect of nerol in mice.
1. INTRODUCTION
The use of bioactive substances in various therapeutic forms by man dates back to ancient times, with descriptions of its use from 4000 BC containing drawings and writings that show that the local culture, even then, it was known about the use of medicinal plants known today such as thyme, opium and licorice [1]. Nowadays, the bioactive compounds extracted from natural products are still part of a considerable portion of the therapeutic arsenal for many different purposes. It is estimated that approximately 25% - 30% of all drugs are evaluated as therapeutic agents derived natural products [2].
The discovery of natural or synthetic compounds with pharmacological properties and its mechanism of action has been one of the biggest challenges for the pharmaceutical chemistry, biochemistry and pharmacology. The natural compounds found in medicinal plants, when evaluated, can make synergistic effects. Thus, it is extremely necessary to understand the composition and chemical structure of these compounds to an elucidation of its potential benefits [2]. The bioactive compounds presents in plants are essential. The comprehension of the role of the maintenance of health is beginning to emerge recently, although the existence of these compounds has been known for a long time [1,3].
The plants biosynthesize a variety of compounds which have no obvious function in the growth and development, called secondary metabolites. These metabolites are also called natural products and their studies led to a current focus on the discovery of new drugs [4]. Within this context, we cite several monoterpenes with different applications, especially in the pharmaceutical industry, food and cosmetics. Many of them have different biological properties, such as antifungal, antioxidant, anticancer, anti-spasmodic and larvicidal activities [5-9]. It was also denoted antinociceptive and antiinflammatory activities, especially on glutamatergic neurotransmission [10]. Monoterpenes are the compounds biosynthesized by some plants which consist of two isoprene units. They are referred to as low molecular weight terpenoids and represent the most diverse class of plant [1]. Monoterpenes also produce significant effects on the cardiovascular system, providing vasorelaxation, decreased heart rate, hypotension and may be useful as agents for the prevention and/or treatment of cardiovascular diseases [11].
Some monoterpenes in many essential oils possess anticonvulsant activity in animal experiments, such as linalool, that has a broad spectrum of action in experimental models of epilepsy in mice, especially protection against seizures induced by pentylenetetrazol, picrotoxin and electroshock, citronellol and limonene have significant anticonvulsant activity by the reduction of neuronal excitability through the channels mainly Na+-dependent voltage [12-14]. The antimicrobial activity is documented by several monoterpenes like menthol, thymol, and linalyl acetate, with a possible mechanism of action due at least in part, a disturbance of the lipid fraction of bacterial plasma membrane which result in membrane permeability [15]. The monoterpene compounds also exhibit various pharmacological properties, such as limonene with antinociceptive effect which mechanism of action can be related to the stimulation of opiate receptors and inhibition of inflammatory mediators [16].
Nerol (cis-2,6-dimethyl-2,6-octadien-8-ol) has the chemical formula C10H18O. It has molecular weight 154.25 g/mol, boiling point 225˚C and solubility in water of about 255.8 mg/L at 25˚C [17]. This monoterpene can be found in several medicinal plants, as Lippia spp and Melissa officinalis L. Previous studies have demonstrated antimicrobial, anxiolytic, antioxidant and antiviral properties for these species can be attributed to the presence of nerol [18-21]. In addition, nerol is used as fragrances, soaps, shampoos, and not cosmetic products such as cleaners and detergents [17]. Observing the pharmacological activities of other monoterpenes, this study aimed to evaluate the acute effect of nerol on the central nervous system by means of behavioral tests with animals.
2. MATERIALS AND METHODS
2.1. Drugs and Reagents
Drugs used pilocarpine hydrochloride (P400), polyoxyethylene-sorbitan mono (Tween 80) and nerol were purchased from Sigma Chemical Co. St. Louis, MO, (USA) and diazepam (DZP) from Cristália (Brazil). The dosage of all drugs was expressed in milligrams per kilogram of body weight. The agents were administered by intraperitoneal route (i.p.) with a dose 0.1 ml/10g.
