Journal of Surface Engineered Materials and Advanced Technology
Vol.08 No.04(2018), Article ID:87814,16 pages
10.4236/jsemat.2018.84010
Effect of Li Ions on Al Electrodeposition from Dimethylsulfone
Sangjae Kim1*, Shota Kumeno1, Kenta Kamebuchi1, Kensuke Kuroda2, Masazumi Okido2
1Department of Materials Science & Engineering, Graduate School of Engineering, Nagoya University, Nagoya, Japan
2Institute of Materials and Systems for Sustainability, IMaSS, Nagoya University, Nagoya, Japan
Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 19, 2018; Accepted: October 13, 2018; Published: October 16, 2018
ABSTRACT
The influence of LiCl coexistence with Al electrodeposition was investigated in a dimethyl sulfone, DMSO2, bath containing AlCl3 at 403 K. The electrochemical behaviors of Li and Al ions were examined using Pt electrodes in the bath and the deposition mechanism was analyzed by cyclic voltammetry, CV, with an Al reference electrode in the bath. The coexistence of LiCl in the AlCl3-DMSO2 bath inhibited the cathodic current corresponding to Al deposition in the CV experiment. The amount of ca. 500 μmol Al deposits was obtained in constant potential electrolysis for 1 h at ?2 V in the bath with 10 mol% AlCl3. However, it decreased to 140 μmol Al in the bath with 10 mol% AlCl3 and 5 mol% LiCl. It was clarified that LiCl addition led to the formation of Li(DMSO2)+ more than the formation of from NMR measurement for the baths. This results in the suppression of Al deposition because LiCl inhibits the formation of complex ions, which is said to be necessary for Al electrodeposition.
Keywords:
Electrodeposition, Aluminum, Lithium, Nuclear Magnetic Resonance, Dimethylsulfone

1. Introduction
Plating technology in surface treatment using aqueous solutions is one of the useful techniques for prolonging the life of metal products. Although aluminum, Al, is a less noble metal, Al reacts with O2 in the air to form a dense oxide film, a passive state, so it is excellent in corrosion resistance. Because Al is also lightweight and has good features such as a glossy and beautiful surface, it is appropriate for use as a plating material. Indeed, galvanized Al plating, i.e., hot dipping, has been applied to a variety of pipes, heat exchange tubes, boilers, bolts, and nuts. Although galvanized and electroplated Zn films have been widely used as anticorrosive plating, Al is attracting attention as an alternative material to Zn because of concerns about exhaustion of Zn resources. It is, therefore, considered that progress in the Al plating processing technique is very important.
Several surface treatment methods, such as ionic liquids [1] - [9] , molten salts [10] [11] [12] , physical vapor deposition [13] [14] , chemical vapor deposition, spray coating [15] [16] , cementation [17] [18] , sputtering [19] [20] , hot dipping [21] [22] , and electroplating [23] [24] [25] , have been proposed to protect substrate against corrosion and to improve its other properties.
In the cases of vacuum plating using chemical or physical vapor depositions, the difficulty of mass production leads to a higher unit production cost, and a thin coating film may result in a stain on the basic material upon postprocessing. Moreover, hot dipping cannot provide a fair-quality coating film. It is also known that unlike other commercialized coating methods, Al metal cannot be obtained from aqueous solution because hydrogen evolution is dominant [26] [27] . The electrolyte must be aprotic. Essentially, two types of electrolytes are suitable: molten salts or electrolytes based on organic aprotic solvents. Both types are sensitive to moisture and air, and avoidance of these factors is essential for the bath life [23] . Accordingly, coating methods using organic compounds such as nonaqueous solvent and ionic fluid have been examined. These compounds are the nonvolatile ionic solvents AlCl3-N-ethylpyridinium bromide, AlCl3-N-ethylpyridinium chloride, AlCl3-1,3-dialkylimidazolium chloride, and AlCl3-1-butyl-1-methylpyrolidinium [28] [29] [30] .
These ionic liquids have been recently proposed as alternative electroplating solutions [31] [32] [33] . In comparison with an ionic liquid, a lower vapor pressure, a higher electric conductivity, a wider electrochemical window, and its high flammability hinder its widespread use in many applications. To solve these problems, AlCl3/dimethyl sulfone has been employed for electroplating at low temperature [34] - [42] . Dimethyl sulfone, (CH3)2SO2 (called DMSO2), is a nontoxic white crystalline powder with high solubility in AlCl3 and in organic solvents such as acetone and ethyl alcohol, and exhibits a low melting point of 382 K [43] .
