 Pharmacology & Pharmacy, 2013, 4, 638-646 Published Online November 2013 (http://www.scirp.org/journal/pp) http://dx.doi.org/10.4236/pp.2013.48090 Open Access PP Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis —II. S1P-S1P1 Axis Induces Pro-Inflammatory Cytokine Production in Astrocytes Noriyasu Seki1, Hirotoshi Kataoka1, Kunio Sugahara1, Atsushi Fukunari2, Kenji Chiba1,2* 1Pharmacology Research Laboratories I, Research Division, Mitsubishi Tanabe Pharma Corporation, Yokohama, Japan; 2Advanced Medical Research Laboratories, Research Division, Mitsubishi Tanabe Pharma Corporation, Yokohama, Japan. Email: *Chiba.Kenji@mk.mt-pharma.co.jp Received October 11th, 2013; revised November 5th, 2013; accepted November 12th, 2013 Copyright © 2013 Noriyasu Seki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Therapeutic administration of fingolimod hydrochloride (FTY720), the functional antagonist at sphingosine 1-phos- phate (S1P) receptor 1 (S1P1) shows a marked improving effect on experimental autoimmune encephalomyelitis (EAE) induced by myelin oligodendrocyte glycoprotein (MOG) in C57BL/6 mice. However, this treatment showed an only partial inhibition of Th1/Th17 cell infiltration into the central nervous system (CNS), suggesting that down-regulation of lymphocytic S1P1 is insufficient to explain the therapeutic effect of FTY720 on EAE. On the other hand, the thera- peutic administration of FTY720 reduced the mRNA expressions of IL-6, CCL2, and glial fibrillary acidic protein, an activation marker of astrocytes, in the CNS of EAE mice. In human astrocytic glyoma, U373MG cells, mRNA expres- sion of S1P1 was higher as compared with those of the other S1P receptor subtypes and phosphorylation of Akt was induced by S1P, FTY720-phosphate (FTY720-P), or an S1P1-selective agonist, SEW2871. FTY720-P appeared to in- duce down-regulation of S1P1 in U373MG cells, implying a functional antagonism at S1P1 on astrocytes. S1P but not FTY720-P induced production of IL-6, IL-8, and CCL2 significantly and treatment with FTY720-P or SEW2871 inhib- ited production of these pro-inflammatory cytokines from U373MG cells stimulated with S1P. These results suggest that S1P-S1P1 axis induces production of pro-inflammatory cytokines by astrocytes. Consequently, it is highly probable that the therapeutic effects of FTY720 on EAE are caused by inhibiting not only egress of myelin-specific Th cells from the draining lymph nodes but also activation of astrocytes in the CNS. Keywords: Sphingosine 1-Phosphosphate Receptor 1; Fingolimod Hydrochloride (FTY720); Experimental Autoimmune Encephalomyelitis; Astrocytes; Pro-Inflammatory Cytokines 1. Introduction A phospholipid mediator, sphingosine 1-phospahte (S1P) and its receptor 1 (S1P1) play an essential role in lym- phocyte egress from the secondary lymphoid organs [1-5]. Fingolimod hydrochloride (FTY720) is an orally active S1P receptor modulator with a structure closely related to sphingosine [6,7]. Phosphorylated FTY720 (FTY720-P, (S)-enantiomer) by sphingosine kinases shows an agonistic activity at four types of S1P receptors (S1P1, S1P3, S1P4, and S1P5) [8]. Notably, FTY720-P induces down-regulation of S1P1 by internalization and subse- quent degradation to reduce S1P responsiveness and acts as a functional antagonist at S1P1 [9-13]. Experimental autoimmune encephalomyelitis (EAE) is a CD4 T cell-dependent animal models for human multi- ple sclerosis (MS) [14-17]. There are several reports that FTY720 is highly effective in EAE in rats and mice [18-23]. As reported previously, prophylactic adminis- tration of FTY720 (0.3 mg/kg, orally) almost completely inhibited development of EAE induced by immunization with myelin proteolipid protein peptide in SJL/J mice [20]. In addition, prophylactic FTY720 administration markedly reduced the infiltration of Th1 and Th17 cells into the central nervous system (CNS) of EAE mice whereas this treatment increased the frequency of Th1 and Th17 cells in the inguinal lymph nodes, implying sequestration of myelin antigen-specific Th1/Th17 cells into draining lymph nodes [24]. From these results, it is *Corresponding author.  Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis 639 suggested that prophylactic effects of FTY720 on EAE are predominantly caused by inhibiting lymphocyte eg- ress from draining lymph nodes. On the other hand, therapeutic administration of FTY720 after establishment of EAE showed significant improvement of EAE symptoms on chronic-progressing EAE induced by immunization with myelin oligodendro- cyte glycoprotein (MOG) in C57BL/6 mice [20,23]. However, we confirmed that therapeutic treatment with FTY720 only showed a partial inhibition of Th1/Th17 cell infiltration into the spinal cord of EAE mice, suggesting involvement of different mechanisms not mediated via lymphocytic S1P1. Recently, Choi et al. have reported that FTY720 efficacy was lost in CNS mutants lacking S1P1 on glial fibrillaly acidic protein (GFAP)-expressing astrocytes but not on neurons [25]. Their studies on S1P1 receptor rescue and pharmacolo- gical experiments revealed the loss of S1P1 on astrocytes through functional antagonism by FTY720-P. In the present study, we found that S1P can induce production of interleukin (IL)-6, IL-8 and CC-chemokine ligand 2 (CCL2) in human astrocytic glyoma, U373MG cells via S1P1. Furthermore, we demonstrated that FTY720-P inhibits S1P-induced pro-inflammatory cyto- kine production in U373MG cells, suggesting a func- tional antagonism at S1P1 on astrocytes. 2. Materials and Methods 2.1. Mice Inbred strains of female C57BL/6 mice were purchased from Charles River Japan and were used at 7 to 12 weeks of age. All animal experiments were performed under an experimental protocol approved the ethics review com- mittee for animal experimentation of Research Division, Mitsubishi Tanabe Pharma Corporation. 2.2. Agents, Cell Lines, and Antibodies FTY720 was synthesized according to the method described previously [6] and was dissolved in distilled water for oral administration. The (S)-enantiomer of FTY720-P (>99.5% enantio excess) synthesized was dissolved in ethanol for in vitro experiments [7]. SEW2871, an S1P1-selective agonist was synthesized according to the method reported previously [26]. S1P was purchased from Sigma-Aldrich. IL-1β and tumor necrosis factor (TNF)-α were purchased from Peprotech. MOG35-55 (MEVGWYRSPFSRVVHLYRNGK) peptide was obtained from Peptide Institute. RPMI 1640 medium was supplemented with 10% fetal calf serum (FCS) which was pretreated with charcoal, 10 mM HEPES, 50 U/ml penicillin, 50 μg/ml streptomycin, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 50 μM 2-mer- captoethanol. Human astrocytic glioma, U373MG cells obtained from American Type Culture Collection were maintained by culturing at 37˚C in 5% CO2 in Dul- becco’s modified eagle medium (DMEM, low glucose) supplemented with 10% FCS pretreated with charcoal, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cy-Chrome-conjugated rat anti-mouse CD4 mono- clonal antibody (mAb) (GK1.5), fluorescein isothocya- nate (FITC)-conjugated rat anti-mouse interferon (IFN)-γ mAb (XMG1.2), phycoerythrin (PE)-conjugated rat anti- mouse IL-17 mAb (TC11-18H10.1), and polyclonal anti- bodies (pAbs) against ionized calcium binding adaptor molecule 1 (Iba1) and GFAP were purchased from BD Bioscience. Mouse anti-human S1P1 pAb (H-60) and rabbit anti-phosphorylated Akt (Thr308) pAb were ob- tained from Santa Cruz and Cell Signaling Technology, respectively. Alexa 594-conjugated anti-rabbit immuno- globulin (Ig) G (Molecular Probes) and horse radish per- oxidase (HRP)-conjugated anti-rabbit IgG (GE Health- care Bioscience) were used as secondary antibodies. 2.3. EAE Induction For induction of EAE, C57BL/6 mice received immuni- zation of MOG35-55 in Freund’s complete adjuvant sub- cutaneously on day 0, followed by intravenous injection with 200 ng of pertussis toxin (List Biological Labor- atories) on day 0 and 2 [20]. Individual mice were scored for clinical signs of EAE using the following criteria: 0, no paralysis; 0.5, stiff tail; 1, limp tail; 1.5, limp tail with inability to right; 2, paralysis of one limb; 2.5, paralysis of one limb and weakness of one other limb; 3, complete paralysis of both hind limbs; 4, moribund state; 5, death. 2.4. Intracellular Cytokines Staining and Flow Cytometry Analysis Lymphocytes prepared from the inguinal lymph nodes or spinal cords were stimulated with 50 ng/ml phorbol-12- myristate-13-acetate and 500 nM ionomycin in the presence of 2 μM monensin for 5 h in RPMI 1640 medium containing 10% FCS at 37˚C in 5% CO2. After blocking with anti-mouse CD16/32 mAb, the cells were stained with Cy-Chrome-conjugated anti-mouse CD4 mAb and permeabilized with 0.5% Triton X-100. The intracellular cytokine staining was performed by using FITC-conjugated anti-mouse IFN-γ mAb and PE-con- jugated anti-mouse IL-17 mAb and flow cytometry analysis was conducted using FACScan (BD Bio- sciences). 