Advances in Biological Chemistry
Vol.06 No.03(2016), Article ID:67480,12 pages
10.4236/abc.2016.63010
The Novel Pyruvated Glucogalactan Sulfate Isolated from the Red Seaweed, Hypnea pannosa
Masakuni Tako1,2*, Rintaro Ohtoshi1, Kazutaka Kinjyo1, Shuntoku Uechi1
1Department of Subtropical Bioscience and Biotechnology, University of the Ryukyus, Okinawa, Japan
2Health and Longevity Research Laboratory, Integrated Innovation Research Center, University of the Ryukyus, Okinawa, Japan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 12 January 2016; accepted 17 June 2016; published 20 June 2016
ABSTRACT
The polysaccharide was isolated from Hypnea pannosa which was grown in Okinawa, Japan. The yield of the polysaccharide was 17.2%, and the total carbohydrates, pyruvic acid, sulfuric acid and ash contents were 55.2%, 3.8%, 35.2% and 24.3%, respectively. 3,6-Anhydro-α-D-galactose, β-D- galactose, α-D-galactose and D-glucose were identified by liquid and thin-layer chromatography. Fourier transform infrared (FTIR) spectra of the polysaccharide resembled that of ι-carrageenan. From the 1H- and 13C-NMR spectra, 1,3-linked β-D-galactose, 1,4-linked anhydro-α-D-galactose, 1,4- linked α-D-galactose, 1,4-linked β-D-glucose and pyruvic acid (carboxyl acetal, methyl proton and methyl carbon) were assigned. Methylation analysis revealed terminal D-galactose 0.1 mol), 1,4-linked D-glucose (1.0 mol) and 1,2,3,4,6-linked D-galactose (3.7 mol) for native polysaccharide, and terminal D-galactose, 1,4-linked D-galactose (1.9 mol), 1,4-linked D-glucose (1.0 mol), 1,3- linked D-galactose (1.7 mol), and 1,3,4,6-linked D-galactose (0.3 mol) which substituted with pyruvate group at 4 and 6 positions for desulfated polysaccharide. The polysaccharide was the novel pyruvated glucogalactan sulfate, the structure of which was proposed.
Keywords:
Hypnea pannosa, Pyruvated Glucogalactan Sulfate, 1H- and 13C-NMR Analysis, Methylation Analysis, Chemical Structure

1. Introduction
One of the authors, Tako, has isolated agar [1] , methylated agar [2] , fucoidan [3] - [6] , alginate [5] [7] , κ-carra- geenan [8] , ι-carrageenan [9] , galactomannan [10] [11] , pectin [12] - [14] , rhamnan sulfate [15] and ulvan [16] from the subtropical biomasses grown in Okinawa Islands, Japan. Recently, we discovered the novel deoxy- D-altrose [17] [18] and α-glucan [19] from the edible mushrooms. Especially, we isolated the novel acetylfucoidan from commercially cultured Cladosiphon okamuranus [5] and patented [20] . The acetyl fucoidan exhibited some biological activities, such as antitumor [21] and immune-enhancing abilities [22] . An over-sulfated acetylfucoidan, the sulfate content of which was 32.8%, showed a significant antitumor activity in vitro [21] . The results suggest that the over-sulfated acetyl fucoidan is applicable as an anticancer drug. The acetylfucoidan is now used as a supplement in health food, food and cosmetic industries in the world.
On the other hand, Tako discussed the structure-function relationship and proposed gelation mechanism of κ-carrageenan [23] - [25] , ι-carrageenan [26] [27] , agarose [28] , gellan gum [29] [30] , amylose [31] [32] , alginate [33] [34] and deacetylated rhamsan gum [35] . We also proposed gelatinization and retrogradation mechanism of amylopectin [36] - [38] and starch [39] - [44] . Tako realizes that there are some basic rules in gel-formation process including water molecules in principle [45] - [47] .
Carrageenans are water-extractable sulfated galactans from red algae Rhodophyta. They are essentially linear polymers composed of repeating disaccharide units of β-(1→3)-linked D-galactose and α-(1→4)-linked 3,6-an- hydro-D-galactose residues. Sulfate groups substituted at C-4 position of D-galactose residue for κ-carrageenan and, C-4 and C-2 position of D-galactose and 3,6-anhydro-D-galactose residue for ι-carrageenan. We report herein the isolation and structural characterization of the pyruvated glucogalactan sulfate from Hypnea pannosa which belong to red seaweed and grow in the coast of Okinawa Island, Japan.
2. Materials and Methods
2.1. Materials
Hypnea pannosa was collected on April 2007 at Uruma, Okinawa Prefecture, Japan. The collected seaweed was washed with tap water and then air-dried at 40˚C for 24 h. The dried seaweed was powdered using a mixer. The powder was stored in refrigerator (4˚C) until extraction.
2.2. Polysaccharide Extraction
The powdered seaweed sample (3 g) was suspended in 500 mL of distilled water and stirred at 100˚C for 1 h. The suspension was filtered, and the filtrate was concentrated at 40˚C using a rotary evaporator. Ethanol (2 - 3 vol.) was added to the concentrated solution to precipitate polysaccharide. The precipitate was washed with ethanol twice and then dried in a vacuum chamber at 40˚C.
The dried precipitate was dissolved in distilled water and then filtered through a suction filter (Celite 545). The filtrate was passed through a column of Amberlite IR-120B (ø 5 × 30 cm, H+ form). The eluate was adjusted to pH 7 with 0.05 m NaOH and concentrated using a rotary evaporator at 40˚C. The concentrated solution was dialyzed against distilled water, and the dialyzed solution was freeze-dried.
