Open Journal of Ecology
Vol.07 No.03(2017), Article ID:75026,11 pages
10.4236/oje.2017.73016
Animal and Plant Species in Antagonistic and Mutualistic Networks, a Multispecies Mean Field Model
Gilberto Corso1*, Claudia P. T. Cruz2, Adriana M. de Almeida3
1Departamento de Biofisica e Farmacologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte, Natal, Brazil
2Escola de Engenharia e Ciências Exatas, Universidade Potiguar, Natal, Brazil
3Departmento de Ecologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte, Natal, Brazil

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 8, 2017; Accepted: March 27, 2017; Published: March 30, 2017
ABSTRACT
We construct a dynamic system model of a web of 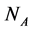 animal species interacting with
animal species interacting with  plant species using a system of coupled differential equations. The model has a parameter which represents the effect of animal on plant species that can be positive (mutualistic interaction) or negative (antagonistic interaction). We work a Multispecies Mean Field Model MMFM in which the full set of
plant species using a system of coupled differential equations. The model has a parameter which represents the effect of animal on plant species that can be positive (mutualistic interaction) or negative (antagonistic interaction). We work a Multispecies Mean Field Model MMFM in which the full set of 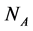 and
and 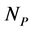 variables are reduced to a couple of an average animal and plant species. We study the conditions for existence of the MMFM and relate the result to the difference between animal and plant species in the network. We compare our results with empirical data from pollinators (mutualistic) and herbivorous insect (antagonistic) networks. We conclude by combining analytical and empirical work that antagonistic networks present fewer animal species in relation to plant species than mutualistic ones.
variables are reduced to a couple of an average animal and plant species. We study the conditions for existence of the MMFM and relate the result to the difference between animal and plant species in the network. We compare our results with empirical data from pollinators (mutualistic) and herbivorous insect (antagonistic) networks. We conclude by combining analytical and empirical work that antagonistic networks present fewer animal species in relation to plant species than mutualistic ones.
Keywords:
Ecological Interactions, Stability Complexity, Plant-Animals Ratio, Pollination, Herbivory

