Journal of Crystallization Process and Technology
Vol. 2 No. 3 (2012) , Article ID: 21361 , 6 pages DOI:10.4236/jcpt.2012.23014
Continuous Melt Suspension Crystallization of Phosphoric Acid
![]()
Chemical Engineering Research Center, East China University of Science and Technology, Shanghai, China.
Email: *jwzhu@ecust.edu.cn
Received April 16th, 2012; revised May 23rd, 2012; accepted May 31st, 2012
Keywords: Phosphoric Acid; Suspension Crystallization; MSMPR Crystallizer; Reflux Ratio
ABSTRACT
A continuous melt suspension crystallization process has been presented for the purification of the phosphoric acid in this study, which is performed in the cascade of a mixed suspension mixed product removal (MSMPR) crystallizer and a gravity wash column for the subsequent solid-liquid separation. Dynamic behavior in the crystallizer and role of reflux ratio on the purification efficiency of column are studied in detail. A reasonable steady state with respect to the liquid phase is achieved after 3 to 4 hrs, which is followed by a solid-phase steady state in terms of the slurry density after 4 hrs. Reflux ratio is the effective parameter for separation and purification by the crystallization equipment from the influences of reflux ratio on the purity of product, the number of theoretical plates and the stability of the operations.
1. Introduction
High-purity phosphoric acid has wide application in many fields such as medical, food, environmental protection and electronic industries. Now it can be produced in two ways: the so-called furnace process and wet process. The former process is energy intensive with a larger environmental impact. Therefore, the latter one tends to be favored, which is much more energy-saving and environment-friendly [1]. In this process phosphoric acid is produced via the acidulation of phosphate rock with sulfuric acid, so many impurities such as iron, aluminum, magnesium, fluorine, and sulfate can be included, and it must be purified before its further application. Several methods, such as, solvent extraction [2-5], ion exchange, electro-osmosis, and crystallization, are employed for the purification of phosphoric acid in order to remove these impurities from the acid [6,7]. Among these technologies crystallization presents several advantages such as higher efficiency, low running cost, and less impact to environment. Therefore, the crystallization technique is mainly employed for the purification of phosphoric acid at the top range.
Several crystallization processes for purification of phosphoric acid have been studied [8-14], such as layer crystallization and recrystallization, they are normally operated in a batch mode and have a low production capability. However, suspension crystallization, which is normally applied in the purification of organic compounds [15], such as canola oil [16] and cocoa butter [17], can be performed continuously at low energy consumption and high product yield. Suspension crystallization has been adopted for the purification of phosphoric acid in the present work. As suspension crystallization continuously generates crystal nuclei, a continuous MSMPR (mixed suspension and mixed product removal) crystallizer has been employed in this study, in which crystals with wider CSD are produced [18-20]. The suspension formed is separated from the mother liquor in a multistage separation process in a wash column, which would probably lead to a final product of higher purity. Dynamic behaviors in the crystallizer including fluid mechanics and the change of liquid concentration and suspension density of crystals during the continuous process are investigated in detail, the number of theoretical plates which can be used to describe the purification capability of column is calculated by the McCabe-Thiele type method, and the effect of reflux ratio as an important operation parameter on the purification efficiency of column is also studied.
2. Experimental
The crystallization equipment includes a MSMPR crystallizer (with a volume of 3 L) and a wash column which was constructed from a tube (20 mm i.d.) with a total length of 1200 mm. Wet process phosphoric acid which had been purified by solvent extraction was provided by Sinochem Fuling Chongqing Chemical Industry Co., Ltd. Concentrated wet process phosphoric acid of about 85 wt% H3PO4 was crystallized in the crystallizer after addition of seed crystals by lowing temperature. Seed crystals were prepared from a solution of reagent grade phosphoric acid (85% H3PO4 and specific gravity of 1.7) which was cooled to and kept at a temperature between –5℃ and –10℃ in plastic beakers, and its main size was about 0.1mm. A transistor relay was used to maintain the liquid level in the crystallizer constant by controlling the feed flow rate. After 1 h of crystallization, magma in the crystallizer was entrained to the top of wash column by the screw propeller stirrer. Because of the density difference between crystal and liquid, crystals in the magma settled down to form a loosely packed bed, which was melted at the bottom of column, and its temperature equaled the melt temperature of phosphoric acid hemihydrate. Part of the melt was withdrawn as purified product, and the remaining was returned to the column (refluxed) as wash liquid. The melt flew upward as the crystals replaced it, countercurrent contact between melt and crystals was enabled. The melt containing impurities was extracted as residual at the top of column. Feeding in the crystallizer and withdrawing of the product and the residual at the bottom and top of the column respectively could allow continuous operations of the crystallization equipment. The purity of the crude crystals descending in the wash column increases due to 1) displacement of the mother liquor, 2) washing of the liquid layer adhering to the crystals, 3) crystallization of pure melt on the cold crystals, 4) sweating of the crystals in contact with the hot melt or adiabatic recrystallization of the crystals.