2.5. Real Time Polymerase Chain Reaction Total RNA was extracted using TRIZOL (Invitrogen) and a tow-step quantitative revers transcriptation-poly- Open Access PP  Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis 640 merase chain reaction (RT-PCR) was performed to deter- mine various molecules mRNA expression using a re- lative standard curve method with cellular housekeeping enzyme (glyceraldehyde 3-phosphate dehydrogenase: GAPDH or β-actin) as normalization control. Comple- mentary DNA was synthesized with TaqMan Reverse Transcription Reagents (Applied Biosystems) using ran- dom hexamers and 0.5 μg of total RNA. Complementary DNA was amplified with various molecules TaqMan probe (6-carboxy-fluorecein label)/primer, cellular house- keeping enzyme TaqMan probe (VIC label)/primer, and TaqMan Universal PCR Master Mix in an ABI PRISM 7900 Sequence Detection System (Applied Biosystems). For analyses of mRNA expressions in mouse tissues, following TaqMan probes/primers were used: CD3 (Mm00599683_m1), IFN-γ (Mm00801778_m1), TNF-α (Mm00443258_m1), T-bet (Mm00450960_m1), a IL-6 (Mm00446190_m1), IL-17 (Mm00439619_m1), CCL2 (Mm00441242_m1), CCL5 (Mm01302427_m1), C-X-C chemokine ligand 10 (CXCL10) (Mm00445235_m1), and GFAP (Mm01253033_m1). GAPDH TaqMan probe/ primer was used as normalization control. For quantifica- tion, standard curves were generated for various molecu- les using serially diluted cDNA samples from the spinal cords in EAE mice. For every sample, a level of mRNA normalized by calculating the ratio of each target mole- cule/GAPDH level. To analyze mRNA expression of retinoic acid receptor-related orphan receptor (ROR) γt in mouse tissues, real time PCR was performed with an ABI PRISM 7700 sequence detector using SYBR Green PCR master mix (Applied Biosystems). The following primer pairs were used (5’→3’): RORγt forward, CCG CTG AGA GGG CTT CAC; RORγt reverse, TGC AGG AGT AGG CCA CAT TAC A [27]. The reaction was incubated for 2 min at 50˚C, denatured for 10 min at 95˚C and subjected to 40 step amplification cycles with annealing/extension at 60˚C for 1 min followed by denaturation at 95˚C for 15 s. Data were analyzed using Sequence Detector software (Applied Biosystems). For analyses of mRNA expressions of human S1P receptors, following the TaqMan probes/ primers were used: S1P1 (Hs00173499_m1), S1P2 (Hs01003373_m1), S1P3 (Hs01019574_m1), S1P4 (Hs00269446_s1), S1P5 (Hs00258220_s1). β-actin TaqMan probe/primer was used as normalization control. For every sample, a level of mRNA normalized by calculating the ratio of each target molecule/β-actin level. 2.6. Immunohistochemical Staining The spinal cord samples were fixed in 10% formalin and embedded in paraffin. Six μm-thickness sections of the spinal cord were stained with hematoxylin and eosin (H&E). For immunochemical staining, 6 μm-thickness frozen sections of the spinal cord were immediately fixed in cold-acetone and incubated with polyclonal antibody against Iba1 or GFAP. The sections were then incubated with secondary antibodies conjugated with amino acid polymer and peroxidase (Histofine Simple Stain MAX- PO kit; Nichirei), colorized with diaminobenzidine in the presence of hydrogen peroxide, and counterstained with hematoxylin. 2.7. Western Blotting Analysis for Akt Phosphorylation After serum starvation for 24 h, U373MG cells (106 cells) were stimulated with S1P or FTY720-P for 10 min and then the cells were lysed. Proteins in the cell lysate were separated by SDS-PAGE and transferred to polyviny- lidene difluoride membrane (Millipore). After blocking, the membrane was incubated with anti-phosphorylated Akt (Thr308) antibody and subsequently with HRP- conjugated secondary antibody. Phosphorylated Akt was visualized with ECL Plus Kit (GE Healthcare Bio- science). 2.8. Immunofluorescence Microscopy U373MG cells were stimulated with FTY720-P at 100 nM for 30 min, and then the cells were washed with phosphate buffered saline (PBS) and fixed with 3.7% formaldehyde in PBS at room temperature for 20 min. Permeabilization was performed in PBS containing 0.5% Triton X-100 and 1 mg/ml bovine serum albumin for 5 min. Immunofluorescence staining was performed by incubation with anti-human S1P1 antibody for 1 h, fol- lowed by detected with alexa-594-conjugated anti-rabbit IgG at room temperature for 30 min. Immunofluo- rescence was digitally captured under a confocal laser microscope (Carl Zeiss). 2.9. Cytokine Production U373MG cells (104 cells) were cultured in the presence of S1P or FTY720-P in DMEM containing with 10% FCS for 48 h at 37˚C in 5% CO2. In other experiments, U373MG cells were pretreated with FTY720-P or SEW2871 for 2 h under serum-free condition, and then the cells were cultured in the presence of 100 nM S1P for 48 h. Concentrations of IL-6, IL-8, and CCL2 in the cul- ture supernatants were determined by BDTM Cytometric Beads Array (BD Biosciences). 