2.3. Chemical Component Analysis
Total carbohydrate was determined by the phenol-sulfuric acid method using D-galactose as a standard [48] . 3,6-Anhydro-D-galactose was determined by method of Yaphe and Arsenault [49] using anhydro-β-D-galactose as a standard. Ash content was determined by incinerating the polysaccharide for 24 h in a muffle furnace at 550˚C and then weighed the residue. Sulfate content was measured by turbidimetric method [50] as follows: the polysaccharide (20 mg) was dissolved in 1.5 mL of distilled water, and 11.5 m HCl solution was added to a final concentration of 3.0 M. The solution was heated at 100˚C for 2 h. The hydrolyzate was dried, then dissolved in 1 mL of distilled water and centrifuged at 2150 × g for 20 min. To the supernatant, 3.8 mL of 4% trichloroacetic acid and 1 mL of 1% gelatin + 2% BaCl2 were added, then mixed sufficiently, and left for 20 min. Absorbance was measured at 360 nm. Pyruvic acid content was measured by using 2,4-dinitrophenyl- hydrazine [51] .
2.4. High-Performance Anion Exchange Chromatography Coupled with a Pulse Amperometric Detector (HPAEC-PAD)
For the quantitative determination of constituent sugar, the polysaccharide (10 mg) was hydrolyzed with 3.0 M trifluoroacetic acid (TFA) at 121˚C for 1 h. The hydrolyzate was dried using a compressor. Isopropanol (500 μL) was added and dried again. The dried hydrolyzate was dissolved in purified water and centrifuged. The supernatant was analyzed by high performance anion exchange chromatography (HPLC) on a DX 500 liquid chromatograph (Dionex Co., Ltd., Sunnyvale, CA), fitted with a column of CarboPac PA1 (ø 4 × 250 mm) and a pulsed amperometric detector. The column was eluted at a flow rate of 1 mL/min at 35˚C with 15 mm NaOH.
2.5. Methanolysis
The polysaccharide (10 mg) was treated with 0.5 m hydrogen chloride in methanol at 105˚C for 12 h in a sealed tube. The reaction mixture was neutralized with silver carbonate at 60˚C, and then filtered and evaporated [2] [7] [8] .
2.6. Thin-Layer Chromatography
Thin-layer chromatography was carried out on glass plate (20 cm in length) treated with silica gel containing calcium sulfate as the binder and using a solvent of butanol-ethanol-water (4:1:5). Chromatograms were sprayed with 10% sulfuric acid in water and heated at 100˚C for 15 min [2] [7] [8] .
2.7. Molecular Mass Determination
The molecular mass of the polysaccharide was determined by high-performance liquid chromatography (HPLC) (Shimadzu SCL-6B; Shimadzu Seisakusho, Co., Ltd, Japan) on a Superdex 200 column (TSK gel G 4000 PWXL) with a sample loop of 200 μL. The HPLC operation was performed at room temperature. The column was developed with 50 mM phosphate buffer, and the same buffer supplemented with 150 mM sodium chloride and fractions (3 mL each) were collected at a flow rate of 0.2 mL/min. Standard pullulan (P-82), P-400 (molecular mass, 4.0 × 105), P-100 (1.0 × 105), P-20 (2.0 × 104), and P-5 (0.5 × 104), (Pharmacia Chemicals Co., Ltd., Sweden), having definite molecular mass were used for calibration.
2.8. Fourier Transform Infrared (FTIR) Spectroscopy and Specific Rotation
FTIR spectrum was measured using an FTS-3000 spectrophotometer (Bio-Rad Laboratories, Hercules, CA) in transmittance mode from 4000 to 400 cm−1 in KBr disc. The KBr disc was prepared by dispersing solid sample in the KBr salt.
The specific rotation of the polysaccharide was measured at 589 nm on a polaimeter (P-1010, Japan Spectroscopic Co., Ltd., Japan) for a 0.2% (W/V) solution in distilled water at 25˚C.
2.9. 1H- and 13C-Nuclear Magnetic Resonance (NMR) Spectroscopy
1H- and 13C-NMR spectra were measured on an FT-NMR spectrometer at 500 and 125 MHz (JNM α500, JEOL Ltd., Ltd., Tokyo, Japan) at 80˚C. The 1H and 13C spectra were recorded using 45˚ pulse width, 32,768 data points, and 1 s pulse delay for 1H; and 60˚ pulse width, 16,384 data points, and 0.5 s pulse delay for 13C, respectively [13] . Sodium 3-(trimethylsilyl)propionic-2,2,3,3,-d4 acid (TSP, 0.00 ppm) was used as an internal standard. As the polysaccharide solution in D2O showed too large viscosity to measure, it was partially hydrolyzed with 0.1 m HCl at 55˚C for 1 h, neutralized with 0.3 m NaOH, and then freeze-dried. The partially hydrolyzed polysaccharide (2.0%) was dissolved in D2O at room temperature [13] [16] .