2.2. Animals
Swiss male mice Mus musculus (25 - 30 g; 2-month-old) were used. Animals were housed in cages with free access to food and water and were kept with standard lightdark cycle (lights on at 07:00 h a.m.). The experimental protocols were approved by the Faculty Ethics Committee (number 003/11). The experiments were performed according to the Guide for the care and use of laboratory of the US Department of Health and Human Services, Washington, DC (1985). All doses are expressed in milligrams per kilogram and were administrated in a volume of 10 ml/kg injected intraperitoneally (i.p.).
2.3. Experimental Protocol
The animals were divided into five groups of eight mice. The groups were treated (i.p.), respectively, 0.05% Tween 80 dissolved in 0.9% saline solution (vehicle group), nerol (30, 60 or 90 mg/kg) or diazepam 2 mg/kg (DZP group). All experiments were performed 30 min after the treatments described above are carried out.
2.4. Open Field Test
The open field test was conducted in acrylic container (transparent walls and black floor, 30 × 30 × 15 cm3) divided into nine squares of equal areas. The test is used to evaluate the exploratory activity of the animal [22]. Each mouse was placed individually in the center of the arena, and allowed him the freedom to explore the environment. The parameters measured were: number of crossings (squares crossed with all four paws), number of groomings (self-cleaning) and number of rearings (act to cram the front legs), registered for the test period of 5 min.
2.5. Elevated plus Maze Test
The elevated plus-maze used is similar to that described by Pellow [23] and co-workers and Lister [24], which was developed from a model developed by Montgomery [25]. The maze consists of two open arms (30 cm × 5 cm × 0.2 cm) and two enclosed arms (30 cm × 15 cm × 5 cm), extended from a central platform raised to a height of 45 cm from the floor. The animals were individually placed in the center of the maze for a period of 5 min observation, recording the percentage of number of entries and time spent in open arms.
2.6. Light/Dark Test
The experimental procedure of the light/dark test was designed by Crawley and Goddwin [26]. The test is to evaluate the behavior of rodents placed individually in an experimental box containing two compartments that vary the brightness of light and dark, so that transitions are made between the compartments measured. Each mouse was individually placed in the center of the illuminated portion facing the opening leading to the dark side of the box. It was observed that the time spent in each compartment for 5 minutes.
2.7. Rota Rod Test
The rota rod test measures ataxia or muscle relaxation effects of drugs produced in animals and was described by Dunhan and Miya [27]. For this test, each mouse was placed with all four feet onto a bar of 2.5 cm diameter, 25 cm high from the floor, in a rotation of 17 rpm for a period of 3 minutes. The duration of permanence in the swivel bar, in second(s), and the number of falls, with three renewals at maximum was recorded.
2.8. Statistical Analysis
For statistical analysis, nonparametric results (percentages) were analyzed by analysis of variance (ANOVA) for multiple comparisons and Student-Newman-Keuls as post hoc test by GraphPad Prism version 3.00 for Windows, GraphPad Software, San Diego California USA. Copyright © 1994-1999 by GraphPad software. Differences were considered statistically significant from p < 0.05.
3. RESULTS
3.1. Open Field Test
The groups treated with nerol (doses of 30, 60 and 90 mg/kg) showed significant decrease in the number of crossings (51.57, 66.45 and 77.42%, respectively) and rearings (67.89, 77.57 and 91.69%, respectively) compared with vehicle group. There was a decrease of 22.84 and 71.43%, respectively, in the groups treated with nerol (doses of 60 and 90 mg/kg) as compared to group treated with DZP. The groups treated with nerol (doses of 60 and 90 mg/kg) showed decrease of 40.98 and 43.77% in the number of groomings when compared with vehicle group (Figure 1).
Experiments were described as materials and methods. Nerol was administered i.p. Values are mean ± S.E.M. of number of crosses, rearings and groomings of mice (8 per group) used in the experiments. ap < 0.05, significantly different Vehicle group. bp < 0.05, significantly different from the Diazepam group. cp < 0.05, significantly different from Nerol 30 mg/kg group. dp < 0.05
(ANOVA followed by Student-Newman-Keuls test, significantly different from Nerol 60 mg/kg group. Drugs were administered 30 min before testing.