2.10. Statistical Analyses Results were express as the mean ± standard error mean (SEM). Statistical differences of EAE clinical scores were analyzed by Mann-Whitney U test. In other experi- Open Access PP 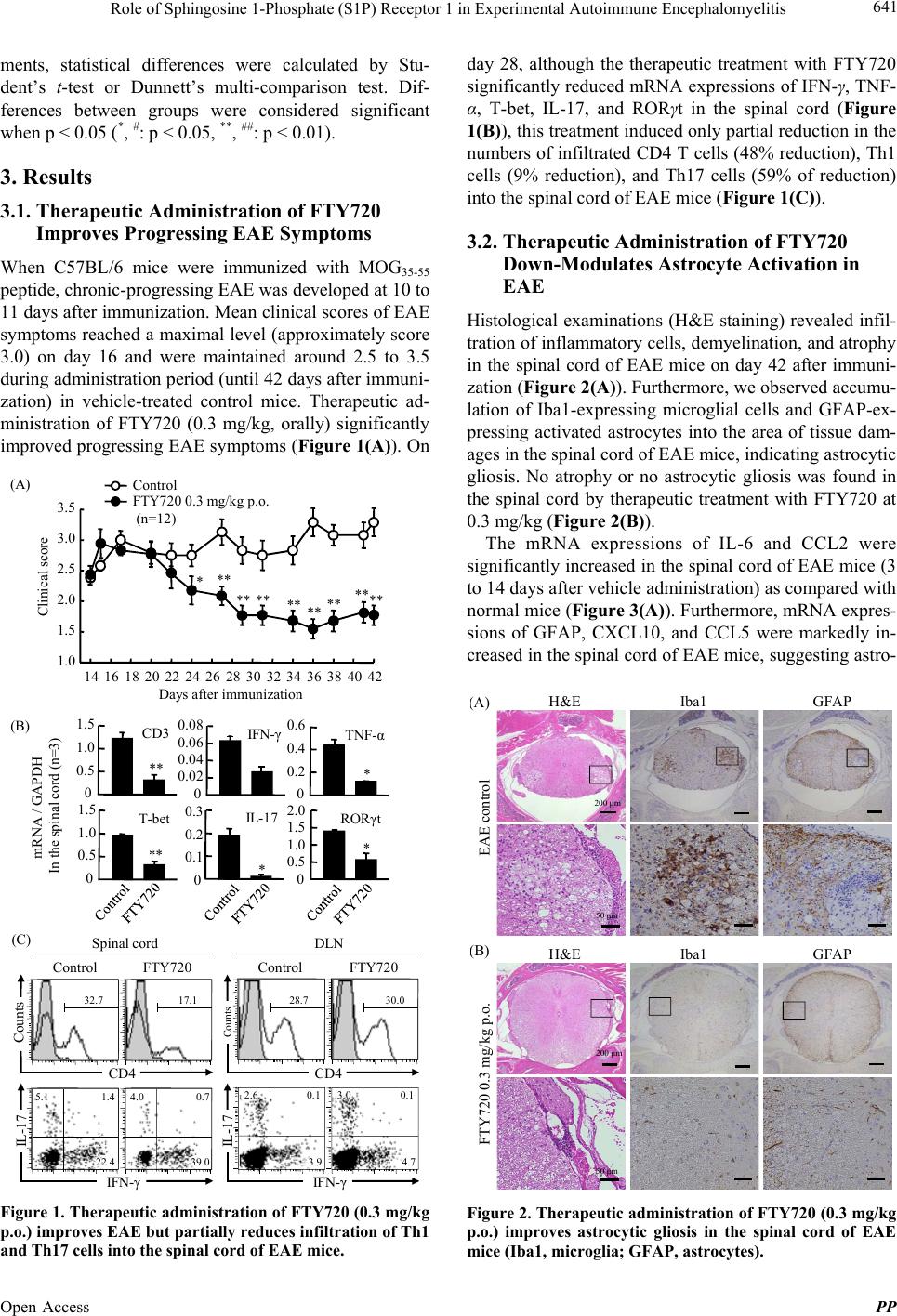 Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis 641 ments, statistical differences were calculated by Stu- dent’s t-test or Dunnett’s multi-comparison test. Dif- ferences between groups were considered significant when p < 0.05 (*, #: p < 0.05, **, ##: p < 0.01). 3. Results 3.1. Therapeutic Administration of FTY720 Improves Progressing EAE Symptoms When C57BL/6 mice were immunized with MOG35-55 peptide, chronic-progressing EAE was developed at 10 to 11 days after immunization. Mean clinical scores of EAE symptoms reached a maximal level (approximately score 3.0) on day 16 and were maintained around 2.5 to 3.5 during administration period (until 42 days after immuni- zation) in vehicle-treated control mice. Therapeutic ad- ministration of FTY720 (0.3 mg/kg, orally) significantly improved progressing EAE symptoms (Figure 1(A)). On (C) Spinal cordDLN Control FTY720 Counts CD4 IL-17 IFN - γ Count s IL-17 Control FTY720 CD4 IFN - γ 5.1 1.4 22.4 4.0 0.7 39.0 0.1 3.9 2.6 0.1 4.7 3.0 32.7 17.128.730.0 (A) (B) IL-17 * RORγt * 0 0.5 1.0 1.5 2.0 0 0.2 0.4 0.6 TNF-α * IFN - γ 0 0.1 0.2 0.3 0 0.02 0.04 0.06 0.08 T-bet ** mRNA / GAPDH In the spinal cord (n=3) 0 0.5 1.0 1.5 0 0.5 1.0 1.5 CD3 ** 14 1618 2022 2426 2830 3234 3638 4042 1.0 1.5 2.0 2.5 3.0 3.5 Clinical score Days after immunization *** ** **** ** ** **** Control FTY720 0.3 mg/kg p.o. (n=12) Figure 1. Therapeutic administration of FTY720 (0.3 mg/kg p.o.) improves EAE but partially reduc es infiltration of Th1 and Th17 cells into the spinal cord of EAE mice. day 28, although the therapeutic treatment with FTY720 significantly reduced mRNA expressions of IFN-γ, TNF- α, T-bet, IL-17, and RORγt in the spinal cord (Figure 1(B)), this treatment induced only partial reduction in the numbers of infiltrated CD4 T cells (48% reduction), Th1 cells (9% reduction), and Th17 cells (59% of reduction) into the spinal cord of EAE mice (Figure 1(C)). 3.2. Therapeutic Administration of FTY720 Down-Modulates Astrocyte Activation in EAE Histological examinations (H&E staining) revealed infil- tration of inflammatory cells, demyelination, and atrophy in the spinal cord of EAE mice on day 42 after immuni- zation (Figure 2(A)). Furthermore, we observed accumu- lation of Iba1-expressing microglial cells and GFAP-ex- pressing activated astrocytes into the area of tissue dam- ages in the spinal cord of EAE mice, indicating astrocytic gliosis. No atrophy or no astrocytic gliosis was found in the spinal cord by therapeutic treatment with FTY720 at 0.3 mg/kg (Figure 2(B)). The mRNA expressions of IL-6 and CCL2 were significantly increased in the spinal cord of EAE mice (3 to 14 days after vehicle administration) as compared with normal mice (Figure 3(A)). Furthermore, mRNA expres- sions of GFAP, CXCL10, and CCL5 were markedly in- creased in the spinal cord of EAE mice, suggesting astro- Iba1 GFAPH&E EAE controlFTY720 0.3 mg/kg p.o. 200 μm 50 μm 50 μm 200 μm A) B) Iba1 GFAPH&E Figure 2. Therapeutic administration of FTY720 (0.3 mg/kg p.o.) improves astrocytic gliosis in the spinal cord of EAE mice (Iba1, microglia; GFAP, astrocytes). Open Access PP  Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis 642 (B) Day 3 CCL2 mRNA / GAPDH in the spinal cord (n=3) IL-6 CCL5CXCL10GFAP * p=0.07 0 0.05 0.10 0.15 0.20 0 0.5 1.0 1.5 0 0.05 0.10 0.15 0 0.1 0.2 0.3 0.4 0 0.1 0.2 0.3 0.4 0 0.05 0.10 0.15 0.20 0.2 0.4 0.6 0 0.2 0.4 0.6 0.8 0 0.5 1.0 1.5 0 * (A) Day 7Day 14 Day 14Day 14Day 14 mRNA / GAPDH in the spinal cord (n=3) Day 3Day 7Day 14 Figure 3. Therapeutic administration of FTY720 (0.3 mg/kg p.o.) decreases mRNA expressions of pro-inflammatory cytokines and GFAP in the spinal cord of EAE mice. cyte activation (Figure 3(B)). Therapeutic administration of FTY720 at 0.3 mg/kg reduced mRNA expressions of IL-6 (40% to 58% reductions) and CCL2 (37% and 48% reduction on day 3 and day 7, respectively) (Figure 3(A)). Similarly, mRNA expressions of GFAP, CXCL10, and CCL5 were lower in the spinal cord of EAE mice after FTY720 administration, suggesting down-modulation of astrocytes activation (Figure 3(B)). 