2.10. Methylation Analysis
Methylation of the polysaccharide was carried out by Ciucanu and Kerek method [52] . The polysaccharide (5 mg) was dissolved in 2 mL of DMSO and powder of NaOH (5 mg) was added to the solution and stirred at room temperature for 90 min. CH3I (1 mL) was added to the solution and stirred for 60 min. Distilled water (4 mL) was added to the solution and dialyzed against tap water followed by distilled water. The dialyzed solution was evaporated to dryness. The methylated polysaccharide was extracted with CHCl3 (2 mL) and washed with distilled water (3 mL) five times. The extracted methylated polysaccharide was hydrolyzed with 2 M TFA (2 mL) at 120˚C for 2 h. The hydrolyzate was dissolved in 1 M NH4OH (100 μL), and then DMSO (500 μL) containing 10 mg of NaBH4 was added. The mixture was incubated at 40˚C for 90 min. Glacial acetic acid (100 μL) was added to the mixture. Anhydrous 1-methylimidazole (100 μL) and acetic anhydride (0.5 μL) were added and then incubated at ambient temperature for 10 min. Partially methylated alditol acetates (PMAAs) were obtained after extracting with CHCl3 and washing with distilled water. The PMAAs in CHCl3 were dried with Na2SO4 and filtered. The PMAAs were analyzed using a GC-MS (GCMS-QP5000, Shimadzu Co., Ltd.) equipped with a capillary column (DB-1, ø 0.25 mm × 30 m, J & W Scientific). Helium was used as carrier gas (125 kPa). The injector and interface temperatures were 210˚C and 270˚C, respectively. Oven temperature was maintained at 150˚C for 5 min after injection, then raised at 5˚C/min to 250˚C, and this temperature was kept for 5 min [13] [16] .
3. Results
3.1. Preparation of Polysaccharide from Hypnea pannosa
One of red seaweed, H. pannosa, which was collected in April from Uruma City, Okinawa Prefecture, Japan, reached 5 - 10 cm long, having many thin branches, which was about φ2.0 - 3.0 mm. The collected seaweeds were washed with tap water and then dried by an air-dried oven at 40˚C for 24 h. The polysaccharide was prepared and purified as described in Materials and Methods. The purified polysaccharide was a colorless, fibrous powder, with yield of 17.2% (w/w) based on dried seaweed. Precipitation or gel-formation did not occur when KCl (1.0%) or CaCl2 (1.0%) was added into the polysaccharide aqueous solution (0.5%) (Table 1).
3.2. Identification of Sugar Components of the Polysaccharide
The total carbohydrate, 3,6-anhydro-α-D-galactose, pyruvic acid, sulfuric acid and ash of the purified polysaccharide were estimated to be 55.2%, 12.5%, 3.8%, 35.2% and 24.3%, respectively.
As shown in Figure 1, the HPLC of the acid hydrolyzate showed the presence of D-galactose and D-glucose the molar ratio of which was estimated to be 2.9:1.0.
The examination of methanolysis product of the polysaccharide by thin-layer chromatography indicated the presence of 3,6-anhydro-methy-α-D-galactoside (spot 2), methyl-β-D-galactoside (4), and methyl-α-D-galactoside (spot 5) [8] [9] . A spot 3, which was located at higher than that of spot 4, might be methyl-α-and/or -β-D-gluco- sides (Figure 2).
Table 1. Chemical components of the polysaccharide isolated from Hypnea panossa.
Figure 1. Liquid chromatogram of hydrolysate of the polysaccharide isolated from H. pannosa.
3.3. Molecular Mass
The molecular mass of purified polysaccharide was measured by the gel chromatography on a Superdex 200 column (Figure 3). According to the standard calibration curve obtained from the definite molecular mass pullulan, the molecular mass of the polysaccharide was calculated to be approximately 7.9 × 105.
Figure 2. Thin-layer chromatogram of methanolyzate of the polysaccharide isolated from H. pannosa. 1. Methyl-3,6- anhydro-β-D-galactoside; 2. Methanolyzate of standard ι-carrageenan; 3. Methanolyzate of the polysaccharide.
Figure 3. Gel permeation chromatogram of the polysaccharide from H. pannosa.
3.4. FTIR Spectrum and Specific Rotation
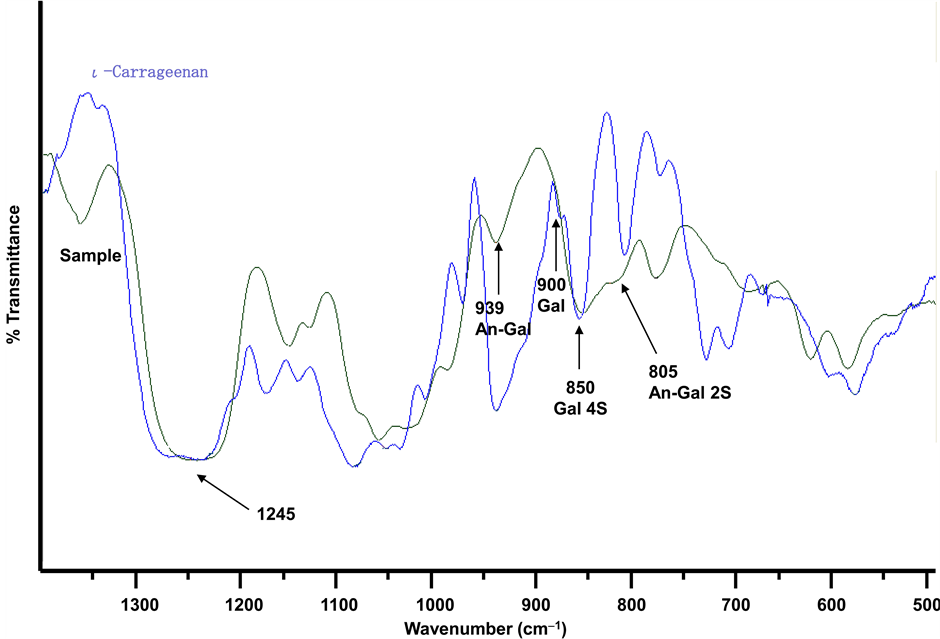
Figure 4 shows spectra of the polysaccharide and standard ι-carrageenan. A broad absorption at 1245 cm−1 was common to all the sulfated polysaccharides due to sulfate absorption. An absorption at 850 cm−1 (strong) was assigned to be ester sulfate on C-4 of 3-linked D-galactose residue. An absorption at 939 cm−1 (weak) was assigned to C-O ether bond of 3,6-anhydro-galactose residue. The peak at 805 cm−1 (medium) was assigned to be an ester sulfate on C-2 of the 4-linked 3,6-anhydro-D-galactose residue. The band at 900 cm−1 (very weak) indicated 3-linked D-galactose residues bearing pyruvate acetal substitution [53] [54] . These data were consistent in part with those of standard ι-carrageenan prepared from Euchemua spinosum.