As crystal moved through the column its temperature raised. At any height the crystal temperature equaled the equilibrium temperature of the melt corresponding to the composition. Thus, a temperature gradient was created. The top was relatively cold and the bottom was at the melt temperature of the purified product. Bed inversion can occur due to density differences caused by differences in temperature. The stirrer was used to reduce this effect also known as axial dispersion.
Samples of magma were taken from the crystallizer at every one hour with a pipette of 25 mL. Solid-liquid separation was performed by filtration, the solid was weighed and can be used to measure crystal size with microscope, while concentration of phosphoric acid in the liquid was determined by gravimetric analysis. Suspension density of crystals MT in the magma can be computed by the mass of crystals and volume of samples. Samples of product and residual were also taken from the column at each hour.
3. Results and Discussion
3.1. Dynamic Behavior in the Crystallizer
In order to achieve iso-kinetic removal of the magma and sample from the crystallizer, it is crucial that a homogeneous suspension of the crystals is maintained. Homogeneous suspension is defined as the condition which exists when the particle concentration and size distribution is constant throughout the vessel. This criterion is of great importance for MSMPR crystallizer. The stirring speed required for homogeneous suspension depends on the type of impeller used as well as on the crystallizer configuration. Properties of the solid-liquid system such as particle density, solids concentration, density of liquid phase, size, size range and shape of solid particles, and viscosity of the liquid phase also affect uniformity of solids suspension [21]. Crystal sizes at different stirring speed in the crystallizer are shown in Table 1, which is important to aid in the design of subsequent downstream processes such as separation of solid and liquid phases and is also crucial in determining end-product quality. It shows that crystals become smaller as stirring in the crystallizer is faster, which is attributed to secondary nucleation at high stirring speed, and there is no significant difference in crystal size at stirring speed of 200 and 300 rpm. Based on the experimental analysis, stirring speed in MSMPR crystallizer can be chosen in the range of 200 - 300 rpm, which ensures full suspension of crystals in the crystallizer and without secondary nucleation.
Successive liquid concentrations and slurry densities at different time are shown in Figures 1 and 2, respectively. It is found that a reasonable steady state with respect to the liquid phase (liquid concentration determination) is

Table 1. Crystal size obtained at different stirring speed.

Figure 1. Liquid concentration profile in the crystallizer at different XF.
achieved after 3 to 4 hrs. Liquid concentration decreases with the increase of feed concentration, which is attributed to the high crystallization yield of phosphoric acid at high feed concentration, and crystals thus obtained is also purer. Fluctuations in the liquor concentration are apparent, possibly due to slight variations in the feed flow rate which is controlled by the transistor relay. A solid-phase steady state in terms of the slurry density is achieved after 4 hrs as shown in Figure 2. High level of supersaturation and crystal growth rate in the crystallizer can be obtained at high feed concentration, so the suspension density of crystals increases with the increase of feed concentration.