3.3. FTY720-P Induces Down-Regulation of S1P1 on Human Astrocytic Glioma Cells Because our results of mouse EAE revealed that FTY720 may down-modulate astrocyte activation, we focused to analyze astrocyte function using human astrocytic glioma, U373MG cells and found that the expression level of S1P1 mRNA was higher as compared with those of the other S1P receptor subtypes in U373MG cells (Figure 4(A)). Furthermore, we demonstrated that phosphoryla- tion of Akt was induced by treatment with S1P (0.1 nM or higher), FTY720-P (0.1 nM or higher), or S1P1-selec- tive agonist, SEW2871 (100 nM or higher) in U373MG cells, suggesting that S1P and FTY720-P induce S1P1- dependent Akt phosphorylation (Figure 4(B)). There was no change in total Akt protein contents in U373MG cells in the presence or absence of these compounds (data not shown). By immunofluorescence using anti-human S1P1 pAb under con-focal microscopy, we confirmed that S1P1 is abundant in cell surface and cytoplasm of S1PR mRNA / β-actin (n=3) S1P1 S1P2 S1P3 S1P4 S1P5 0.008 0.010 0 0.002 0.004 0.006 S1P FTY720-P SEW2871 (A) mRNA expression of S1P receptors 0nM 101001000 10000 00.1110100 nM (B) Akt phosphorylation in U373MG cells (C) S1P1 expression in U373MG cells Control FTY720-P U373MG cells Figure 4. The expression level of S1P1 mRNA is higher in U373MG cells and FTY720-P induces Akt phosphorylation and down-regulation of S1P1. U373MG cells (Figure 4(C), left). By contrast, S1P1 expression was markedly reduced by treatment with 100 nM FTY720-P, indicating internalization (down-regula- tion) of S1P1 in U373MG cells (Figure 4(C), right). 3.4. FTY720-P Inhibits Pro-Inflammatory Cytokine Production Induced by S1P in Astrocytic Glyoma, U373MG Cells Because U373MG cells are reported to produce high level of IL-6 by stimulation with IL-1β [28], we examined the ability of S1P to induce production of pro- inflammatory cytokines including IL-6 in this cell line. We found that S1P at 100 nM or higher markedly induce production of IL-6, IL-8, and CCL2 cells whereas FTY720-P up to 1000 nM showed no clear effect in U373MG cells (Figure 5(A)). Treatment with FTY720-P (100 nM or higher) significantly inhibited S1P-induced production of IL-6, IL-8, and CCL2 in a dose-dependent manner (Figure 5(B)). Similar results were obtained when SEW2781 at 10000 nM was treated, suggesting that S1P induces production of IL-6, IL-8 and CCL2 via Open Access PP  Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis 643 Pro-inflammatory cytokine (ng/mL) 0 1 2 3 4 5 6 CCL2 10 10 3 FTY720-P 10 2 S1P - 10 10 3 10 2 (nM) 0 5 10 15 20 IL-8 0 0.5 1.0 1.5 2.0 2.5 (A) IL-6 0 5 10 15 20 0 0.2 0.4 0.6 0.8 1.0 1.2 0 1 2 3 4 5 - 010 10 3 10 4 FTY720-P SEW 10 4 10 2 S1P (100 nM) CCL2 *** ** ## IL-6 ** ** ** ## IL- 8 ** ** ** # (nM) Pro-inflammatory cytokine (ng/mL) (B) Figure 5. S1P induces IL-6, IL-8 and CCL2 produc tion and FTY720-P inhibits production of these pro-inflammatory cytokines induced by S1P in U373MG cells. S1P1 (Figure 5(B)). On the other hand, FTY720-P did not affect IL-1β- or TNF-α-induced IL-6 production in U373 MG cells (data not shown). 4. Discussion In this study, we demonstrated that therapeutic adminis- tration of FTY720 showed a significant improvement of chronic EAE induced by MOG in C57BL/6 mice; however this treatment partially reduced infiltration of Th1 and Th17 cells into the CNS. On the other hand, the therapeutic treatment with FTY720 resulted in no atro- phy of the spinal cord or no astrocytic gliosis, and re- duced mRNA expressions of GFAP and several pro- inflammatory cytokines such as IL-6 and CCL2 in the CNS of EAE mice. Based on these results, it is thought that down-regulation of lymphocytic S1P1 is insufficient to explain the therapeutic effect of FTY720 on EAE. Namely, this means that the improvement of chronic EAE by FTY720 is likely due to not only a functional antagonism at lymphocytic S1P1 but also other me- chanisms. Foster et al. reported that in chronic EAE induced by MOG in DA rats, therapeutic treatment with FTY720 after establishment of the diseases markedly improves EAE symptoms [21]. They proposed the possibility that FTY720-P directly acts on neural cells in the CNS as well as lymphocytes because the concentrations of FTY720 and FTY720-P in the brain was higher 10 times than in peripheral blood [21]. Recently, Choi et al. demonstrated that FTY720 efficacy was lost in CNS mutants lacking S1P1 on neural cells, particularly GFAP- expressing astrocytes but not on neurons [25]. Their studies on S1P1 receptor rescue and pharmacological experi- ments revealed the loss of S1P1 on astrocytes through functional antagonism by FTY720-P as one of primary mechanism of FTY720. From these findings, it is highly probable that the functional antagonism at