The specific rotation [α]589 of the polysaccharide at 25˚C was estimated to be a value of +16.8˚ (c 0.2%, H2O). The value was lower than that of standard ι-carrageenan (+26.5˚). The result suggests that D-glucosyl residue consisted of β-conformation on the polysaccharide.
3.5. 13C- and 1H-NMR Spectra Analysis
As the polysaccharide solution in D2O showed too large viscosity to measure, it was partially hydrolyzed with 0.1 M HCl at 55˚C for 1 h. The partially hydrolyzed polysaccharide (2.0%) was dissolved in D2O. The seven sugar moieties were designated as residues A, B, C, D, E, F and G according to their decreasing anomeric carbon chemical shifts. Figure 5 shows 13C-NMR spectrum. From published papers [53] - [59] , the signal at B (105.753 ppm)), C (103.526), D (103.102), E (97.266), F (97.009) and G (96.700) was assigned to be anomeric carbon of 3-linked β-D-galactopyranose adjacent to 4-linked α-D-galactopyranose (B), β-D-galactopyranose (C), pyruvated β-D-galactopyranose (D), anhydro-α-D-galactopyranose (E), sulfated anhydro-α-D-galactopyranoe (F) and α-D-galactopyranose (G), respectively. The chemical shift at 106.49 ppm (A) is assigned to β-1,4-linked D-glucopyranosyl residue [60] [61] . The carboxyl, acetal and methyl carbon was assigned at 186.673, 101.361 and 27.919 ppm, respectively [53] [54] [57] .
1H-NMR spectrum is shown in Figure 6. The chemical shift of the envelope of anomeric signals are consis-
Figure 4. Infrared spectra of the polysaccharide from H. pannosa and standard ι-carrageenan.
Figure 5. 13C NMR Spectrum of the polysaccharide isolated from H. pannosa at 60˚C.
Figure 6. 1H NMR Spectrum of the polysaccharide isolated from H. pannosa at 60˚C.
tence with presence of α-D-galactopyranose (G), C-2 sulfated 3,6-anhydro-α-D-galactopyranose (F), 3,6-anhydro- α-D-galactopyranose (E), and β-D-galactopyranose (B, C and D) at 5.690, 5.425, 5.337, and 4.625 ppm, respectively [53] [57] [59] [62] . The anomeric proton of β-D-glucopyranose was assigned at 4.454 ppm [60] [61] . The signal at 1.493 ppm can be assigned to methyl proton of pyruvic acid [53] [54] [57] . The ring proton signals of the spectrum were overlapped due to high viscosity even after partially hydrolysis. The results are summarized in Table 2.
3.6. Methylation Analysis
The native and desulfated polysaccharide was methylated according to the procedure described by Ciucanu & Kerek [52] . The obtained permethylated polysaccharide was subjected to complete acid hydrolysis to furnish mixtures of the methylated sugars, which were analyzed as the corresponding alditol acetates using gas-liquid chromatography (GC) and combined gas-liquid chromatography/mass spectroscopy (MS). The chromatogram is shown in Figure 7 (desulfated polysaccharide). Partially methylated alditol acetates were identified using published data [63] [64] . For the native polysaccharide (not shown in Figure), the 3 peaks were observed: 1,5-di-O- acetyl-2,3,4,6-tetra-O-methyl-D-galactose (peak 1), 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl-D-glucose (3), and 1,2, 3,4,5,6-hexa-O-acetyl-D-galactose (8) which originated from terminal D-galactose (0.1 mol), 1,4-linked D-glucose (1.0 mol) and 1,2,3,4,5,6-linked D-galactose (3.8 mol) residues, respectively.
After desulfation (Figure 7), 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl-D-glucose (peak 3; 1.0 mol) 1,4,5-tri-O- acetyl-2,3,6-tri-O-methyl-D-galactose (peak 2: 1.9 mol)=1,5-di-O-acetyl-2.3.4.6-tetra-O-methyl-D-galactose (peak 1; 0.6 mol), 1,3,5-tri-O-acetyl-2,4,6-O-methyl-D-galactose (peak 4:1.7 mol), 1,3,4,5-tetra-O-acetyl-2,6-di-O- methyl-D-galactose (peak 5; 0.3 mol), 1,4,5,6-tetra-O-acetyl-2,3-di-O-methyl-D-galactose (peak 6; 0.3 mol), and 1,3,4,5,6-penta-O-acetyl-2-mono-O-methyl-D-galactose (peak 7; 0.3 mol) were observed, but peak 8 was disappeared. The results indicated that the 1,4-linked D-glucose residue (peak 3) was free from sulfuric acid and pyruvic acid. The peak 7 suggested that the pyruvate group substituted with acetal linkage on the position of C-4 and C-6 of 1,3-linked D-galactose. The small amount of peak 7 (0.3 mol) was due to association of pyruvate group even after desulfation. The results are summarized in Table 3.