3.2. Elevated plus Maze Test
In this test, the number of entries into open arms (NEOA) was recorded. Animals treated with nerol 90 mg/kg showed increase of 279.19% and 157.54% in the number of entries in open arms compared to vehicle and DZP group, respectively.
In relation to time of permanence in the open arms (TPOA), the groups treated with nerol 90 mg/kg showed an increase of 71.75% when compared with vehicle group (Figure 2).
Experiments were described as materials and methods. Nerol was administered i.p. Values are mean ± S.E.M. the percentage of the number of entries into open arms (NEOA) and the time of permanence in open arms (TPOA) of mice (5 per group) used in the experiments.
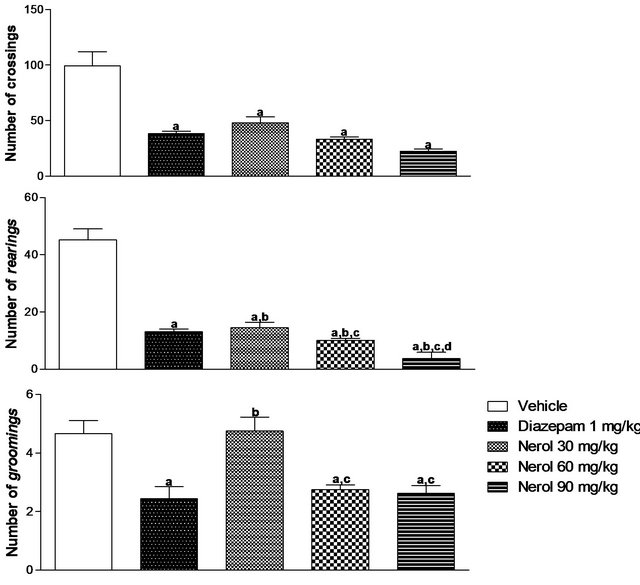
Figure 1. Effects of nerol in mice in the open field test.
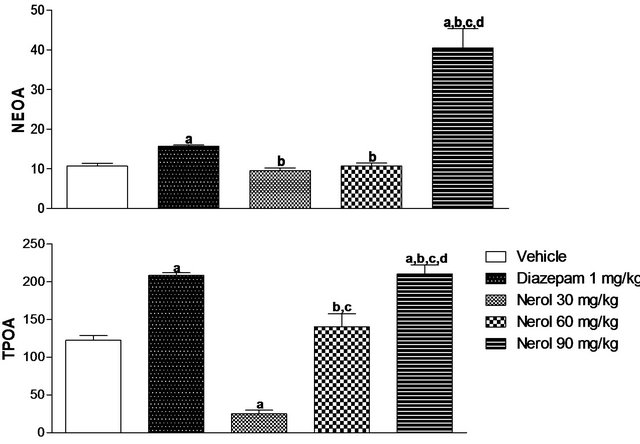
Figure 2. Effects of nerol in the plus maze model in mice.
ap < 0.05, significantly different from Vehicle group. bp < 0.05, significantly different from Diazepam group. cp < 0.05, significantly different from Nerol 30 mg/kg group. dp < 0.05 (ANOVA followed by Student-Newman-Keuls test, significantly different from Nerol 60 mg/kg group. Drugs were administered 30 min before testing.
3.3. Light/Dark Test
In this test, the groups treated with nerol 60 mg/kg or nerol 90 mg/kg showed a significant increase of 47.60 and 64.30%, respectively, in the time spent in the light compartment in relation to vehicle group (Figure 3).