S1P1 on astrocytes by FTY720-P partly attribute the improvement of EAE symptoms by therapeutic treatment with FTY720 after disease establishment. To examine the direct effect of FTY720-P on astrocyte functions via S1P1, we used human astrocytic glioma, U373MG cells because this cell line expressed higher mRNA level of S1P1 rather than the other S1P receptors. Therefore, U373MG cells are thought to be useful for analysis of astrocyte functions mediated by S1P-S1P1 axis. In this study, we confirmed that phosphorylation of Akt was induced by S1P, FTY720-P, or SEW2871 in U373MG cells, suggesting that S1P and FTY720-P act as an agonist at S1P1 [26,29]. Consistent with the results using human S1P1-stably expressing CHO cells [8,30] or human astrocytes [25], our data clearly demonstrated that FTY720-P induces and maintains down-regulation of S1P1 in U373MG cells, suggesting a functional anta- gonism at S1P1 on astrocytes. Similar results have been reported that FTY720 desensitizes cell surface S1P1 on astrocytes derived from human fetal CNS specimens for more than 24 h [31]. Pro-inflammatory cytokines such as IL-6, CCL2, and CXCL10 are considered to play an important role in progression of EAE based on the studies using knockout mice of these molecules [32-35]. Furthermore, it has been shown that IL-6 derived from astrocytes can induce EAE [36] and that CXCL10 mRNA is expressed mainly in astrocytes in MOG-induced EAE mice [37]. Based on these findings, it is considerably meaningful to elucidate the role of S1P1-S1P axis in pro-inflammatory cytokine/ chemokine productions by astrocytes. Doorn et al. re- ported that TNF-α can induce CCL2 production by primary human astrocytes or U373MG cells [38]. How- ever, there is no report revealing whether S1P at physiol- ogical concentrations can induce production of pro-in- flammatory cytokines by astrocytes. In this study, we found that S1P 100 nM or higher can induce production of IL-6, IL-8, and CCL2 significantly in U373MG cells. Furthermore, pretreatment with FTY720-P (100 nM or higher) or SEW2871 (10000 nM) inhibited production of IL-6, IL-8, and CCL2 by S1P- activated U373MG cells, implying the involvement of S1P-S1P1 axis in astrocyte activation. Trough concen- trations of FTY720-P in the brain reached to approxi- mately 900 nM in rodents when FTY720 (0.3 mg/kg, Open Access PP  Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis 644 orally) was administrated daily for 3 weeks [21]. There- fore, it is highly possible that FTY720-P inhibits pro- duction of IL-6, IL-8 and CCL2 by S1P-activated astro- cytes in EAE mice if S1P concentrations are elevated to more than 100 nM by neuroinflammation [39]. Although it was reported that FTY720 (10 to 100 μM) partially reduced TNF-α-induced CCL2 production in human astrocytes or U373 MG cells [38], this effect may be unlikely because of its extremely high concentrations. A mechanism underlying S1P-induced production of IL-6, IL-8, and CCL2 in U373MG cells is probably mediated through a signal transduction pathway via NF- κB because NF-κB is shown to be activated by down- stream signaling of S1P1 [40,41]. Unlike S1P, FTY720-P did not induce IL-6, IL-8, or CCL2 by U373MG cells. FTY720-P may not efficiently bring about production of these cytokines because of down-regulation of cell sur- face S1P1 expression [9-13]. In conclusion, our findings suggest that FTY720-P down-modulates production of pro-inflammatory cyto- kines by S1P-activated astrocytes via S1P1. Conse- quently, it is highly probable that the therapeutic effects of FTY720 on EAE are caused by inhibiting not only egress of myelin-specific Th cells from the draining lymph nodes but also activation of astrocytes in the CNS. 5. Acknowledgements The authors are grateful thank for their excellent assis- tance of Mamoru Koyama, Yasuko Ogawa, and Dr. Hiroyuki Utsumi in Advanced Medical Research Labora- tories, and Dr. Hirofumi Matsuyuki in Pharamacology Research Laboratories. We also thank Dr. Kunitomo Adachi at Medicinal Chemistry Laboratories I, Mitsu- bishi Tanabe Pharma Corporation for synthesizing (S)- enantiomer of FTY720-P and SEW2871. REFERENCES [1] S. Mandala, R. Hajdu, J. Bergstrom, E. Quackenbush, J. Xie, J. Milligan, R. Thornton, G. J. Shei, D. Card, C. Keohane, M. Rosenbach, J. Hale, C. L. Lynch, K. Rup- precht, W. Parsons and H. Rosen, “Alteration of Lym- phocyte Trafficking by Sphingosine-1-Phosphate Recep- tor Agonists,” Science, Vol. 296, No. 5566, 2002, pp. 346-349. http://dx.doi.org/10.1126/science.1070238 [2] M. Matloubian, C. G. Lo, G. Cinamon, M. J. Lesneski, Y. Xu, V. Brinkmann, M. L. Allende, R. L. Proia