None of the less noble metals, such as aluminum and lithium, can be electrodeposited from a system using water as a solvent. Therefore, by dissolving chlorides such as aluminum chloride, AlCl3, or lithium chloride, LiCl, using DMSO2 instead of water and performing electrolysis, it is expected that the reduction reaction of metal ions occurs on the cathode electrode and electroplating can be performed.
27Al NMR [33] and Raman analyses [38] of the AlCl3-DMSO2 mixtures indicate that there are two main soluble aluminum species, namely and , formed according to the following reaction, although some researchers suggest the coexistence of [44] :
(1)
The electrodeposition of Al can occur from the solvated cation , whereas the reduction of is not observed within the electrochemical window of the electrolytes. It has been demonstrated that dense, uniform Al coatings with a high corrosion resistance can be electrodeposited from AlCl3-DMSO2 baths at 383 K [33] [43] [45] [46] .
In this study, we investigated the electrochemical behavior in an AlCl3-DMSO2 bath with LiCl addition to obtain the effect of Li ions on the formation and Al deposition from NMR analysis for the bath and constant potential electrolysis, respectively. The possibility of Li electrodeposition was also examined in a LiCl-DMSO2 bath.
2. Experimental
2.1. Electrode Preparation
Platinum plate and copper plate (>99% purity, Nilaco) were used as working electrodes for cyclic voltammetry, CV, measurement and electrolysis at a constant potential. They were masked with insulating tape, Nitoflon, leaving an exposed surface area of 1.0 cm2. The Cu plate was wet-polished with 400 emery paper, and the Pt plate was also wet-polished, immersed in hydrochloric acid, washed with distilled water, and then washed with acetone for 20 min before tape masking. An Al rod (>99.99% purity, Nilaco) was immersed in the bath as a reference electrode, Al/Al3+. Pt plate was also used as a counter electrode.
2.2. Bath Preparation
To remove moisture of ca. 300 ppm contained in DMSO2 (99 mass%, Tokyo Chemical Industry, Japan), the DMSO2 powder was maintained at 353 K for 72 h in a constant temperature drier (DO-450 A, AS ONE Corporation) and melted at 403 K. The amount of DMSO2 used in each experiment corresponded to 0.2 mol.
A bath with 10 mol% or 20 mol% AlCl3 was prepared by adding aluminum chloride, AlCl3 (98 mass%, Nacalai Tesque, crystallized), to the melted DMSO2. Further, a Li-Al bath was prepared by adding 0 - 20 mol% of lithium chloride, LiCl (98 mass%, Nacalai Tesque, crystallized) to each Al bath. Bath preparation was conducted in an Ar-filled glove box.
2.3. Electrochemical Measurements and Characterization of Deposits
Electrochemical properties were measured in the Ar-filled glovebox with a potentiostat/galvanostat (BioLogic, SP 150). Cyclic voltammograms were obtained at the potential region between ?3.5 V and 3.5 V vs. Al/Al3+ at a scan rate of 100 mV∙s?1. Electrodeposition was attempted on the Cu plate by constant potential electrolysis under ?2 V for 1 h. The temperature was maintained at 403 K using a hotplate. The electrolyte was agitated at 150 rpm during the experiment using a magnetic stirring device at the bottom of the beaker.
2.4. Bath Characterization
7Li and 27Al NMR spectra were obtained at 130.3 MHz using a 500 MHz NMR spectrometer (Agilent Technology). All chemical shifts were referenced to D2O containing 1.5 M Al(NO3)3, which was used as an external reference. Samples were placed in 10 mm NMR tubes with a 5-mm coaxial tube filled with DMSO-d6 as a lock solvent. Spectra were gathered at 403 K, as in the electrochemical measurements.
2.5. Characterization of Electrodeposits
After electrolysis, deposits were washed with an AlCl3?DMSO2 solution and vacuum-dried prior to characterization. Crystal orientation and surface morphology of the deposits were characterized by X-ray diffraction, XRD (Rigaku Ultima IV; 40 kV, ?30 mA, 0.4 deg∙min?1), and the nitric acid solution was collected by suction and analyzed for Al and Li by inductively coupled plasma-atomic emission spectrometry, ICP-AES (Seiko Instruments, SPS 7800).