Experiments were described as materials and methods. Nerol was administered i.p. Values are mean ± S.E.M. of time spent by mice (eight per group) in the light room (eight per group). ap < 0.05 (ANOVA followed by Student-Newman-Keuls test, significantly different from Vehicle group. bp < 0.05 (ANOVA followed by Student-Newman-Keuls test, significantly different from Diazepam group. cp < 0.05 (ANOVA followed by Student-Newman-Keuls test, significantly different from Nerol 30 mg/kg group. dp < 0.05 (ANOVA followed by Student-New-man-Keuls test, significantly different from Nerol 60 mg/kg group. Drugs were administered 30 min before testing.
3.4. Rota Rod Test
In this test, the groups treated with nerol didn’t show significant difference in the time of permanence and number of falls when compared with vehicle or DZP groups (Figure 4).
Experiments were described as materials and methods. Nerol was administered i.p. Values are mean ± S.E.M. of time of permanence and number of falls of mice (eight per group). ap < 0.05, significantly different from Vehicle. bp < 0.05, significantly different from Diazepam group. cp < 0.05, significantly different from Nerol 30 mg/kg group. dp < 0.05 (ANOVA followed by StudentNewman-Keuls test, significantly different from Nerol 60 mg/kg group. Drugs were administered 30 min before testing.
4. DISCUSSION
In this study, the pharmacological effects of monoterpene nerol were investigated in animal models to inves-
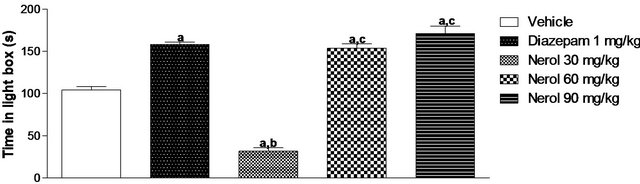
Figure 3. Effects of nerol in the light/dark test in mice.
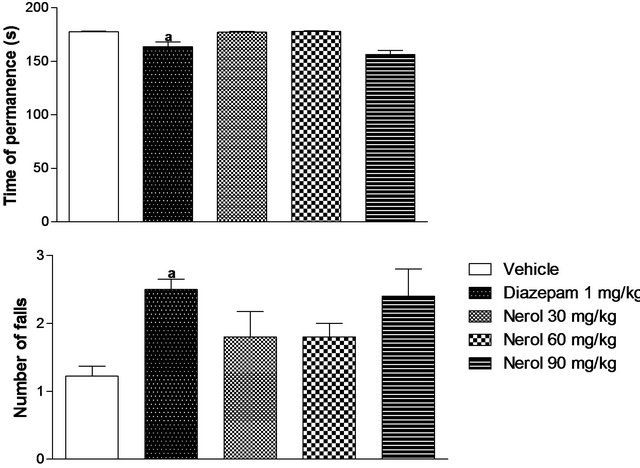
Figure 4. Effects of nerol in the rota rod test in mice.
tigate psychopharmacological effects of this substance on the CNS. The results obtained were similar to those observed for other monoterpenes [28-30]. Moreover, nerol does not produce twisting, tremors, seizures, stereotyped behavior and catalepsy, suggesting that the effect isn’t possibly toxic.
To identify possible changes resulting from administration of the compound under study, it is necessary to carry out behavioral tests, such as open field, elevated plus maze, light-dark and rota rod tests. In these tests, it is possible observe behavioral changes in mice that arise under possible actions on the CNS of nerol in the experiments.
In the open field test, three parameters were evaluated: the total number of crossings, assessing the animal’s exploratory activity; the total number of rearings (cram the front legs) and total number of groomings (self-cleaning of the animal), which assess degree of sedation or fear (anxiety) and can be altered by drugs with anxiolytic/anxiogenic activity [22,31]. A significant reduction of the functional aspects of spontaneous movement of mice in this test, after administration of doses of nerol, suggested a possible anxiolytic action of nerol, which corroborates the hypothesis that the nerol reduces the CNS activity. Similar result was reported where there was reduced ambulation of the animals, this being a feature of psychiatric drugs [32,33]. The reduction in locomotor activity was observed in many essential oils containing terpene derivatives [28] and may be due to an inhibitory effect of nerol on the central nervous system. We suggest that nerol could possess an activity or a neuro-sedative profile of action tending to other hypnotic drugs [34].