and J. G. Cyster, “Lymphocyte Egress from Thymus and Peripheral Lymphoid Organs Is Dependent on S1P Receptor 1,” Nature, Vol. 427, No. 6972, 2004, pp. 355-360. http://dx.doi.org/10.1038/nature02284 [3] J. G. Cyster, “Chemokines, Sphingosine-1-Phosphate, and Cell Migration in Secondary Lymphoid Organs,” Annual Review of Immunology, Vol. 23, 2005, pp. 127- 159. http://dx.doi.org/10.1146/annurev.immunol.23.021704.11 5628 [4] C. G. Lo, Y. Xu, R. L. Proia and J. G. Cyster, “Cyclical Modulation of Sphingosine-1-Phosphate Receptor 1 Sur- face Expression during Lymphocyte Recirculation and Relationship to Lymphoid Organ Transit,” Journal of Experimental Medicine, Vol. 201, No. 2, 2005, pp. 291- 301. http://dx.doi.org/10.1084/jem.20041509 [5] T. H. Pham, T. Okada, M. Matloubian, C. G. Lo and J. G. Cyster, “S1P1 Receptor Signaling Overrides Retention Mediated by G Alpha i-Coupled Receptors to Promote T Cell Egress,” Immunity, Vol. 28, No. 1, 2008, pp. 122- 133. http://dx.doi.org/10.1016/j.immuni.2007.11.017 [6] K. Adachi, T. Kohara, N. Nakao, M. Arita, K. Chiba, T. Mishina, S. Sasaki and T. Fujita, “Design, Synthesis, and Structure Activity Relationships of 2-Substitued-2-Amino- 1, 3-Propanediols: Discovery of a Novel Immunosup- pressant, FTY720,” Bioorganic & Medicinal Chemistry Letters, Vol. 5, 1995, pp. 853-856. http://dx.doi.org/10.1016/0960-894X(95)00127-F [7] M. Kiuchi, K. Adachi, A. Tomatsu, M. Chino, S. Takeda, Y. Tanaka, Y. Maeda, N. Sato, N. Mitsutomi, K. Suga- hara and K. Chiba, “Asymmetric Synthesis and Biologi- cal Evaluation of the Enantiomeric Isomers of the Immu- nosuppressive FTY720-Phosphate,” Bioorganic and Me- dicinal Chemistry, Vol. 13, No. 2, 2005, pp. 425-432. http://dx.doi.org/10.1016/j.bmc.2004.10.008 [8] K. Chiba, “FTY720, a New Class of Immunomodulator, Inhibits Lymphocyte Egress from Secondary Lymphoid Tissues and Thymus by Agonistic Activity at Sphingosine 1-Phosphate Receptors,” Pharmacology and Therapeutics, Vol. 108, No. 3, 2005, pp. 308-319. http://dx.doi.org/10.1016/j.pharmthera.2005.05.002 [9] K. Chiba, H. Matsuyuki, Y. Maeda and K. Sugahara, “Role of Sphingosine 1-Phosphate Receptor Type 1 in Lymphocyte Egress from Secondary Lymphoid Tissues and Thymus,” Cellular & Molecular Immunology, Vol. 3, No. 1, 2006, pp. 11-19. [10] Y. Maeda, H. Matsuyuki, K. Shimano, H. Kataoka, K. Sugahara and K. Chiba, “Migration of CD4 T Cells and Dendritic Cells toward Sphingosine 1-Phosphate (S1P) Is Mediated by Different Receptor Subtypes: S1P Regulates the Functions of Murine Mature Dendritic Cells via S1P Receptor Type 3,” Journal of Immunology, Vol. 178, No. 6, 2007, pp. 3437-3446. [11] S. Thangada, K. M. Khanna, V. A. Blaho, M. L. Oo, D. S. Im, C. Guo, L. Lefrancois and T. Hla, “Cell-Surface Resi- dence of Sphingosine 1-Phosphate Receptor 1 on Lym- phocytes Determines Lymphocyte Egress Kinetics,” Jour- nal of Experimental Medicine, Vol. 207, No. 7, 2010, pp. 1475-1483. http://dx.doi.org/10.1084/jem.20091343 [12] F. Mullershausen, F. Zecri, C. Cetin, A. Billich, D. Gue- rini and K. Seuwen, “Persistent Signaling Induced by FTY720-Phosphate Is Mediated by Internalized S1P1 Receptors,” Nature Chemical Biology, Vol. 5, No. 6, 2009, pp. 428-434. http://dx.doi.org/10.1038/nchembio.173 [13] M. L. Oo, S. Thangada, M. T. Wu, C. H. Liu, T. L. Mac- donald, K. R. Lynch, C. Y. Lin and T. Hla, “Immunosup- pressive and Anti-Angiogenic Sphingosine 1-Phosphate Open Access PP  Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis 645 Receptor-1 Agonists Induce Ubiquitinylation and Protea- somal Degradation of the Receptor,” Journal of Biologi- cal Chemistry, Vol. 282, No. 12, 2007, pp. 9082-9089. http://dx.doi.org/10.1074/jbc.M610318200 [14] D. A. Hafler, “Multiple Sclerosis,” Journal of Clinical Investigation, Vol. 113, No. 6, 2004, pp. 788-794. http://dx.doi.org/10.1172/JCI21357 [15] V. K. Kuchroo, A. C. Anderson, H. Waldner, M. Munder, E. Bettelli and L. B. Nicholson, “T Cell Response in Ex- perimental Autoimmune Encephalomyelitis (EAE): Role of Self and Cross-Reactive Antigens in Shaping, Tuning, and Regulating the Autopathogenic T Cell Repertoire,” Annual Review of Immunology, Vol. 20, 2002, pp. 101- 123. http://dx.doi.org/10.1146/annurev.immunol.20.081701.14 1316 [16] R. Martin and H. F. McFarland, “Immunological Aspects of Experimental Allergic Encephalomyelitis and Multiple Sclerosis,” Critical Reviews in Clinical Laboratory Sci- ences, Vol. 32, No. 2, 1995, pp. 121-182. http://dx.doi.org/10.3109/10408369509084683 [17] L. Steinman, “Assessment of Animal Models for MS and Demyelinating Disease