3. Results and Discussion
3.1. Electrochemical Behavior in DMSO2 Bath Containing LiCl Only
The possible cathodic reactions on Pt electrode were examined, initially, by CV measurements in a DMSO2 electrolyte containing LiCl at concentrations between 5 and 20 mol% at 403 K. The electrode potential was scanned from the open-circuit potential near 1 V in the negative direction to −3.5 V followed by scanning in the positive direction to 3.5 V and then cycled.
Figure 1 shows the voltammograms recorded after 20 cycles. The shapes of the curves did not change after five cycles. Several peaks of redox reactions were obtained. Compared with the result in the bath with AlCl3 only as described later in Figure 5(a), Figure 5(f), anodic and cathodic currents at potentials between −2 V and +2 V may not clearly depend on LiCl concentration. They may correspond to the redox reactions by moisture in the bath. The cathodic current seems to increase with LiCl concentration at a potential less than −2 V. These currents are considered to be Li deposition concerning the complex ion accompanied with the DMSO2 ligand. The cathodic current density at −3.5 V is smallest for the bath containing 5 mol% LiCl and largest for 15 mol% LiCl.
7Li NMR spectroscopy was next employed as this technique allows the environment of Li atoms to be unambiguously determined in most cases. For consideration of the reaction mechanism of Li electrodeposition, NMR analysis was conducted as a state investigation of ions in the bath. The NMR analysis results for 7Li and 7Li chemical shift are shown in Figure 2. A peak was detected in the
Figure 1. Cyclic voltammograms of Pt electrode in DMSO2 baths with various LiCl concentrations at 403 K. (a) 5; (b) 10; (c) 15; and (d) 20 mol%.
Figure 2. 7Li NMR spectra of 15 mol% LiCl/DMSO2 electrolytes.
vicinity of −2 ppm in the bath containing 15 mol% LiCl. Because this peak coincides with the chemical shift [47] of solvated ions, it is considered that Li+ and DMSO2 as well as Al3+ in DMSO2 form complex ions with the reaction represented in Equation (2).
(2)
It is considered that Li was electrodeposited from the complex ion at the cathode by the reaction in Equation (3).
(3)
To confirm Li deposition, electrolysis was conducted at potentials of −2.0 V, −2.5 V, −3.0 V, and −3.5 V for 1 h using a Cu plate as a working electrode. The appearance of the electrode surface is shown in Figure 3. No deposits were observed on the surface at −2.0 V, slightly black at −2.5 V, and silver film whitened after washing at −3.0 V and −3.5 V. The whitened deposits are thought to be Li metal reacting with moisture in the air to form hydroxide after electrolysis. Gas evolution was also observed at the Cu electrode during electrolysis at every potential, which may be H2 caused by the moisture contained in the LiCl resource. In addition, to determine which electrochemical reaction occurred at several potentials for 1 h from the DMSO2 bath with 15 mol% LiCl, XRD analysis was employed. Figure 4 shows that LiOH.H2O was slightly detected on the deposits obtained at −2.5 V, and a multiplicity of peaks of Li compounds such as LiOH and LiOH.H2O are identified at −3.0 V and −3.5 V.
Figure 3. Surface photographs of deposits on Cu substrate for 1 h in 15 mol% LiCl/DMSO2 baths at 403 K under constant potential electrolysis at (a) −2.0 V; (b) −2.5 V; (c) −3.0 V; and (d) −3.5 V.
Figure 4. XRD patterns for the deposits obtained on Cu electrodes at 403 K for 1 h in DMSO2 bath with 15 mol% LiCl at (a) −2.0 V; (b) −2.5 V; (c) −3.0 V; and (d) −3.5 V.
As a confirmation that the precipitate must be Li metal, the deposits were dissolved in 1 M HCl and diluted 100-fold, then qualitative and quantitative analyses were carried out using ICP-AES analysis. The results by ICP-AES analysis of the amount of Li contained in the film electrodeposited on the Cu substrate are summarized in Table 1. The lithium component is obtained at potentials less than −2.5 V. The cathodic current in electrolysis at −2.0 V corresponds to the H2 evolution without Li deposition.