1. Introduction
Since the seminal paper [1] , the stability complexity debate has evolved to a hot topic in theoretical ecology [2] [3] . From a mathematical perspective, the sta- bility complexity problem can be posed in at least two different contexts: firstly, the traditional approach of population dynamics, a set of coupled differential equations representing the density of a set of many interacting species [1] [4] [5] [6] . In this framework, the main idea is to work the stability of the fixed points of the equations describing the time evolution of the populations [7] . On the other side, the stability complexity problem can be formulated using a more contemporary framework, namely the network theory [8] [9] [10] . In this approach, the focus is on the number, distribution, or assortativity of interac- tions, in one word: the pattern of connections in the network [11] [12] .
In this work, we explore both mathematical approaches. Firstly, the coupled dynamic system is a flexible schema that permits a modeling of complex systems using phenomenological parameters such as the intrinsic growth rate of a popu- lation, its carrying capacity, or the interaction strength among species. All cited parameters can, in principle, be empirically estimated [13] . In addition, most empirical data available to compare with dynamic system models are records of food webs or pollinator networks which indicate the presence of interaction among a set of species. Confident time series records of populations interacting in a community are not available, but network inventories of interaction among species are common in the literature. We cite as examples web interaction inven- tories [14] , pollinators flower networks [15] host parasite networks [16] , and herbivory networks [17] .
In this manuscript, we work with a dynamic multi-species model representing two different situations: mutualistic and antagonistic interactions. Pollination webs are formed by species which both profit from the interaction and then should be favored by adaptive forces. On the other hand, Herbivory (trophic) webs are formed by bipartite webs in which only herbivores profit. This is dif- ferently from pollination where in this case the plants are injured in the inter- action and adaptive forces involved in the interaction are opposite to what found in pollination. We consider a set of 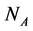 animal species and a set of
animal species and a set of 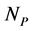 plant species that interact with each other, but do not interact among themselves. The objective of this paper is to construct a Multispecies Mean Field Model MMFM reducing the original
plant species that interact with each other, but do not interact among themselves. The objective of this paper is to construct a Multispecies Mean Field Model MMFM reducing the original 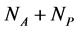 equations to two equations that represent the average animal and plant species. In this approach, the set of coupled dynamic equations may have an intricate topology with many fixed points, limit cycles, strange attractors, or even chaos because of the intrinsic non-linearity [18] . The reduced MMFM, as we shall see, has just one single fixed point of interest. Despite the strong simplification in this methodology, we extract useful hints about network properties, espeacially regarding the network pattern related to the stability of interacting systems, a key point in the complexity stability debate. We are aware that the MMFM that we present here lacks a rigorous mathe- matical foundation. However, we persist in this theoretic field because of the promising results we have found until now.
equations to two equations that represent the average animal and plant species. In this approach, the set of coupled dynamic equations may have an intricate topology with many fixed points, limit cycles, strange attractors, or even chaos because of the intrinsic non-linearity [18] . The reduced MMFM, as we shall see, has just one single fixed point of interest. Despite the strong simplification in this methodology, we extract useful hints about network properties, espeacially regarding the network pattern related to the stability of interacting systems, a key point in the complexity stability debate. We are aware that the MMFM that we present here lacks a rigorous mathe- matical foundation. However, we persist in this theoretic field because of the promising results we have found until now.
In this paper, we consider a MMFM of a dynamic system of coupled Lotka Volterra like equations. The MMFM is a simplified two-dimension version of a multiple dimension system. The Lotka Volterra equations we deal with in our manuscript correspond to linear functional responses in the interaction term [19] [20] , while we discuss more involved non-linear responses in the text. The analysis of the conditions for existence of the MMFM reveals some properties of the complex many dimensional dynamic network underlying the complex dyna- mics.
The main result of the cited paper of May [1] is that ecological networks are sparse. This conclusion is somewhat unexpected, we cite MacArthur that beli- eved that large number of interaction will stabilize networks and not the oppo- site [21] . Our work also uses a coupled of differential equations to model ecolo- gical webs. In contrast, we use different technique and our main findings con- cern differences between antagonistic and mutualistic networks. Our manuscript shows a theoretical model that explains the difference between the number of plant and animals in herbivorous and pollinator networks. The rest of the paper is organized as follows: in Section 2, we present the full multiple dimension dynamic system used to describe the model and the associated MMFM. In Section 3 we separately compare the existing conditions for mutualistic and antagonistic networks with empirical data. Finally, we present our main findings in Section 4 and discuss the number of animal and plants in the community.
2. Methodology
This section is divided in two parts. Initially, we expose the biological data used to test the methodology, namely the set of binary and quantitative interaction networks. The second part of the methodology is devoted to describe the mean field model that is in the center of our analysis.
2.1. The Biological Data Set
In this work, we employed a set of quantitative webs extracted from the lite- rature. We use a total of 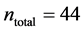 quantitative webs divided into two catego- ries: pollinators
quantitative webs divided into two catego- ries: pollinators 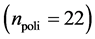 and herbivory
and herbivory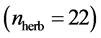 . Pollinator webs are a typical example of a mutualistic networks formed by flowering plants and polli- nator animal species, [22] [23] . Herbivory webs are a well studied example of antagonistic interaction, these networks are formed by herbivorous insect spe- cies and their respective host plants [24] .
. Pollinator webs are a typical example of a mutualistic networks formed by flowering plants and polli- nator animal species, [22] [23] . Herbivory webs are a well studied example of antagonistic interaction, these networks are formed by herbivorous insect spe- cies and their respective host plants [24] .
The empirical objects used in this work are ecological interaction webs which essentially are matrices of size 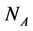 versus
versus 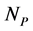 corresponding to two groups of interacting animal and plant species, respectively. In the case of an interaction between species
corresponding to two groups of interacting animal and plant species, respectively. In the case of an interaction between species 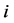 of the first set and species
of the first set and species 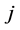 of the second the matrix element is
of the second the matrix element is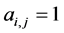 , otherwise
, otherwise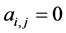 , for the binary case. In the more general case of quantitative matrices
, for the binary case. In the more general case of quantitative matrices 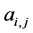 can assume any positive value. The connec- tivity of an animal species is defined as
can assume any positive value. The connec- tivity of an animal species is defined as 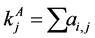 and for a plant species
and for a plant species


species

2.2. The Mean Field Approximation
We employ a model that is a close extension of the multispecies model develo- ped in the reference [26] . We extend the previous work to a model that encom- passes both mutualistic and antagonistic interactions. The theory is based on a two-species model proposed in the reference [2] for the mutualistic case but it is even older in the antagonist situation [27] . The evolution of the animal 


where 














We adopt a mean field approach that simplifies the model allowing further analytical insight. The reference [26] used a similar reduction technique to approximate modeling. Indeed, such reductions are common in theoretical eco- logy [27] . We assume that all plant species are equivalent, which means