Seed crystals are always used to relieve supersaturation in the crystallizer and prohibit spontaneous crystallization in industry. Amount of seed crystals depends on the supersaturation in the crystallizer, size and purity of seed crystals, and the time crystal growth needed. The effect of seed crystals on the liquid concentration and suspension density of crystals are illustrated in Figures 3
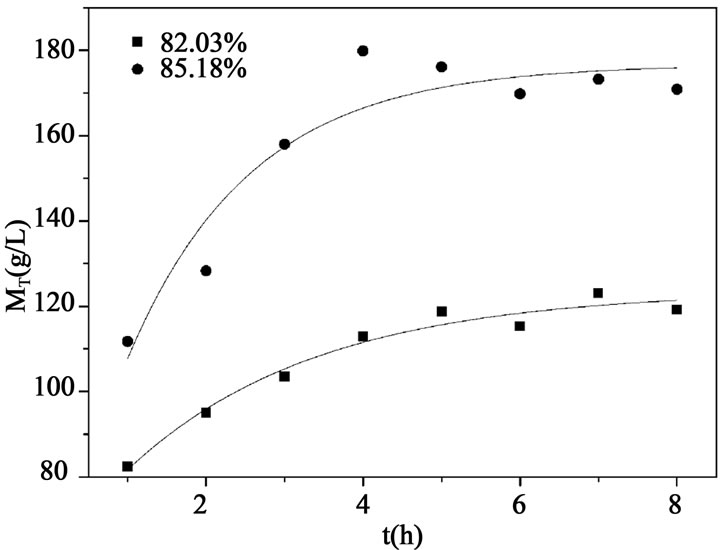
Figure 2. Suspension density profile in the crystallizer at different XF.
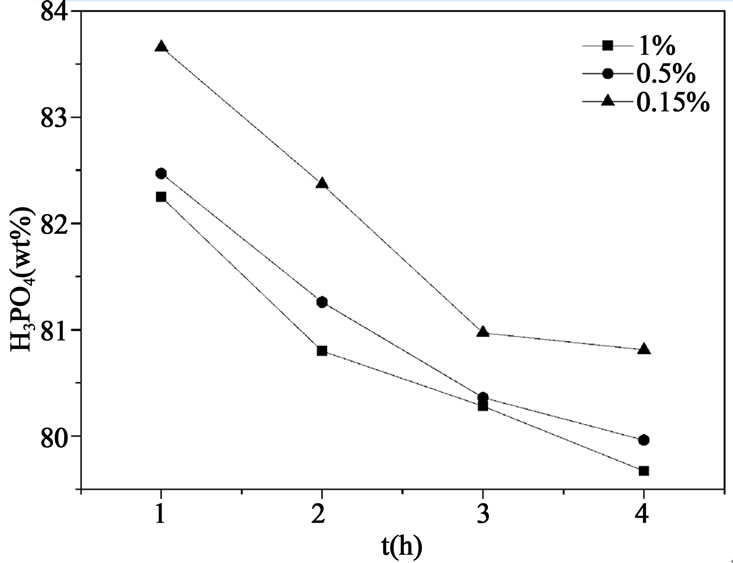
Figure 3. Liquid concentration profile in the crystallizer at different seed crystal.
and 4, respectively. It can be seen that liquid concentration becomes lower and suspension density of crystals increases when larger amount of seed crystals is added, which provides larger surface for crystal growth. Crystals thus obtained are large and uniform, and it can be purified more thoroughly in the wash column. However, adding too much seed crystals may cause secondary nucleation and devastate crystal growth, which can be indicated in Figure 4, suspension density changes little with the increase of crystal seed added. In order to shorten the time that crystallization equipment needs to achieve a steady state and prevent scaling of crystals in the wash column, MPSMPR crystallizer should be operated at a solid content between 30% - 40% of crystallizer volume. So it indicates that mass of seed crystals equals to 0.5 wt% of feed acid is much enough to eliminate the supersaturation and without induce secondary nucleation.