3.2. Electrochemical Behavior in DMSO2 Bath Containing AlCl3 and LiCl
It is known that Al is electrodeposited from a bath of AlCl3-DMSO2 [33] [43] [45] [46] . Therefore, it was verified whether it could be precipitated as an Al alloy by adding another metal chloride to this system. An attempt was made to determine by CV the oxidation-reduction peak of Al and the oxidation-reduction peak of the mixed metal species. The deposition/dissolution reaction of Al ion/metal takes place at around 0 V based on the redox potential of Al as a reference electrode. Cyclic voltammograms were measured on Pt electrodes in the potential range from −2 V to +2 V at 403 K in DMSO2 baths containing 10 mol% AlCl3 and 20 mol% AlCl3, accompanied with 0 to 20 mol% LiCl. The electrode potential was scanned from the open-circuit potential near 0 V to the negative direction, followed by scanning to the positive direction and then cycled. As shown in Figure 5, deposition and dissolution currents for Al were observed around 0 V in 10 mol% and 20 mol% AlCl3 baths without LiCl. The first voltammogram (Figure 5(a)) exhibited one reduction peak and a broad oxidation peak. In 10 mol% AlCl3 bath, the currents corresponding to Al redox reaction were obviously suppressed in peak magnitude by Li coexistence of 5 mol% or more of LiCl (Figures 5(b)-(e)). In contrast, several redox peaks newly appeared in the potential range between −1.5 V and −0.5 V. In 20 mol% AlCl3 bath, redox currents for Al were also clearly observed from around 0 V in the bath with LiCl below 10 mol% and few changes (Figure 5(f) and Figure 5(g)). LiCl addition above 10 mol% (Figures 5(h)-(j)) led to the decrease in the Al current. This phenomenon means that Li coexistence inhibits the formation of complex ions, which is said to be necessary for Al electrodeposition.
Constant potential electrolysis was carried out for 1 h at −2 V in all baths to clarify the Al and Li deposition. The surface state of Cu electrodes after constant potential electrolysis in each bath is shown in Figure 6. In baths (a) and (f)
Table 1. Results of ICP analysis.
Figure 5. Cyclic voltammograms of Pt electrode at 403 K in baths containing various concentrations of AlCl3 and LiCl in mol%.
without LiCl, a black deposit and a glossy deposit were obtained, respectively. These deposits must be Al containing many impurities. With the increase in the amount of LiCl in the bath, the surface of the deposits after washing became whitened (Figures 5(b)-(e)). These white precipitates seem to be lithium hydroxide reacted with moisture in the air after electrolysis. Glossy deposits (Figure 5(f) and Figure 5(g)) also changed to whitened ones with the addition of LiCl. A smooth black precipitate was obtained in the baths containing 15 mol% LiCl and more (Figure 5(i) and Figure 5(j)).
To identify the electrodeposits, XRD analysis was carried out as qualitative analysis of precipitates, and analysis by ICP-AES was decided as quantitative analysis. In Figure 7, Al peaks decreased and multiple peaks of LiOH and LiOH∙H2O increased with the increase in LiCl in the bath. In particular,
Figure 6. Surface photographs of deposits on Cu electrode formed at −2 V, 403 K in DMSO2 bath with various concentrations of AlCl3 and LiCl.
Figure 7. XRD patterns for deposits obtained on Cu electrodes at −2 V, 403 K for 1 h in baths with various concentrations of AlCl3 and LiCl in mol%.
LiOH∙H2O peaks were detected in the results of 20 mol% LiCl. It is considered that Li metal electrodeposited on the Cu electrode reacts with moisture and appears on the surface as hydroxide.
To calculate the amounts of Al and Li contained in the deposits on the Cu substrate, ICP-AES quantitative analysis was performed as shown in Figure 8. In both 10 mol% AlCl3 and 20 mol% AlCl3 baths, the Al amount decreased with increase in LiCl in the bath. In addition, Li increased accompanied with LiCl concentration in the bath. Although Al and Li were contained in deposits from Figure 8, it was not possible to identify the XRD peaks corresponding to Al-Li alloys such as Al2Li3 or Al3Li in the electrodeposit from Figure 7.
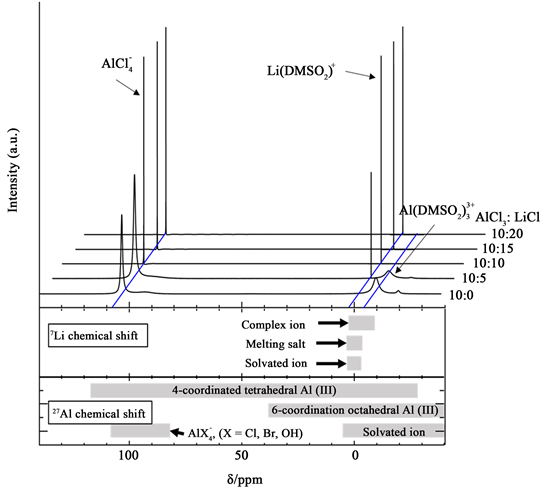
In addition, to estimate the effect of ions present in the bath on the deposit component, the NMR analysis on all electrolytic baths was carried out for 10 mol% AlCl3 bath (Figure 9) and for 20 mol% AlCl3 bath (Figure 10), where it is clarified that , Li(DMSO2)+, and exist in the baths.