4. Discussion
The polysaccharide isolated from Hypnea pannosa had high content of sulfate (35.2%) combination with pyruvic acid (3.8%). The polysaccharide consisted of 3,6-anhydro-α-D-galactose (12.5%), α-D-galactose, β-D-galactose, and D-glucose residues. IR spectrum indicated the presence of β-D-galactose 4 sulfate and 3,6-anhydro-α-D-galactose 2-sulfate residues. The result suggested that the polysaccharide involved ι-carrabiose units. 13C and 1H NMR spectra showed that it contained 1,3-linked β-D-galactose, 1,4-linked α-D-galactose, 1,4-linked 3,6-anhydro-D-galactose, 1,4-linked β-D-glucose, and pyruvic acid residues, respectively. The pyruvate group was associated in acetal linkage on the main chain. The methylation analysis of native polysaccharide showed all hydroxyl groups of D-galactose residues substituted with sulfate or pyruvate groups, because 2,3,4,6- tetra-O-methyl-D-galactose (peak 1: 0.1 mol), 2,3,6-tri-O-methyl-D-glucose (peak 3: 1.0 mol) and D-galactose
Table 2. Chemical shifts of resonances in the 13C and 1HNMR spectra of the polysaccharide isolated from H. pannosa.
*Sulfated β-D-galactose, **Pyruvated β-D-galactose, ***Sulfated Anhydro-α-D-galactose, ****Sulfated α-D-galactose.
Figure 7. Gas chromatogram of partially methylated alditol acetates of the desulfated polysaccharide isolated from Hypnea pannosa.
Table 3. Methylation analysis of the polysaccharide isolated from Hypnea pannosa.
aPeak number in Figure 7.
Figure 8. The chemical structure of the pyruvated glucogalactan sulfate isolated from Hypnea pannosa.
(peak 8: 3.8 mol) were identified for native polysaccharide. After desulfation, peak 8 was disappeared, and 2,3,6-tri-O-methyl-D-galactose (peak 2: 1.9 mol), 2,4,6-tri-O-methyl-D-galactose (peak 4: 1.7 mol) and 2-mono-O-methyl-D-galactose (peak 7: 0.3 mol) were identified. The pyruvate group might substitute at 4 and 6 positions of the 3-linked D-galactose (peak 7) residue. About 50% of 2,3,6-tri-O-methyl-D-galactose (peak 2) might be derived from 3,6-anhydro-α-D-galactose (12.5%) and the rest from α-D-galactose residue. The results suggested that both ι-carrabiose derivative, λ-carrabiose derivative and 1,4-linked β-D-glucose units are involved in the polysaccharide (five sugar repeating units).
5. Conclusion
From the results and discussion, we concluded that the polysaccharide isolated from Hypnea pannosa was the pyruvated glucogalactan sulfate. The chemical structure of the polysaccharide was illustrated in Figure 8. The polysaccharide consisted of ι-carrabiose derivative, λ-carrabiose derivative and 1,4-linked β-D-glucose units. However, it was not neglected that the polysaccharide from Hypnea pannosa was the mixture of ι-carrageenan derivative, λ-carrageenan derivative and 1,4-linked β-D-glucan, because such pyruvated glucogalactan sulfate had not been reported.
Cite this paper
Masakuni Tako,Rintaro Ohtoshi,Kazutaka Kinjyo,Shuntoku Uechi, (2016) The Novel Pyruvated Glucogalactan Sulfate Isolated from the Red Seaweed, Hypnea pannosa. Advances in Biological Chemistry,06,114-125. doi: 10.4236/abc.2016.63010
References
- 1. Tako, M. (1994) Isolation of an Agar from Gracilaria blodgettii and Its Gelling Properties. Journal of Applied Glycoscience, 41, 305-310.
- 2. Tako, M., Higa, M., Medoruma, K. and Nakasone, Y. (1999) A Highly Methylated Agar from Red Seaweed Gracilaria arcuata. Botanica Marina, 42, 513-517.
http://dx.doi.org/10.1515/BOT.1999.058 - 3. Tako, M., Uehara, M., Kawashima, Y., Chinen, I. and Hongo, F. (1996) Isolation and Identification of Fucoidan from Okinawamozuku (Cladosiphon okamuranus TOKIDA). Journal of Applied Glycoscience, 43, 143-148.
- 4. Tako, M., Nakada, T. and Hongo, F. (1999) Chemical Characterization of Fucoidan from Commercially Cultured Nemacystus decipiens. Bioscience, Biotechnology, and Biochemistry, 63, 1813-1815.
http://dx.doi.org/10.1271/bbb.63.1813 - 5. Tako, M., Yoza, E. and Tohma, S. (2000) Chemical Characterization of Acetylfucoidan and Alginate from Commercially Cultured Cladosiphon okamuranus. Botanica Marina, 43, 393-398.
http://dx.doi.org/10.1515/BOT.2000.040 - 6. Shiroma, R., Konishi, T. and Tako, M. (2008) Structural Study of Fucoidan from the Brown Seaweed Hijikia fusiformis. Food Science and Technology Research, 14, 176-182.
http://dx.doi.org/10.3136/fstr.14.176 - 7. Tako, M., Kiyuna, S. and Hongo, F. (2001) Isolation and Characterization of Alginic Acid from Commercially Cultured Nemacystus decipiens. Bioscience, Biotechnology and Biochemistry, 65, 654-657.