The elevated plus maze is another of the main models used in the study of compounds that act on the CNS. In previous works [25,35], there was less use of the open arms in relation to enclosed arms. It is hypothesized that the stimulation caused by the new environment produced reactions of a conflict between fear and curiosity, demonstrated a tendency toward behavioral approach and avoidance, respectively [25,35]. Initial work with an elevated plus-maze, similar to that currently exists, was developed by Handley and Mithani [36], as a model for the study of anxiety. This maze, after suffering a change that gave the existing form today, was validated behavioral, physiological and pharmacologically to rats [23] and to mice [37]. Other studies have linked the observations made in this test with anxiolytic drugs [38,39]. In this test, nerol increased the number of entries and time spent in open arms. Nerol may have some activity at the central level, a similar result was obtained with other monoterpenes such as borneol [40], isopulegol [41] and α,β- epoxy-carvone [42].
In light-dark test, we found the natural aversion that animals have to the ambient light. This model allows us to assess levels of anxiety with time spent in the illuminated environment. When an animal shows a decrease in time spent in the illuminated environment, it is said that this animal is with high levels of anxiety. However, the administration of anxiolytic drugs induce increased activity of the animals in the room clear [43,44]. The results show that nerol was able to increase the residence time in the light room similar to other compounds isolated that had anxiolytic effect by increasing this parameter [45,46]. Moreover, both in the acute treatment [47], and in chronic treatment [48], these substances prevented the escape latency without altering escape Tmaze test, changing the transition between light and dark compartments of the light/dark test and time spent on the course, therefore having anxiolytic, similar behavior adopted by animals treated with nerol.
The lack of coordination in the rota rod test is characteristic of a drug that reduces the CNS activity, such as neuroleptics, anxiolytics, sedatives and hypnotics [49]. The rotating bar is able to detect myorelaxant activity of test compound as well as in identifying minimal neurological damage such as ataxia, sedation, hyperexcitability, and since normal animals can be kept for a long period of time on the swivel bar [50]. Animals treated with nerol didn’t modify their behavior during the test, suggesting no myorrelaxant activity. Aqueous extract of Orbignya phalerata Mart, widely used in Brazilian flok medicine, also did not significantly change the motor activity of animals when compared with the control group, up to 24 h after administration and did not alter the remaining time of the animals on the rota-rod apparatus [51].
5. CONCLUSION
This work evidence that nerol possesses a possible anxiolytic effect in mice. The monoterpene will can be used, after other studies, in the treatment of anxiety, contributing to the improvement of the harms to health of people in this context.
6. ACKNOWLEDGEMENTS
This work was funded by the National Council for Scientific and Technological Development (CNPq). Rivelilson Mendes de Freitas is a fellow of CNPq.
REFERENCES
- Bernhoft, A. (2010) Bioactive compounds in plants— Benefits and risks for man and animals. The Norwegian Academy of Science and Letters, Oslo.
- Calixto, J.B. (2000) Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Brazilian Journal of Medical and Biological Research, 33, 179-189. doi:10.1590/S0100-879X2000000200004
- Patil, B.S., Jayaprakasha, G., Murthy, K.N.C. and Vikram, A. (2009) Bioactive compounds: Historical perspectives, opportunities, and challenges agric. Food Chemistry, 57, 8142-8160. doi:10.1021/jf9000132
- Turner, G.W. and Croteau, R. (2004) Organization of Monoterpene biosynthesis in mentha. Immunocytochemical localizations of geranyl diphosphate synthase, limonene6-hydroxylase, isopiperitenol dehydrogenase, and pulegone reductase. Plant Physiology, 136, 4215-4227. doi:10.1104/pp.104.050229
- Costa, J.G.M., Rodrigues, F.F.G., Angélico, E.C., Silva, M.R., Mota, M.L., Santos, N.K.A., Cardoso, A.L.H. and Lemos, T.L.G. (2005) Estudo químico-biológico dos óleos essenciais de Hyptis martiusii, Lippia sidoides e Syzigium aromaticum frente às larvas do Aedes aegypti. Revista Brasileira de Farmacognosia, 15, 304-309. doi:10.1590/S0102-695X2005000400008
- Karkabounas, S., Kostoula, O.K., Daskalou, T., Veltsistas, P., Karamouzis, et al. (2006) Anticarcinogenic and antiplatelet effects of carvacrol. Experimental Oncology, 28, 121-125.