Figure 8. Amount of Al and Li in deposits by ICP analysis.
Figure 9. 7Li and 27Al NMR spectra of 10 mol% AlCl3/DMSO2 containing from 0 to 20 mol% LiCl.
was never observed. DMSO2 is therefore found to act as a Lewis base weaker than Cl? but stronger than leading to the following equilibria in DMSO2-based electrolytes [38] .
(1)
(4)
A peak corresponding to was detected in the vicinity of 104 ppm in all baths [33] . In the bath containing LiCl, a peak corresponding to Li(DMSO2)+ was detected around −2 ppm [47] . The peak of appearing between −22 ppm and −2 ppm decreased with increase in LiCl. The peak of was not detected in 10 mol% AlCl3 baths with 10 mol% or more LiCl and in 20 mol% AlCl3 baths with 20 mol% LiCl. Table 2 lists data for relative species abundances corresponding to the spectra of Figure 9 and Figure 10, where the contributions from underlying peaks were estimated by deconvolution of shoulders in some cases if sufficiently distinct. These ratios were calculated from the compositional ratio (AlCl3/LiCl) of the baths determined by titration and alternately from the integrated areas under the assigned NMR peaks, and are compared here. The values determined from titration and the NMR signals for a given bath should agree; thus, comparing them, should serve as a cross-check of the composition. Since decreased and Li(DMSO2)+ increased with the addition of LiCl, Li+ tended to form a complex with DMSO2, and it is considered that LiCl inhibits the formation of .
Figure 10. 7Li and 27Al NMR spectra of 20 mol% AlCl3/DMSO2 containing from 0 to 20 mol% LiCl.
Table 2. Analysis of relative abundance of ions in baths by 7Li and 27Al NMR data.
4. Conclusions
In this study, in addition to how Li ion behaves in a LiCl-containing DMSO2 bath, we also investigated how to change the behavior of Al ions and Li ions by adding LiCl in an AlCl3-DMSO2 bath capable of Al electrodeposition. Furthermore, after conducting constant potential electrolysis in each bath, surface analysis of the working electrode and analysis of ions in the electrolytic bath were carried out to obtain the following findings.
1) In the CV measurement in a LiCl-containing DMSO2 bath, the rise of current from around −1.4 V, which is considered to be the redox of Li ions, was observed. Li can also be precipitated from DMSO2 as in Al from the analysis result of electrodeposition obtained by constant potential electrolysis in DMSO2-15 mol% LiCl bath. From the NMR analysis in a DMSO2-15 mol% LiCl bath, both Li form a complex ion with DMSO2.
2) In CV measurement in a bath containing LiCl added to an AlCl3-DMSO2 bath, current responses considered as oxidation and reduction of Al ions and Li ions are observed simultaneously. In the constant potential electrolysis in a bath in which LiCl was added to an AlCl3-DMSO2 bath, the state of the electrodeposition changed as the amount of LiCl added increased. Because LiCl inhibits the formation of , which is necessary for Al electrodeposition from DMSO2, it inhibits the electrodeposition of Al.