3.2. Influence of Reflux Ratio on Purification of Phosphoric Acid
In the melt suspension crystallization process, reflux ratio is an important parameter which can be changed over a wide range, compared to others, such as feed concentration, stirring speed in the column and crystal bed height. At the melting section, the crystals arrived at the heater and were melted. Then, a part of melt was withdrawn as the product, while the rest was returned to the column to wash settling crystals. The reflux ratio, R, is defined as,
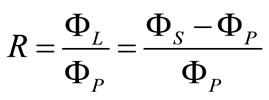 (1)
(1)
In which, ΦS, ΦL and ΦP are mass flow rate of crystal, reflux melt and product, respectively. The effect of reflux ratio on the purity of product is shown in Figure 5. Impurity F concentration was analyzed by fluorine elec-
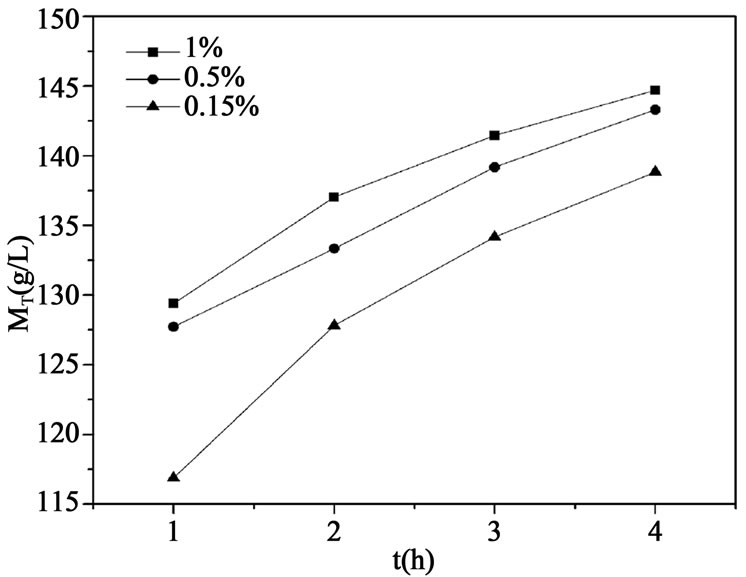
Figure 4. Suspension density profile in the crystallizer at different seed crystal.
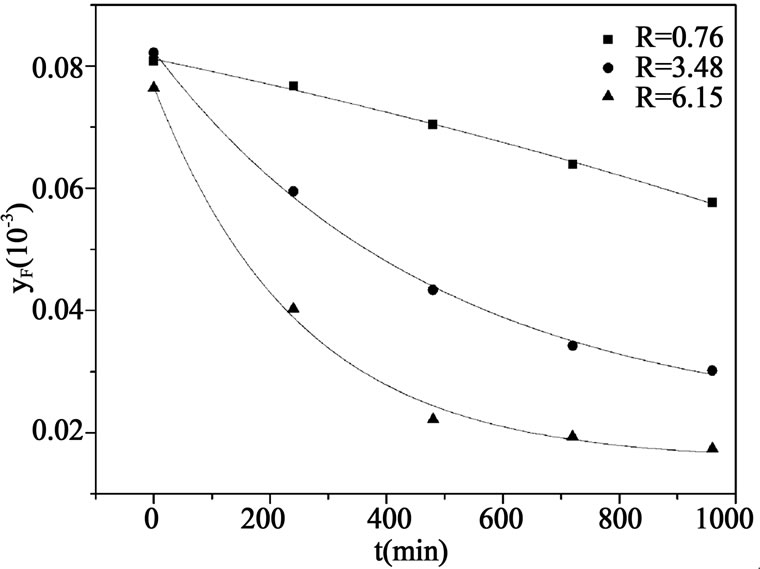
Figure 5. Impurity F concentration profile in the product at different R.
trode method. When reflux ratio is small, operation of the crystallization equipment becomes unstable and the impurity F concentration in the product is higher, because of the unsatisfactory countercurrent contacts between rising melt and settling crystals. Impurity F concentration in the product is found to be lower when the reflux ratio is larger, and this reduction becomes faster at higher reflux ratio, which is indicated by the transition of straight line to exponential curve. Because as the amount of reflux increases, the counter-current contacts between the settling crystals and the rising melt would be enhanced and consequently the purification of crystals would be more effective. The suspension density is also higher at large reflux ratio, and it was reported that impurity concentration of product is lower as the suspension density is higher [22].
The effect of reflux ratio on the concentration of phosphoric acid in the product is shown in Figure 6. Concentration of phosphoric acid in the product increases with the increase of reflux ratio, when reflux ratio is larger than 6, further increase in reflux ratio has no significant influence on the concentration of product. This can be attributed to that the purification mechanism is transferred from countercurrent washing to sweating as R increases, which tends to purify phosphoric acid in a much slower way.