- Garcia, R., Alves, E.S.S., Santos, M.P., Aquije, G.M.F.V., Fernandes, A.R.R., Santos, R.B., Ventura, J.A. and Fernandes, P.M.B., (2008) Antimicrobial activity and potential use of monoterpenes as tropical fruits preservatives. Brazilian Journal of Microbiology, 39, 163-168. doi:10.1590/S1517-83822008000100032
- Singh, P., Shukla, R., Prakash, B., Kumar, A., Singh, S., Mishraa, P.K. and Dubey, N.K. (2010) Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food and Chemical Toxicology, 48, 1734-1740. doi:10.1016/j.fct.2010.04.001
- Consolini, A.E., Berardi, A., Rosella, M.A. and Volonté, M. (2011) Antispasmodic effects of Aloysia polystachya and A. gratissima tinctures and extracts are due to noncompetitive inhibition of intestinal contractility induced by acethylcholine and calcium. Revista Brasileira de Farmacognosia, 21, 889-900. doi:10.1590/S0102-695X2011005000137
- Rocha, M.L. (2010) Estudo da atividade antinociceptiva e anti-inflamatória do monoterpeno α,β-epoxi-carvona e seu efeito sobre a neurotransmissão glutamatérgica. Universidade Federal da Paraíba, João Pessoa.
- Santos, M.R.V., Moreira, F.V., Fraga, B.P., De Sousa, D.P., Bonjardim, L.R. and Quintans-Junior, L.J. (2011) Cardiovascular effects of monoterpenes: A review. Brazilian Journal of Pharmacognosy, 21, 764-771.
- Viana, G.S., Vale, T.G., Silva, C.M. and Matos, F.J. (2000) Anticonvulsant activity of essential oils and active principles from chemotypes of Lippia alba (MILL.) NE Brown. Biological and Pharmaceutical Bulletin, 23, 1314- 1317. doi:10.1248/bpb.23.1314
- Brum, L.F.S., Emanuelli, T., Souza, D.O. and Elisabetsky, E. (2001) Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochemical Research, 26, 191-194. doi:10.1023/A:1010904214482
- De Sousa, D.P., Gonçalves, J.C.R., Quintans-Júnior, L., Cruz, J.S., Araújo, D.A.M. and Almeida, R.N. (2006) Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neuroscience Letters, 401, 231-235. doi:10.1016/j.neulet.2006.03.030
- Trombetta, D., Castelli, F., Sarpietro, M.G., Venuti, V., Cristani, M., Daniele, C., Saija, A., Mazzanti, A. and Bisignano, G. (2005) Mechanisms of antibacterial action of three monoterpenes. Antimicrobial Agents and Chemotherapy, 49, 2474-2478. doi:10.1128/AAC.49.6.2474-2478.2005
- Amaral, J.F., Silva, M.I.G., Neto, M.R.A., Neto, P.F.T., et al. (2007) Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biological and Pharmaceutical Bulletin, 30, 1217-1220. doi:10.1248/bpb.30.1217
- Lapczynski, A., Foxenberg, R.J., Bhatia, S.P., Letizia, C.S. and Api, A.M. (2008) Fragrance material review on nerol. Food and Chemical Toxicology, 46, 241-244. doi:10.1016/j.fct.2008.06.062
- Kennedy, D.O., Wake, G., Savelev, S., et al. (2003) Modulation of mood and cognitive performance following acute administration of single doses of Melissa officenalis (lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology, 28, 1871-1881. doi:10.1038/sj.npp.1300230
- Allahverdiyev, A., Duran, N., Ozguven M. and Koltas, S. (2004) Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine, 11, 657-661. doi:10.1016/j.phymed.2003.07.014
- Topal, U., Sasaki, M., Goto, M. and Otles, S. (2008) Chemical compositions and antioxidant properties of essential oils from nine species of Turkish plants obtained by supercritical carbon dioxide extraction and steam distillation. International Journal of Food Sciences and Nutrition, 59, 619-634. doi:10.1080/09637480701553816
- Escobar, P., Leal, S.M., Herrera, L.V., Martinez, J.R. and Stashenko, E. (2010) Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Memórias do Instituto Oswaldo Cruz, 105, 184-190. doi:10.1590/S0074-02762010000200013
- Archer, J. (1973) Tests for emotionality in rats and mice: A review. Animal Behavior, 21, 205-235.