Acknowledgements
We thank Mie Torii for her experimental help in NMR measurements. And we also gratefully acknowledge that Otsuka Toshimi Scholarship Foundation for supporting our study in Japan.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Kim, S., Kumeno, S., Kamebuchi, K., Kuroda, K. and Okido, M. (2018) Effect of Li Ions on Al Electrodeposition from Dimethylsulfone. Journal of Surface Engineered Materials and Advanced Technology, 8, 110-125. https://doi.org/10.4236/jsemat.2018.84010
References
- 1. El Abedin, S.Z., Moustafa, E.M., Hempelmann, R., Natter, H. and Endres, F. (2005) Additive Free Electrodeposition of Nanocrystalline Aluminium in a Water and Air Stable Ionic Liquid. Electrochemistry Communications, 7, 1111-1116. https://doi.org/10.1016/j.elecom.2005.08.010
- 2. Chang, J.K., Chen, S.Y., Tsai, W.T., Deng, M.J. and Sun, I.W. (2007) Electrodeposition of Aluminium on Magnesium Alloy in Aluminium Chloride (AlCl3)-1-Ethyl-3-Methylimidazolium Chloride (EMIC) Ionic Liquid and Its Corrosion Behavior. Electrochemistry Communications, 9, 1602-1606. https://doi.org/10.1016/j.elecom.2007.03.009
- 3. Endres, F., Abbott, A.P. and MacFarlane, D.R. (2008) Electrodeposition from Ionic Liquids. Wiley-VCH, Weinheim. https://doi.org/10.1002/9783527622917
- 4. Barchi, L., Bardi, U., Caporali, S., Fantini, M., Scrivani, A. and Scrivani, A. (2010) Electroplated Bright Aluminium Coatings for Anticorrosion and Decorative Purposes. Progress in Organic Coatings, 67, 146-151. https://doi.org/10.1016/j.porgcoat.2009.10.017
- 5. Abbott, A., Qiu, F., Abood, H., Ali, M. and Ryder, K. (2010) Double Layer, Diluent and Anode Effects upon the Electrodeposition of Aluminium from Chloroaluminate Based Ionic Liquids. Physical Chemistry Chemical Physics, 12, 1862-1872. https://doi.org/10.1039/B917351J
- 6. Takahashi, S., Akimoto, K. and Saeki, I. (1989) Aluminum Plating From The Room Temperature Molten Salt Electrolyte. Journal of the Surface Finishing Society of Japan, 40, 134-135.
- 7. Abbott, A.P., Eardley, C.A., Farley, N.R.S., Griffith, G.A. and Pratt, A. (2001) Electrodeposition of Aluminium and Aluminium/Platinum Alloys from AlCl3/Benzyltrime-thylammonium Chloride Room Temperature Ionic Liquids. Journal of Applied Electrochemistry, 31, 1345-1350. https://doi.org/10.1023/A:1013800721923
- 8. Liao, Q., Pitner, W.R., Stewart, G., Hussey, C.L. and Stafford, G.R. (1997) Electrodeposition of Aluminum from the Aluminum Chloride-1-Methyl-3-Ethylimidazolium Chloride Room Temperature Molten Salt + Benzene. Journal of Electrochemical Society, 14, 4936-4943.
- 9. Hurley, F.H. and Wier, T.P. (1951) The Electrodeposition of Aluminum from Nonaqueous Solutions at Room Temperature. Journal of Electrochemical Society, 98, 207-212. https://doi.org/10.1149/1.2778133
- 10. Jafarian, M., Gobal, F., Danaee, I. and Mahjani, M.G. (2007) Impedance Spectroscopy Study of Aluminum Electrocrystallization from Basic Molten Salt (AlCl3-NaC1-KC1). Electrochimica Acta, 52, 5437-5443. https://doi.org/10.1016/j.electacta.2007.02.068
- 11. Jafarian, M., Mahjani, M.G., Gobal, F. and Danaee, I. (2006) Electrodeposition of Aluminum from Molten AlCl3-NaC1-KC1 Mixture. Journal of Applied Electrochemistry, 36, 1169-1173. https://doi.org/10.1007/s10800-006-9192-1
- 12. Fukumoto, M., Suzuki, T., Hara, M. and Narita, T. (2009) Effect of the Electrodeposition Temperature on the Cyclic-Oxidation Resistance of Ni Aluminide Containing Zr Formed by Molten-Salt Electrodeposition. Materials Transactions, 50, 335-340.