For the solid-liquid mixing system, crystallization in the column is similar to rectification, and multistage crystallization is needed to gain product of high purity, length of purification section is determined, so the number of theoretical plates can be used to describe purification capability of column. The more number of theoretical plates, the higher separation efficiency of column has. Mass balances around the melting section are expressed as Total:
 (2)
(2)
Phosphoricacid:
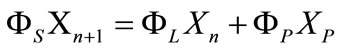 (3)
(3)
in which XP is mass concentration of phosphoric acid in the product. Therefore, the enriching line is calculated by the equation after substitution of equation (1), where XC, XL are mass concentration of phosphoric acid in the crystal and melt, respectively,
 (4)
(4)
The q-line is represented as, in which XF represents mass concentration of phosphoric acid in the feed acid,
 (5)
(5)
q is the value that indicates the thermal condition of the feed, for the feed at saturated temperature, q = 1.
The number of theoretical plates can be computed by graphical method, which is shown in Figure 7, AB is the phase diagram of the phosphoric acid-H2O system, BC is diagonal, FG is the enriching line, EF is q line, while DF
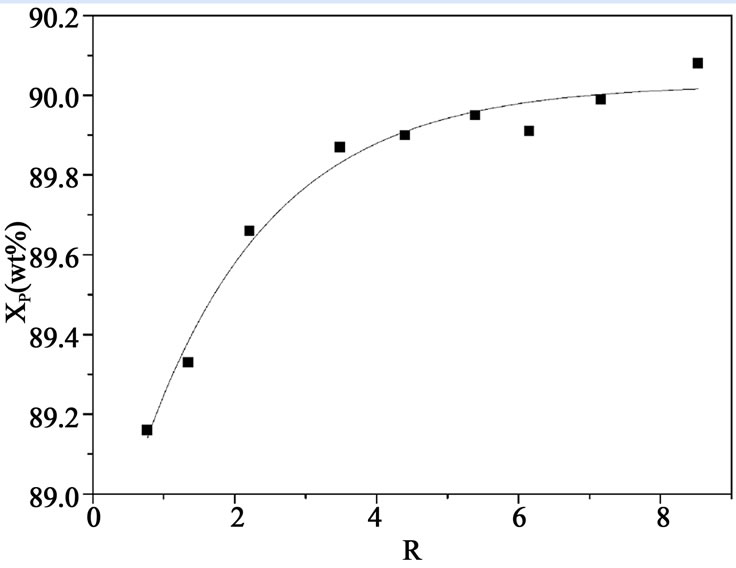
Figure 6. The relationship between product concentration and R.
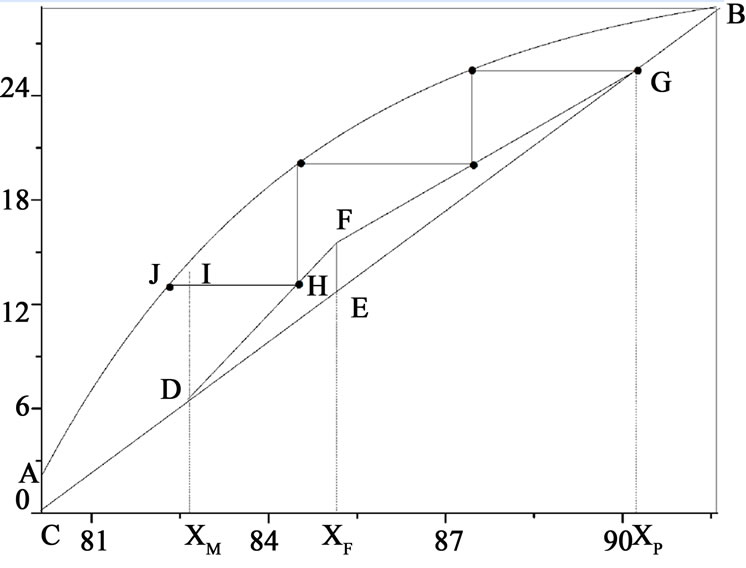
Figure 7. Calculation of the number of theoretical plates.
is the recovery line, which could be drawn by a straight line that connects the point (xM, xM) and the point where the q line and enriching line intersect, xM is the concentration of phosphoric acid in the residual stream. The number of theoretical steps, N, is calculated by the McCabe-Thiele type method. For instance, N is about 2.8 for Figure 7.