Moreover, we assume in the mean field approach that



We perform the equations’ average for the set of animal and plant species in
the following way. We add all 
by






Finally, we use 


We are aware that the mean field statement employed in the analytical treat- ment that reduces the dimension of the system from 




There are four solutions for this pair of coupled system: the trivial null solu- tion






We note that any solution is biologically feasible only for 




2.3. Discussion of a Nonlinear Model
We may also consider more sophisticated mutualistic models that take into account a non-linear saturation process as in the reference [19] [20] . We remark that the nonlinear model, as any ordinary differential equation model, is a mean field approach; to expand our methodology to a non mean field regime we would have to explicitly include the space by using differential partial equations or cellular automata models [12] . The linear mutualistic model presents an exp- losive behavior that can be solved using saturation in the interaction response between animal-plant. In reference [20] the mutualistic interaction for one animal and one plant species 


However, for low handling time
3. Condition for Existence of the Mean Field Model
In our work, we study network properties and analyze what the MMFM can tell about network patterns that produce viable communities. In this way, we do not focus on species parameter








We distinguish two situations in the study: the mutualistic interactions and the antagonistic interactions. The first is characterized by a positive effect of the plant animal encounter on plant population growth 

3.1. Mutualism
The condition for non-trivial existence of the system formed by Equations (7) and (8) assumes that both 


This inequality imposes that the quantities







3.2. Antagonism
In the case of antagonistic interactions, we perform the transformation


This new condition imposed to antagonistic systems implies that 

3.3. Data Comparison
The condition for existence (11) which is exclusive of antagonistic networks claims that 
The relative difference between animals and plants is presented in Figure 1. In this picture we show a bar-plot comparing the ratio 






4. Discussion
In this paper, we work a multidimensional dynamic model that encompasses
Figure 1. The figure shows the ratio