- 13. Charrier, C., Jacquot, P., Denisse, E., Millet, J.P. and Mazille, H. (1997) Aluminium and Ti/Al Multilayer PVD Coatings for Enhanced Corrosion Resistance. Surface and Coatings Technology, 90, 29-34. https://doi.org/10.1016/S0257-8972(96)03080-0
- 14. Yang, D., Jonnalagadda, R., Rogers, B.R., Hillman, J.T., Foster, R.F. and Cale, T.S. (1998) Texture and Surface Roughness of PRCVD Aluminum Films. Thin Solid Films, 332, 312-318. https://doi.org/10.1016/S0040-6090(98)01034-7
- 15. Paredes, R.S.C., Amico, S.C. and d’Oliveira, A.S.C.M. (2006) The Effect of Roughness and Pre-Heating of the Substrate on the Morphology of Aluminium Coatings Deposited by Thermal Spraying. Surface and Coatings Technology, 200, 3049-3055. https://doi.org/10.1016/j.surfcoat.2005.02.200
- 16. Hussey, T.S., Koczak, M.J., Smith, R.W. and Kalidindi, S.R. (1997) Electrodeposition of Aluminum in Molten AlCl3-n-Butylpyridinium Chloride Electrolyte. Materials Science and Engineering: A, 229, 137-146. https://doi.org/10.1016/S0921-5093(97)80109-8
- 17. Bose, S. (2007) High Temperature Coatings. Elsevier, Butterworth Heinemann, 71-97. https://doi.org/10.1016/B978-075068252-7/50007-X
- 18. Houngninou, C., Chevalier, S. and Larpin, J.P. (2004) Synthesis and Characterisation of Pack Cemented Aluminide Coatings on Metals. Applied Surface Science, 236, 256-269. https://doi.org/10.1016/j.apsusc.2004.04.026
- 19. Chu, M.S. and Wu, S.K. (2003) The Improvement of High Temperature Oxidation of Ti-50Al by Sputtering Al Film and Subsequent Interdiffusion Treatment. Acta Materialia, 51, 3109-3120. https://doi.org/10.1016/S1359-6454(03)00123-X
- 20. Zhong, D., Moore, J.J., Disam, J., Thiel, S. and Dahan, I. (1999) Deposition of NiAl Thin Films from NiAl Compound Target Fabricated via Combustion Synthesis. Surface and Coatings Technology, 120-121, 22-27. https://doi.org/10.1016/S0257-8972(99)00334-5
- 21. Wang, D., Shi, Z. and Zou, L. (2003) A Liquid Aluminum Corrosion Resistance Surface on Steel Substrate. Applied Surface Science, 214, 304-311. https://doi.org/10.1016/S0169-4332(03)00505-1
- 22. Kobayashi, S. and Yakou, T. (2002) Control of Intermetallic Compound Layers at Interface between Steel and Aluminum by Diffusion-Treatment. Materials Science and Engineering: A, 338, 44-53. https://doi.org/10.1016/S0921-5093(02)00053-9
- 23. Gálová, M. (1980) Electrodeposition of Aluminium from Organic Aprotic Solvents. Surface Technology, 11, 357-369. https://doi.org/10.1016/0376-4583(80)90053-9
- 24. Zhao, Y. and VanderNoot, T.J. (1997) Electrodeposition of Aluminium from Nonaqueous Organic Electrolytic Systems and Room Temperature Molten Salts. Electrochimica Acta, 42, 3-13. https://doi.org/10.1016/0013-4686(96)00080-1
- 25. Lehmkuhl, H., Mehler, K. and Landau, U. (1990) The Principles and Techniques of Electrolytic Aluminum Deposition and Dissolution in Organoaluminum Electrolytes. In: Gerischer, H. and Tobias, C.W., Eds., Advances in Electrochemical Science and Engineering, Vol. 3, VCH Verlagsgesellschaft, Weinheim, 165.
- 26. Simka, W., Puszczyk, D. and Nawrat, G. (2009) Electrodeposition of Metals from Non-Aqueous Solutions. Electrochimica Acta, 54, 5307-5319. https://doi.org/10.1016/j.electacta.2009.04.028
- 27. Nakamura, K. and Kitada, A. (1997) Electrodeposition of Aluminum from AlCl3/Glyme Solutions at Room Temperature. Electrochimica Acta, 42.
- 28. Liu, L., Lu, X., Cai, Y., Zheng, Y. and Zhang, S. (2012) Influence of Additives on the Speciation, Morphology, and Nanocrystallinity of Aluminium Electrodeposition. Australian Journal of Chemistry, 65, 1523-1528. https://doi.org/10.1071/CH12305
- 29. Wilkes, J.S. (2007) Molten Salts and Ionic Liquids: Are They Not the same Thing? ECS Transactions, 35, 3-7.