The number of theoretical plates N at different reflux ratio is shown in Figure 8. It can be seen that N is larger as the reflux ratio increases, and remains almost constant at last. As the reflux ratio increases, more fresh melts are provided by the reflux flows of the melts, it is possible to increase the amount of settling crystals and the suspension density is higher. So the crystal bed height increases, which is the real site of purification takes place, and hence more efficient purifying process is being carried out within in the bed. The number of theoretical plates N changes little after R is larger than 6, because the purification mechanism of countercurrent washing transfers to sweating, and impurities diffuse from crystals to melt slowly which tends to decrease the impurity removal efficiency, and this is consistent with the result indicated in Figure 6. Although product is much purer and the column has higher purification efficiency under high reflux ratio, the product yield decreases, so it should be determined by throughput of product.
4. Conclusion
Continuous suspension crystallization to purify phosphoric acid were conducted in a cascade of MSMPR crystallizer and a gravity wash column, dynamic behavior in the crystallizer and the influence of reflux ratio on the separation and purification capability of column were studied experimentally. A reasonable steady state with respect to the liquid phase (solution concentration determination) is achieved after 3 to 4 hrs, and this is followed by a solid-phase steady state in terms of the slurry density
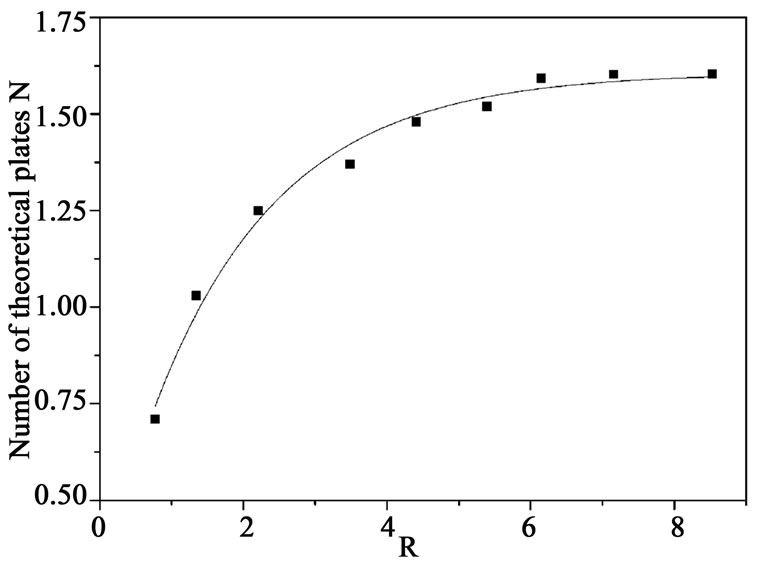
Figure 8. Effect of R on the number of theoretical plates.
after 4 hrs. Stirring speed in MSMPR crystallizer is 200 - 300 rpm, which ensures full suspension of crystals and without secondary nucleation. Impurity concentration of products is lower and consequently the number of theoretical plates is larger as the reflux ratio increases. It is found that reflux ratio is the effective parameter for separation and purification by the crystallization equipment from the influences of reflux ratio on the purity of product, the number of theoretical plates and the stability of the operations.
5. Acknowledgements
The authors gratefully acknowledge the financial support from The Eleventh Five-year National Science and Technology Supporting Major Project (2007BAE58B03) PRC and West Region Project of Shanghai Science and Technology (10195801600).
REFERENCES
- P. Becker, “Phosphates and Phosphoric Acid,” Marcel Decker Inc., New York, 1983.
- M. Feki, “Contribution to the Phosphoric-Acid Purification of Wet Process by extraction with the Methyl-Isobutylcetone,” Ph.D. Thesis, University of Sciences, Tunis, 2001.
- R. Dhouib-Sahnoun, M. Feki and H. F. Ayedi, “Extractive purification of Wet Phosphoric Acid with tributylphosphate: study of the Impurities Distribution,” Journal of the Science of Tunisia, Vol. 8, 2000, pp. 873-885.
- A. M. Urtiaga, A. Alonso, I. Ortiz, J. A. Daoud and S. A. El-Reefy, “Comparison of Liquid Membrane Processes for the removal of cadmium from Wet Phosphoric Acid,” Journal of Membrane Science, Vol. 164, No. 1, 2000, pp. 229-240. doi:10.1016/S0376-7388(99)00197-0
- V. Kislik and A. Eyal, “Aqueous Hybrid Liquid Membrane Process for Metal Separation. Part II. Selectivity of Metals Separation from Wet-Process Phosphoric Acid,” Journal of Membrane Science, Vol. 169, No. 1, 2000, pp. 133-146. doi:10.1016/S0376-7388(99)00332-4
- J. F. McCullough, “Phosphoric Acid Purification: Comparing the Process Choices,” Chemical Engineering, Vol. 83, 1976, pp. 101-106.