- 8. Qi, Z.-Q., Tako, M. and Toyama, S. (1997) Chemical Characterization of κ-Carrageenan of Ibaranori (Hypnea charoides Lamouroux). Journal of Applied Glycoscience, 44, 137-142.
- 9. Lin, L.-H., Tako, M. and Hongo, F. (2000) Isolation and Characterization of ι-Carrageenan Isolated from Eucheuma serra. Journal of Applied Glycoscience, 47, 303-310.
http://dx.doi.org/10.5458/jag.47.303 - 10. Pakdee, P., Kinjyo, K., Tako, M., Tamaki, Y., Tomita, Y. and Yaga, S. (1995) Water-Soluble Polysaccharide from Seeds of Trees I. Galactomannan from Seeds of Leucaena leucocephala de WIT. Mokuzai Gakkaishi, 41, 440-443.
- 11. Tamaki, Y., Teruya, T. and Tako, M. (2010) Chemical Structure of Galactomannan from Delonix regia. Bioscience, Biotechnology, and Biochemistry, 74, 1110-1112.
http://dx.doi.org/10.1271/bbb.90935 - 12. Tamaki, Y., Uechi, S., Taira, T., Ishihara, M., Adaniya, S., Uesato, K., Fukuta, M. and Tako, M. (2004) Isolation and Characterization of Pectin from Pericarp of Citrus depressa. Journal of Applied Glycoscience, 51, 19-25.
http://dx.doi.org/10.5458/jag.51.19 - 13. Tamaki, Y., Konishi, T., Fukuta, M. and Tako, M. (2008) Isolation and Structural Characterization of Pectin from Endocarp of Citrus depressa. Food Chemistry, 107, 352-364.
http://dx.doi.org/10.1016/j.foodchem.2007.08.027 - 14. Tamaki, Y. and Tako, M. (2008) Isolation and Characterization of Pectin from Peel of Citrus tankan. Bioscience, Biotechnology and Biochemistry, 72, 896-899.
http://dx.doi.org/10.1271/bbb.70706 - 15. Nakamura, M., Yamashiro, Y., Konishi, T., Hanashiro, I. and Tako, M. (2011) Structural Characteristics of Rhamnan Sulfate from Commercially Cultured Monostroma nitidum. Nippon Shokuhin Kagaku Kogaku Kaishi, 58, 245-251.
http://dx.doi.org/10.3136/nskkk.58.245 - 16. Tako, M., Tamanaha, M., Tamashiro, Y. and Uechi, S. (2015) Struc-ture of Ulvan Isolated from the Edible Green Seaweed, Ulva pertusa. Advances in Bioscience and Biotechnology, 6, 645-655.
http://dx.doi.org/10.4236/abb.2015.610068 - 17. Tako, M., Dobashi, Y., Tamaki, Y., Konishi, T., Yamada, M., Ishida, H. and Kiso, M. (2012) Identification of Rare 6- Deoxy-D-altrose from an Edible Mushroom (Lactarius lividatus). Carbohydrate Research, 350, 25-30.
http://dx.doi.org/10.1016/j.carres.2011.12.016 - 18. Tako, M., Shimabukuro, J., Jiang, W., Yamada, M., Ishida, H. and Kiso, M. (2013) Rare 6-Deoxy-D-altrose from the Folk Medicinal Mushroom Lactarius akahatsu. Biochemical Compounds, 1, 1-6.
http://dx.doi.org/10.7243/2052-9341-1-5 - 19. Tako, M., Dobashi, Y., Shimabukuro, J., Yogi, T., Uechi, K., Tamaki, Y. and Konishi, T. (2013) Structure of a Novel α-Glucan Substitute with the Rare 6-Deoxy-D-altrose from Lactarius lividatus (Mushroom). Carbohydrate Polymers, 92, 2135-2140.
http://dx.doi.org/10.1016/j.carbpol.2012.11.010 - 20. Tako, M. (2002) Acetyl Fucoidan and Its Manufacturing Methods. Patent No. 3371124.
- 21. Teruya, T., Konishi, T., Uechi, S., Tamaki, H. and Tako, M. (2007) An-ti-Proliferative Activity of Oversulfated Fucoidan from Commercially Cultured Cladosiphon okamuranus TOKIDA in U937 Cells. International Journal of Biological Macromolecules, 41, 221-226.
http://dx.doi.org/10.1016/j.ijbiomac.2007.02.010 - 22. Teruya, T., Tatemoto, H., Konishi, T. and Tako, M. (2009) Structural Characteristics and in Vitro Macrophage Activation of Acetyl Fucoidan from Cladosiphon okamuranus. Glycoconjugate Journal, 26, 1019-1018.
http://dx.doi.org/10.1007/s10719-008-9221-x - 23. Tako, M. and Nakamura, S. (1986) Indicative Evidence for a Conformational Transition in κ-Carrageenan from Studies of Viscosity-Shear Rate Dependence. Carbohydrate Research, 155, 200-205.
http://dx.doi.org/10.1016/S0008-6215(00)90146-0 - 24. Tako, M. and Nakamura, S. (1986) Synergistic Interaction between Kappa-Carrageenan and Locust-Bean Gum in Aqueous Media. Agricultural and Biological Chemistry, 50, 2817-2822.