- Pellow, S., Chopin, P., File S.E. and Briley, M. (1985) Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods, 14, 149-167. doi:10.1016/0165-0270(85)90031-7
- Lister, R.G. (1987) The use of a plus-maze to measure anxiety in the mouse. Psichopharmacology, 92, 180-185.
- Montgomery, K.C. and Monkman, J.A. (1955) The relation between fear and exploratory behavior. Journal of Comparative and Physiological Psychology, 48, 132-136. doi:10.1037/h0048596
- Crawley, J.N. and Goodwin, F.K. (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology Biochemistry and Behavior, 13, 167-170. doi:10.1016/0091-3057(80)90067-2
- Dunham, N.W. and Miya, T.S. (1957) A note on simple apparatus for detecting neurological deficit in rats and mice. Journal of Pharmaceutical Science, 46, 208-209. doi:10.1002/jps.3030460322
- Umezu, T., Sakata, A. and Ito, H. (2001) Ambulationpromoting effect of peppermint oil and identification of its active constituents. Pharmacology Biochemistry Behavior, 69, 383-390. doi:10.1016/S0091-3057(01)00543-3
- Farhat, G.N., Affara, N.I. and Gali-Muhtasib, H.U. (2001) Seasonal changes in the composition of the essential oil extract of East Mediterranean sage (Salvia libanotica) and its toxicity in mice. Toxicon, 39, 1601-1605. doi:10.1016/S0041-0101(01)00143-X
- Almeida, A.A.C., Costa, J.P., Carvalho, R.B.F., De Sousa, D.P. and Freitas, R.M. (2012) Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Research, 1448, 56-62. doi:10.1016/j.brainres.2012.01.070
- Siegel, P.S. (1946) A simple electronic device for the measurement of gross bodily activity of small animals. Journal of Psychology, 21, 227-236. doi:10.1080/00223980.1946.9917283
- Fernández, A., Álvarez, A., García, D. and Sáenz, T. (2001) Antiinflammatory effect of Pimenta racemosa var. ozua and isolation of the triterpene lupeol. Ⅱ Farmaco, 56, 335-338. doi:10.1016/S0014-827X(01)01080-1
- Argal, A. and Pathak, A.K. (2006) CNS activity of Calotropis gigantea roots. Journal of Ethnopharmacology, 106, 142-145. doi:10.1016/j.jep.2005.12.024
- Santos, F.A., Rao, V.S.N. and Silveira, E.R., (1996) Studies on the neuropharmacological effects of Psidium guyanensis and Psidium pholianum essential oils. Phytotherapy Research, 10, 655-658. doi:10.1002/(SICI)1099-1573(199612)10:8<655::AID-PTR933>3.0.CO;2-X
- Montgomery, K.C. (1955) The relation between fear induced by novel stimulation and exploratory behavior. Journal of comparative and Physiological Psychology, 48, 254-260. doi:10.1037/h0043788
- Handley, S.L. and Mithani, S. (1984) Effects of alphaadrenoceptor agonists and antagonists in a maze-explomration model of “fear”—motivated behavior. NaunynSchmiedeberg’s Archives of Pharmacology, 327, 1-5. doi:10.1007/BF00504983
- Lister, R.G. (1990) Ethological-based animal models of anxiety disorders. Pharmacological Therapy, 46, 321-340.