- 30. Endres, F. and El Abedin, S.Z. (2006) Air and Water Stable Ionic Liquids in Physical Chemistry. Physical Chemistry Chemical Physics, 8, 2101. https://doi.org/10.1039/b600519p
- 31. Yang, C.-C. (1994) Electrodeposition of Aluminum in Molten AlCl3-n-Butylpyridinium Chloride Electrolyte. Materials Chemistry and Physics, 37, 355-361. https://doi.org/10.1016/0254-0584(94)90175-9
- 32. Yue, G., Lu, X., Zhu, Y., Zhang, X. and Zhang, S. (2006) Electrodeposition of Aluminium from Ionic Liquids: Part I Electrodeposition and Surface Morphology of Aluminium from Aluminium Chloride (AlCl3)–1-Ethyl-3-Methylimidazolium Chloride ([EMIm]Cl) Ionic Liquids. Surface and Coatings Technology, 201, 1-9. https://doi.org/10.1016/j.surfcoat.2005.10.046
- 33. Legrand, L., Heintz, M., Tranchant, A. and Messina, R. (1995) Sulfone-Based Electrolytes for Aluminum Electrodeposition. Electrochimica Acta, 40, 1711-1716. https://doi.org/10.1016/0013-4686(95)00019-B
- 34. Hirato, T., Fransaer, J. and Celis, J.-P. (2001) Electrolytic Codeposition of Silica Particles with Aluminum from AlCl3-Dimethylsulfone Electrolytes. Journal of the Electrochemical Society, 148, C280-C283. https://doi.org/10.1149/1.1354616
- 35. Miyake, M., Tajikara, S. and Hirato, T. (2011) Fabrication of TiAl3 Coating on TiAl-Based Alloy by Al Electrodeposition from Dimethylsulfone Bath and Subsequent Annealing. Surface and Coatings Technology, 205, 5141-5146. https://doi.org/10.1016/j.surfcoat.2011.05.019
- 36. Jiang, T., Brym, M.J.C., Dube, G., Lasia, A. and Brisard, G.M. (2007) Studies on the AlCl3/Dimethylsulfone (DMSO2) Electrolytes for the Aluminum Deposition Processes. Surface and Coatings Technology, 201, 6309-6317. https://doi.org/10.1016/j.surfcoat.2006.11.035
- 37. Legrand, L., Tranchant, A. and Messina, R. (1994) Electrodeposition Studies of Aluminum on Tungsten Electrode from DMSO2 Electrolytes Determination of AlIII Species Diffusion Coefficients. Journal of the Electrochemical Society, 141, 378-382. https://doi.org/10.1149/1.2054735
- 38. Legrand, L., Tranchant, A., Messina, R., Romain, F. and Lautie, A. (1996) Raman Study of Aluminum Chloride-Dimethylsulfone Solutions. Inorganic Chemistry, 35, 1310-1312. https://doi.org/10.1021/ic941455q
- 39. Miyake, M., Motonami, H. and Hirato, T. (2012) Iron Aluminide Coatings by Electrodepsotion of Aluminum from an Organic Bath and Subsequent Annealing. ISIJ International, 52, 2273-2277.
- 40. Shiomi, S., Miyake, M. and Hirato, T. (2012) Electrodeposition of Bright Al-Zr Alloy Coatings from Dimethylsulfone-Based Baths. Journal of the Electrochemical Society, 159, D225-D229. https://doi.org/10.1149/2.079204jes
- 41. Fransaer, J., Leunis, E., Hirato, T. and Celis, J.-P. (2002) Aluminium Composite Coatings Containing Micrometre and Nanometre-Sized Particles Electroplated from a Non-Aqueous Electrolyte. Journal of Applied Electrochemistry, 32, 123-128. https://doi.org/10.1023/A:1014738011603
- 42. Legrand, L., Chassaing, E., Chausse, A. and Messina, R. (1998) RDE and Impedance Study of Anodic Dissolution of Aluminium in Organic AlCl3/Dimethylsulfone Electrolytes. Electrochimica Acta, 43, 3109-3115. https://doi.org/10.1016/S0013-4686(98)00065-6
- 43. Gaylord Chemical Company (2007) Dimethyl Sulfone (DMSO2) Physical Properties.
- 44. Nakayama, Y., Senda, Y., Kawasaki, H., Koshitani, N., Hosoi, S., Kudo, Y., Morioka, H. and Nagamine, M. (2015) Sulfone-Based Electrolytes for Aluminium Rechargeable Batteries. Physical Chemistry Chemical Physics, 17, 5758. https://doi.org/10.1039/C4CP02183E
- 45. Pereira-Ramos, J.P., Messina, R. and Perichon, J. (1986) Electrochemical Formation of LiAl Alloy in Molten Dimethylsulfone at 150 °C. Journal of Electroanalytical Chemistry, 209, 283-296. https://doi.org/10.1016/0022-0728(86)80554-X
- 46. Legrand, L., Tranchant, A. and Messina, R. (1996) Aluminium Behaviour and Stability in AlCl3DMSO2 Ctrolyte. Electrochimica Acta, 41, 2715-2720. https://doi.org/10.1016/0013-4686(96)00126-0
- 47. Kitagawa, S., Mizuno, M. and Maekawa, M. (2008) Solution and Solid-State NMR of Multinuclear Species. Sankyo Publishing Co.