- 25. Qi, Z.-Q., Tako, M. and Toyama, S. (1997) Molecular Origin of the Rheological Characteristics of κ-Carrageenan Isolated from Ibaranori (Hypnea charoides Lamouroux). Journal of Applied Glycoscience, 44, 531-536.
- 26. Tako, M., Nakamura, S. and Kohda, Y. (1987) Indicative Evidence for a Conformational Transition in ι-Carrageenan. Carbohydrate Research, 161, 247-253.
http://dx.doi.org/10.1016/S0008-6215(00)90081-8 - 27. Lin, L.-H., Tako, M. and Hongo, F. (2001) Molecular Origin for Rheo-logical Characteristics of ι-Carrageenan Isolated from Eucheuma serra. Food Science and Technology Research, 17, 176-180.
http://dx.doi.org/10.3136/fstr.7.176 - 28. Tako, M. and Nakamura, S. (1988) Gelation Mechanism of Agarose. Carbohydrate Research, 180, 277-283.
http://dx.doi.org/10.1016/0008-6215(88)80084-3 - 29. Tako, M., Sakae, A. and Nakamura, S. (1989) Rheological Properties of Gellan Gum in Aqueous Media. Agricultural and Biological Chemistry, 53, 771-776.
- 30. Tako, M., Teruya, T., Tamaki, Y. and Konishi, T. (2009) Molecular Origin for Rheological Characteristics of Native Gellan Gum. Colloid and Polymer Science, 287, 1445-1454.
http://dx.doi.org/10.1007/s00396-009-2112-2 - 31. Tako, M. and Hizukuri, S. (1995) Evidence for Conformational Transition in Amylose. Journal of Carbohydrate Chemistry, 14, 613-622.
http://dx.doi.org/10.1080/07328309508005362 - 32. Tamaki, Y., Konishi, T. and Tako, M. (2011) Gelation and Retrogradation Mechanism of Wheat Amylose. Materials, 4, 1763-1775.
http://dx.doi.org/10.3390/ma4101763 - 33. Tako, M. and Kohda, Y. (1997) Calcium Induced Association Characteristics of Alginate. Journal of Applied Glycoscience, 44, 153-159.
- 34. Teruya, T., Tamaki, Y., Konishi, T. and Tako, M. (2010) Rheological Characteristics of Alginate Isolated from Commercially Cultured Nemacystus decipiens (Itomozuku). Journal of Applied Glycoscience, 57, 7-12.
http://dx.doi.org/10.5458/jag.57.7 - 35. Tako, M., Tohma, S., Taira, T. and Ishihara, M. (2003) Gelation Mechanism of Deacetylated Rhamsan Gum. Carbohydrate Polymers, 54, 279-285.
http://dx.doi.org/10.1016/S0144-8617(03)00029-8 - 36. Tako, M. (1999) Molecular Origin for Thermal Stability of Waxy-Rice (Kogane) Starch. Starch/Starke, 48, 414-417.
http://dx.doi.org/10.1002/star.19960481106 - 37. Tako, M. and Hizukuri, S. (1997) Molecular Origin for the Thermal Stability of Rice Amylopectin. Journal of Carbohydrate Chemistry, 16, 655-666.
http://dx.doi.org/10.1080/07328309708007343 - 38. Tako, M. and Hizukuri, S. (2000) Molecular Origin for Thermal Stability of Koshihikari Rice Amylopectin. Food Research International, 33, 35-40.
http://dx.doi.org/10.1016/S0963-9969(00)00021-1 - 39. Tako, M. and Hizukuri, S. (1999) Gelatinization Mechanism of Rice Starch. Journal of Carbohydrate Chemistry, 18, 573-584.
http://dx.doi.org/10.1080/07328309908544020 - 40. Tako, M. (2000) Gelatinization Characteristics of Rice Starch. Journal of Applied Glycoscience, 47, 187-192.
http://dx.doi.org/10.5458/jag.47.187 - 41. Tako, M. and Hizukuri, S. (2000) Retrogradation Mechanism of Rice Starch. Cereal Chemistry, 77, 473-477.
http://dx.doi.org/10.1094/CCHEM.2000.77.4.473 - 42. Tako, M. and Hizukuri, S. (2003) Gelatinization Mechanism of Potato Starch. Carbohydrate Polymers, 48, 397-401.
http://dx.doi.org/10.1016/S0144-8617(01)00287-9 - 43. Tako, M., Tamaki, Y., Konishi, T., Shibanuma, K., Hanashiro, I. and Takeda, Y. (2008) Gelatinization and Retrogradation Characteristics of Wheat (Rosella) Starch. Food Research International, 41, 797-802.
http://dx.doi.org/10.1016/j.foodres.2008.07.002 - 44. Tako, M., Tamaki, Y., Teruya, T., Konishi, T., Shibanuma, K., Hanashiro, I. and Takeda, Y. (2009) Rheological Characteristics of Halberd Wheat Starch. Starch/Starke, 61, 275-281.
http://dx.doi.org/10.1002/star.200800073 - 45. Tako, M. (2000) Structural Principles of Polysaccharide Gels. Journal of Applied Glycoscience, 47, 49-53.
http://dx.doi.org/10.5458/jag.47.49 - 46. Tako, M., Tamaki, Y., Teruya, T. and Takeda, Y. (2014) The Principles of Starch Gelatinization and Retrogradation. Food and Nutrition Sciences, 5, 280-291.
http://dx.doi.org/10.4236/fns.2014.53035 - 47. Tako, M. (2015) The Principle of Polysaccharide Gels. Advances in Bioscience and Biotechnology, 6, 22-36.
http://dx.doi.org/10.4236/abb.2015.61004 - 48. Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F. (1956) Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry, 28, 350-356.