mutualistic and antagonistic interactions in a single framework. The MMFM is based on reducing a set of 
The essence of Mean Field approach in theoretical science consists in erasing individual differences to capture overall features of the system. In a mean field approximation, the interaction details among individuals in a network are less important than the average properties of the system dynamics. The challenge of the Mean Field Theory consists in not taking into account superfluous heter- ogeneities of the problem, but instead focusing on overall aspects of the pheno- menon. The objective of the Mean Field theory is neither to give answers to in- dividual differences, nor to look at small differences in the system. In this pers- pective, the MMFM still gives answers that can be empirically verified, despite obvious limitations and strong reductionism. The dependence asymmetry and the sparsity of the networks are positive results of the Mean Field approach and have already been discussed in the literature [26] . The difference between animal and plant species has not previously explored, and our work is the first to point out this theoretical result of the MMFM. In summary, the Mean Field Theory should be judged a posteriori. If the theoretical model gives a positive answer to empirical data, only then can it be taken seriously.
The idea that antagonistic interactions are more modular like, whereas mu- tualistic ones are more nested was extensively explored in the literature [15] [30] . The antagonistic relations tend to follow an arms race co-evolutionary strategy that produces a formation of cliques in the network, while the mutua- listic interactions point to a generalist plus sub-generalist co-evolutionary strate- gy [31] . Our manuscript works out another, not so explored, difference between antagonistic and mutualistic networks: the number of animal and plants in their composition. In fact, an alternative and appealing title for our paper could be: “Why pollinator networks have more animals than plants in their structures than herbivorous networks?” This paper essays an explanation for this question, in fact, this is the objective of our work.
It is well known that biodiversity of animal species is larger than plant species [32] [33] [34] . Following this simple rule, we should generally expect that the number of animal species should surpass the number of plant species in intera- cting networks. This expectation could be used as a null model for plant and animal species occupancy in an interacting network. Our theoretical results suggest that it is not valid for antagonistic interaction networks, which is in agreement with empirical results. In this way, as stated in Figure 1, pollinator networks are closer to a null model of animal and plant occupancy than antag- onistic networks which present a strong misbalance in their composition.
Finally, we believe that this work is important for the discussion of the com- plexity stability debate. We are conscious of the strong simplification of our mathematical framework. Initially we propose a model of coupled differential equations that is already a mean field approach because it does not take into account the spatial structure of the landscape. Over this model we proceed to perform a further simplification, reducing the number of variables from 
Acknowledgements
Financial support to Gilberto Corso from CNPq (Conselho Nacional de Desen- volvimento Científico e Tecnológico) is acknowledged.
Cite this paper
Corso, G., Cruz, C.P.T. and de Almeida, A.M. (2017) Animal and Plant Species in Antagonistic and Mutualistic Networks, a Multispecies Mean Field Model. Open Journal of Ecology, 7, 211-221. https://doi.org/10.4236/oje.2017.73016
References
- 1. May, R.M. (1972) Will a Large Complex System Be Stable? Nature, 238, 413-414.
https://doi.org/10.1038/238413a0 - 2. May, R.M. (1974) Stability and Complexity in Model Ecosystems. Princeton University Press, Princeton.
- 3. Bastolla, U., Fortuna, M.A., Pascual-García, A., Ferrera, A., Luque, B. and Bascompte, J. (2009) The Architecture of Mutualistic Networks Minimizes Competition and Increases Biodiversity. Nature, 458, 1018-1020.
https://doi.org/10.1038/nature07950 - 4. Case, T.J. (2000) An Illustrated Guide to Theoretical Ecology. Oxford University Press, Oxford.
- 5. Bastolla, U., Lässig, M., Manrubia, S.C. and Valleriani, A. (2005) Biodiversity in Model Ecosystems, I: Coexistence Conditions for Competing Species. Journal of Theoretical Biology, 235, 521-530.
https://doi.org/10.1016/j.jtbi.2005.02.005 - 6. James, A., Pitchford, J.W. and Plank, M.J. (2012) Disentangling Nestedness Disentangled. Nature, 487, 227-230.
https://doi.org/10.1038/nature11214 - 7. Lässig, M., Bastolla, U., Manrubia, S.C. and Valleriani, A. (2001) Shape of Ecological Networks. Physical Review Letters, 86, 4418-4421.