- Kharade, S.M., Khetmar, S.S., Desai, P.S., Lokhande, R.S. and Patil, S.S. (2011) Evaluation of anxiolytic activeity of Carum copticum by using elevated plus maze and open field method. International Research Journal of Pharmacy, 2, 165-168. doi:10.1016/0163-7258(90)90021-S
- Ajayi, S.A. and Nwoha, P.U. (2011) “The use of elevated plus maze to study the effects of aqueous extract of Garcinia kola (Linn) on the anxiety status of malnourished mice. Eletronic Journal of Biomedicine, 2, 63-67.
- Granger, R.E., Campbell, E.L. and Johnston, G.A.R. (2005) (+)- and (−)-borneol: Efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochemical Pharmacology, 69, 1101- 1111. doi:10.1016/j.bcp.2005.01.002
- Silva, M.I., Silva, M.A., et al. (2009) Effects of isopulegol on pentylenetetrazol-induced convulsions in mice. Fitoterapia, 80, 506-513. doi:10.1016/j.fitote.2009.06.011
- De Sousa, D.P., Nóbrega, F.F.F., Claudino, F.S., Almeida, R.N., Leite, J.R. and Mattei, R. (2007) Pharmacological effects of the monoterpene α,β-epoxy-carvone in mice. Revista Brasileira de Farmacognosia, 17, 170-175. doi:10.1590/S0102-695X2007000200006
- Cross, J.H., Vieira, P., Miranda, H.P., Cerqueira, M., et al. (2007) Latent schistosomiasis in portuguese soldiers, Military medicine, 172, 144-146.
- Bourin, M. and Hascoët, M. (2003) The mouse light/dark box test. European Journal of Pharmacology, 463, 55-65. doi:10.1016/S0014-2999(03)01274-3
- Flausino, O.A.J., Pereira, A.M., da Silva, V.B. and Nunes-de-Souza, R.L. (2007) Effects of erythrinian alkaloids isolated from Erythrina mulungu (Papilionaceae) in mice submitted to animal models of anxiety. Biological Pharmacological Bulletin, 30, 375-378. doi:10.1248/bpb.30.375
- Flausino, O.A.J., Santos, L.A., Verli, H., Pereira, A.M., Bolzani, V.S. and Nunes-De-Souza, R.L. (2007) Anxiolytic effects of erythrinian alkaloids from Erythrina mulungu. Journal of Natural Products, 71, 48-53. doi:10.1021/np060254j
- Onusic, G.M., Nogueira, R.L., Pereira, A.M. and Viana, M.B. (2002) Effect of acute treatment with a water-alcohol extract of Erythrina mulungu on anxiety-related responses in rats. Brazilian Journal of Medical and Biological Research, 35, 473-477. doi:10.1590/S0100-879X2002000400011
- Onusic, G.M., Nogueira, R.L., Pereira, A.M., Flausino, O.A.J. and Viana, M.B. (2003) Effects of chronic treatment with a water-alcohol extract from Erythrina mulungu on anxiety-related responses in rats. Biological Pharmacological Bulletin, 26, 1538-1542. doi:10.1248/bpb.26.1538
- Sen, T. and Chaudhuri, K.N. (1992,) Studies on the neuropharmacological aspects of Pluchea indica root extract. Phytotherapy Research, 6, 175-179. doi:10.1002/ptr.2650060402
- Swinyard, E.A. and Kupperberg, H.J. (1985) Antiepileptic drugs: Detection, quantification and evaluation. Federation Proceedings, 44, 2629-2633.
- Silva, A.P.S., Cerqueira, G.S., Nunes, L.C.C. and Freitas, R.M. (2012) Effects of an aqueous extract of Orbignya phaletara Mart on locomotor activity and motor coordination in mice and as antioxidant in vitro. Die Pharmazie, 67, 260-263.
NOTES
*Corresponding author.