- 49. Yaphe, Y. and Arsenault, G.P. (1965) Improved Resorcinol Reagent for the Determination of Fructose, and of 3,6-Anhydrogalactose in Polysaccharides. Analytical Biochemistry, 13, 143-148.
http://dx.doi.org/10.1016/0003-2697(65)90128-4 - 50. Dodgson, K.S. and Price, R.C. (1962) A Note on the Determination of the Ester Sulfate Content of Sulfated Polysaccharides. Biochemical Journal, 84, 106-110.
http://dx.doi.org/10.1042/bj0840106 - 51. Sloneker, J.H. and Jeanes, A. (1962) Pyruvic Acid, a Unique Component of an Exocellular Bacterial Polysaccharide. Nature, 194, 478-479.
http://dx.doi.org/10.1038/194478a0 - 52. Ciucanu, I. and Kerek, K. (1984) A Simple and Rapid Method for the Permethylation of Carbohydrates. Carbohydrate Research, 131, 209-217.
http://dx.doi.org/10.1016/0008-6215(84)85242-8 - 53. Chiovitti, A., Bacic, A., Craik, D.J., Kraft, G.T., Liao, M.L., Falshaw, R. and Furneaux, R.H. (1998) A Pyruvated Carrageenan from Australian Specimens of the Red Alga Sarconema filiforme. Carbohydrate Research, 310, 77-83.
http://dx.doi.org/10.1016/S0008-6215(98)00170-0 - 54. Chiovitti, A., Bacic, A., Craik, D.J., Munro, S.L.A., Kraft, G.T. and Liao, M.L. (1998) Carrageenans with Complex Substitution Patterns from Red Algae of the Genus Erythroclonium. Carbohydrate Research, 305, 243-252.
http://dx.doi.org/10.1016/S0008-6215(97)00000-1 - 55. Greer, C.W. and Yaphe, W. (1984) Hybrid (Iota-nu-Kappa) Carrageenan from Eucheuma nudum (Rhodophyta, Solieriaceae), Identified Using Iota- and Kappa-Carrageenases and 13C-NMR Magnetic Resonance Spectroscopy. Botanica Marina, 27, 479-484.
- 56. Van de Velde, F., Peppelman, H.A., Rollema, H.A. and Tromp, R.H. (2001) On the Structure of κ/ι-Hybrid Carrageenans. Carbohydrate Research, 331, 271-283.
http://dx.doi.org/10.1016/S0008-6215(01)00054-4 - 57. Falshaw, R., Furneaux, R.H. and Wong, H. (2003) Analysis of Pyruvylated β-Carrageenan by 2D NMR Spectroscopy and Reductive Partial Hydrolysis. Carbohydrate Research, 338, 1403-1414.
http://dx.doi.org/10.1016/S0008-6215(03)00171-X - 58. Van de Velde, F., Pereira, L. and Rollema, H.S. (2004) The Revised NMR Chemical Shift Data of Carrageenans. Carbohydrate Research, 339, 2309-2313.
http://dx.doi.org/10.1016/j.carres.2004.07.015 - 59. Guibet, M., Kervarec, N., Génicot, S., Chevolot, Y. and Helbert, W. (2006) Complete Assignment of 1H and 13C NMR Spectra of Gigartina skottsbergii λ-Carrageenan Using Carrabiose Oligosaccharides Prepare by Enzymatic Hydrolysis. Carbohydrate Research, 341, 1859-1869.
http://dx.doi.org/10.1016/j.carres.2006.04.018 - 60. Roubroeks, J.P., Andersson, R. and Aman, P. (2000) Structural Features of (1→3),(1→4)-β-D-glucan and Arabinoxylan Fractions Isolated from Rye Bran. Carbohydrate Polymers, 47, 3-11.
http://dx.doi.org/10.1016/S0144-8617(99)00129-0 - 61. Liu, Y. and Wang, F. (2007) Structural Characterization of an Active Polysaccharide from Phellinus ribis. Carbohydrate Polymers, 70, 386-392.
http://dx.doi.org/10.1016/j.carbpol.2007.04.019 - 62. Tojo, E. and Prado, J. (2003) A Simple 1H NMR Methods for the Quantification of Carrageenans in Blends. Carbohydrate Polymers, 53, 325-329.
http://dx.doi.org/10.1016/S0144-8617(03)00080-8 - 63. Jansson, P.-E., Kenne, L., Liedgren, H., Lindberg, B. and Lonngren, J. (1976) A Practical Guide to the Methylation Analysis of Carbohydrates. Chemical Communications (University of Stockholm), No. 8, 1-75.
- 64. Sassaki, G.L., Gorin, P.A.J., Souza, L.M., Czelusniak, P.A. and Iacomini, M. (2005) Rapid Synthesis of Partially O-Methylated Alditol Acetate Standards for GC-MS: Some Relative Activities of Hydroxyl Groups of Methyl Glycopyranosides on Purdie Methylation. Carbohydrate Research, 340, 731-739.
http://dx.doi.org/10.1016/j.carres.2005.01.020
NOTES

*Corresponding author.