https://doi.org/10.1103/PhysRevLett.86.4418 - 8. Strogatz, S.H. (2001) Exploring Complex Networks. Nature, 410, 268-276.
https://doi.org/10.1038/35065725 - 9. Newman, M.E.J. (2003) The Structure and Function of Complex Networks. SIAM Review, 45, 167-256.
https://doi.org/10.1137/S003614450342480 - 10. Ings, T.C., Montoya, J.M., Bascompte, J., Blüthgen, N., Brown, L., Dormann, C.F., et al. (2009) Ecological Networks—Beyond Food Webs. Journal of Animal Ecology, 78, 253-269.
https://doi.org/10.1111/j.1365-2656.2008.01460.x - 11. Albert, R. and Barabási, A.-L. (2002) Statistical Mechanics of Complex Networks. Reviews of Modern Physics, 74, 47-98.
https://doi.org/10.1103/RevModPhys.74.47 - 12. Solé, R.V. and Bascompte, J. (2006) Self-Organization in Complex Ecosystems. Princeton University Press, Princeton.
- 13. Bascompte, J. (2009) Disentangling the Web of Life. Science, 325, 416-419.
https://doi.org/10.1126/science.1170749 - 14. Guimarães, P.R., Raimundo, R.L.G. and Cagnolo, L. (2015) Interaction Web Database. National Center for Ecological Analysis and Synthesis.
https://www.nceas.ucsb.edu/interactionweb/index.html - 15. Bascompte, J. and Jordano, P. (2013) Mutualistic Networks. Princeton University Press, Princeton.
https://doi.org/10.1515/9781400848720 - 16. Vázquez, D.P., Poulin, R., Krasnov, B.R. and Shenbrot, G.I. (2005) Species Abundance and the Distribution of Specialization in HOST-PARASITE Interaction Networks. Journal of Animal Ecology, 74, 946-955.
https://doi.org/10.1111/j.1365-2656.2005.00992.x - 17. Lewinsohn, T.M., Novotny, V. and Basset, Y. (2005) Insects on Plants: Diversity on Herbivore Assemblages Revisited. Annual Review of Ecology, Evolution, and Systematics, 36, 597-620.
https://doi.org/10.1146/annurev.ecolsys.36.091704.175520 - 18. Alligood, K.T., Sauer, T.D. and Yorke, J.A. (1997) Chaos: An Introduction to Dynamical Systems. Springer, New York.
https://doi.org/10.1007/978-3-642-59281-2 - 19. Holland, J.N., DeAngelis, D.L. and Bronstein, J.L. (2002) Population Dynamics and Mutualism: Functional Responses of Benefits and Costs. The American Naturalist, 159, 231-244.
https://doi.org/10.1086/338510 - 20. Holland, J.N., Okuyama, T. and DeAngelis, D.L. (2006) Comment on “Asymmetric Coevolutionary Networks Facilitate Biodiversity Maintenance”. Science, 313, 1887.
https://doi.org/10.1126/science.1129547 - 21. MacArthur, R.H. (1972) Geographical Ecology. Princeton University Press, Princeton.
- 22. Jordano, P. (1987) Patterns of Mutualistic Interactions in Pollination and Seed Dispersal: Connectance, Dependence Asymmetries, and Coevolution. American Naturalist, 129, 657-677.
https://doi.org/10.1086/284665 - 23. Memmott, J. (1999) The Structure of a Plant-Pollinator Food Web. Ecology Letters, 2, 276-280.
https://doi.org/10.1046/j.1461-0248.1999.00087.x - 24. Nascimento, A.R., Neto, M.A., Almeida, A.M., Fonseca, C.R., Lewinsohn, T.M. and Penteado-Dias, A. (2014) Parasitoid Wasps in Flower Heads of Asteraceae in the Brazilian Cerrado: Taxonomical Composition and Determinants of Diversity. Neotropical Entomology, 43, 298-306.
https://doi.org/10.1007/s13744-014-0218-9 - 25. Corso, G., Cruz, C.P.T., Pinto, M.P., de Almeida, A.M. and Lewinsohn, T.M. (2015) Binary versus Weighted Interaction Networks. Ecological Complexity, 23, 68-72.
https://doi.org/10.1016/j.ecocom.2015.04.003 - 26. Bascompte, J., Jordano, P. and Olesen, J.M. (2006) Asymmetric Coevolutionary Net-works Facilitate Biodiversity Maintenance. Science, 312, 431-433.
https://doi.org/10.1126/science.1123412 - 27. Murray, J.D. (2002) Mathematical Biology: An Introduction. Springer-Verlag, Berlin.
- 28. Goldenfeld, N. (1992) Lectures on Phase Transitions and the Renormalization Group (Frontiers in Physics). Addison-Wesley, Boston.
- 29. Marro, J. and Dickmann, R. (1999) Nonequilibrium Phase Transitions in Lattice Models. Cambridge University Press, Cambridge.
https://doi.org/10.1017/CBO9780511524288 - 30. Thébault, E. and Fontaine, C. (2008) Does Asymmetric Specialisation Differ between Mutualistic and Trophic Networks? Oikos, 117, 555-563.
https://doi.org/10.1111/j.0030-1299.2008.16485.x - 31. Thébault, E. and Fontaine, C. (2010) Stability of Ecological Communities and the Architecture of Mutualistic and Trophic Networks. Science, 329, 853-856.
https://doi.org/10.1126/science.1188321 - 32. May, R.M. and Beverton, R.J.H. (1990) How Many Species? Philosophical Transactions of the Royal Society B, 330, 293-304.
https://doi.org/10.1098/rstb.1990.0200 - 33. Wilson, E.O. (1999) The Diversity of Life. W. W. Norton & Company, New York.
- 34. Mora, C., Tittensor, D.P., Adl, S., Simpson, A.G.B. and Worm, B. (2011) How Many Species Are There on Earth and in the Ocean? PLoS ONE, 9, e1001127.
https://doi.org/10.1371/journal.pbio.1001127