- W. J. Huang, “Progress in the field of Purifying WetProcess Phosphoric Acid by Solvent Precipitation,” Chemical Industry and Engineering Progress, Vol. 6, 1997, pp. 39-43.
- J. Zhu, “Preparation of Electric-Grade Phosphoric Acid by Melt Crystallization,” CN Patent No. 1843900, 2006.
- H. y. Wei and L. p. Dang, “Purification of phosphoric acid by crystallization,” CN Patent No. 1730385, 2006.
- K. C. Ung and K. J. Cheon, “Purification method of Phosphoric Acid through Layer Crystallization Including Washing Operation,” KR Patent No. 20060111282(A), 2006.
- Aaltonen, Jarmo and Sakari, “Process for production of Phosphoric Acid by crystallization of phosphoric acid hemihydrates,” WO Patent No. 00/59827, 2000.
- Y. Yasuo, T. Seikichi and N. Katsuyuki, “High purity Phosphoric Acid,” JP Patent No. 2000026111, 2000.
- K. Tadeusz, Wiewiorowski, P. D. Mollere, V. C. Astley and D. M. Dyer, “Phosphoric Acid Crystallization Process,” US Patent No. 4655789, 1987.
- H. Hisao, Y. Akihisa and K. Masao, “Production of Highly Purified Phosphoric Acid,” JP Patent No. 2225309, 1990.
- M. Matsuoka, T. Fukuda, Y. Takagi and H. Takiyama, “Purification of Organic Solid Solutions by Melt Crystallization: Comparison between Layer and Suspension Crystallization,” Journal of Crystal Growth, Vol. 166, No. 1- 4, 1996, pp. 1035-1039. doi:10.1016/0022-0248(95)00481-5
- A. P. B. Ribeiro, R. C. Basso and R. Grimaldi, “Influence of Chemical Interesterification on Thermal Behavior, Microstructure, Polymorphism and Crystallization Properties of Canola Oil and Fully Hydrogenated Cottonseed Oil Blends,” Food Research International, Vol. 42, No. 8, 2009, pp. 1153-1162. doi:10.1016/j.foodres.2009.05.016
- L. Svanberg, L. Ahrné, N. Lorén and E. Windhab, “Effect of Sugar, Cocoa Particles and lecithin on Cocoa Butter Crystallization in seeded and Non-Seeded Chocolate Model Systems,” Journal of Food Engineering, Vol. 104, No. 1, 2011, pp. 70-80. doi:10.1016/j.jfoodeng.2010.09.023
- H. Miki, R. Fukunaga, Y. Asakuma, K. Maeda and K. Fukui, “Distribution of dye into KDP crystals in a continuous MSMPR crystallizer,” Separation and Purification Technology, Vol. 43, No. 1, 2005, pp. 77-83. doi:10.1016/j.seppur.2004.10.006
- H. Miki, T. Terashima, Y. Asakuma, K. Maeda and K. Fukui, “Inclusion of Mother Liquor inside KDP crystals in a continuous MSMPR crystallizer,” Separation and Purification Technology, Vol. 43, No.1, 2005, pp. 71-76. doi:10.1016/j.seppur.2004.10.002
- Y. Asakuma, T. Terashima, K. Maeda and K. Fukui, “Attrition behavior by Micro-Hardness Parameter in Suspension Crystallization Processes,” Powder Technology, Vol. 171, 2007, pp. 75-80. doi:10.1016/j.powtec.2006.10.001
- V. W. Uhl and J. B. Gray, “Mixing-Theory and Practice,” Academic Press, New York, 1966.
- M. Nagashima, K. Takegami, H. Takiyama and M. Matsuoka, “Mechanisms of purification of Crystalline Particles in an Inclined Column Crystallizer,” Proceedings of the 14th International Symposium on Industrial Crystallization, Cambridge, 12-16 September 1999, pp. 1861- 1871.
NOTES
*Corresponding author.