in the Design of rational Ther- apy,” Neuron, Vol. 24, No. 3, 1999, pp. 511-514. http://dx.doi.org/10.1016/S0896-6273(00)81107-1 [18] M. Fujino, N. Funeshima, Y. Kitazawa, H. Kimura, H. Amemiya, S. Suzuki and X. K. Li, “Amelioration of Ex- perimental Autoimmune Encephalomyelitis in Lewis Rats by FTY720 Treatment,” Journal of Pharmacology and Experimental Therapeutics, Vol. 305, No. 1, 2003, pp. 70-77. http://dx.doi.org/10.1124/jpet.102.045658 [19] M. Webb, C. S. Tham, F. F. Lin, K. Lariosa-Willingham, N. Yu, J. Hale, S. Mandala, J. Chun and T. S. Rao, “Sphingosine 1-Phosphate Receptor Agonists Attenuate Relapsing-Remitting Experimental Autoimmune Ence- phalitis in SJL Mice,” Journal of Neuroimmunology, Vol. 153, No. 1-2, 2004, pp. 108-121. http://dx.doi.org/10.1016/j.jneuroim.2004.04.015 [20] H. Kataoka, K. Sugahara, K. Shimano, K. Teshima, M. Koyama, A. Fukunari and K. Chiba, “FTY720, Sphin- gosine 1-Phosphate Receptor Modulator, Ameliorates Ex- perimental Autoimmune Encephalomyelitis by Inhibition of T Cell Infiltration,” Cellular & Molecular Immunology, Vol. 2, No. 6, 2005, pp. 439-448. [21] C. A. Foster, L. M. Howard, A. Schweitzer, E. Persohn, P. C. Hiestand, B. Balatoni, R. Reuschel, C. Beerli, M. Schwartz and A. Billich, “Brain Penetration of the Oral Immunomodulatory Drug FTY720 and Its Phosphoryla- tion in the Central Nervous System during Experimental Autoimmune Encephalomyelitis: Consequences for Mode of Action in Multiple Sclerosis,” Journal of Pharmacol- ogy and Experimental Therapeutics, Vol. 323, No. 2, 2007, pp. 469-475. http://dx.doi.org/10.1124/jpet.107.127183 [22] B. Balatoni, M. K. Storch, E. M. Swoboda, V. Schonborn, A. Koziel, G. N. Lambrou, P. C. Hiestand, R. Weissert and C. A. Foster, “FTY720 Sustains and Restores Neu- ronal Function in the DA Rat Model of MOG-Induced Experimental Autoimmune Encephalomyelitis,” Brain Research Bulletin, Vol. 74, No. 5, 2007, pp. 307-316. http://dx.doi.org/10.1016/j.brainresbull.2007.06.023 [23] D. Papadopoulos, J. Rundle, R. Patel, I. Marshall, J. Stretton, R. Eaton, J. C. Richardson, M. I. Gonzalez, K. L. Philpott and R. Reynolds, “FTY720 Ameliorates MOG- Induced Experimental Autoimmune Encephalomyelitis by Suppressing Both Cellular and Humoral Immune Re- sponses,” Journal of Neuroscience Research, Vol. 88, No. 2, 2010, pp. 346-359. http://dx.doi.org/10.1002/jnr.22196 [24] K. Chiba, H. Kataoka, N. Seki, K. Shimano, M. Koyama, A. Fukunari, K. Sugahara and T. Sugita, “Fingolimod (FTY720), Sphingosine 1-Phosphate Receptor Modulator, Shows Superior Efficacy as Compared with Interferon- Beta in Mouse Experimental Autoimmune Encephalo- myelitis,” International Immunopharmacology, Vol. 11, No. 3, 2011, pp. 366-372. http://dx.doi.org/10.1016/j.intimp.2010.10.005 [25] J. W. Choi, S. E. Gardell, D. R. Herr, R. Rivera, C. W. Lee, K. Noguchi, S. T. Teo, Y. C. Yung, M. Lu, G. Ken- nedy and J. Chun, “FTY720 (Fingolimod) Efficacy in an Animal Model of Multiple Sclerosis Requires Astrocyte Sphingosine 1-Phosphate Receptor 1 (S1P1) Modula- tion,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 108, No. 2, 2011, pp. 751-756. http://dx.doi.org/10.1073/pnas.1014154108 [26] M. G. Sanna, J. Liao, E. Jo, C. Alfonso, M. Y. Ahn, M. S. Peterson, B. Webb, S. Lefebvre, J. Chun, N. Gray and H. Rosen, “Sphingosine 1-Phosphate (S1P) Receptor Sub- types S1P1 and S1P3, Respectively, Regulate Lympho- cyte Recirculation and Heart Rate,” Journal of Biological Chemistry, Vol. 279, No. 14, 2004, pp. 13839-13848. http://dx.doi.org/10.1074/jbc.M311743200 [27] M. Veldhoen, R. J. Hocking, C. J. Atkins, R. M. Locksley and B. Stockinger, “TGFbeta in the Context of an In- flammatory Cytokine Milieu Supports de Novo Differen- tiation of IL-17-Producing T Cells,” Immunity, Vol. 24, No. 2, 2006, pp. 179-189. http://dx.doi.org/10.1016/j.immuni.2006.01.001 [28] R. O. Carlson and S. H. Aschmies, “Tyrosine Kinase Activity Is Essential for Interleukin-1 Beta—Stimulated Production of Interleukin-6 in U373 Human Astrocytoma Cells,” Journal of Neurochemistry, Vol. 65, No. 6, 1995, pp. 2491-2499. http://dx.doi.org/10.1046/j.1471-4159.1995.65062491.x [29] C. G. Jung, H. J. Kim, V. E. Miron, S. Cook, T. E. Ken- nedy, C. A. Foster, J. P. Antel and B. Soliven, “Func- tional Consequences of S1P Receptor Modulation in Rat Oligodendroglial Lineage Cells,” Glia, Vol. 55, No. 16, 2007, pp. 1656-1667. http://dx.doi.org/10.1002/glia.20576 [30] H. Matsuyuki, Y. Maeda, K. Yano, K. Sugahara, K. Chiba, T. Kohno and Y. Igarashi, “Involvement of Sphin- gosine 1-Phosphate (S1P) Receptor Type 1 and Type 4 in Migratory Response of Mouse T Cells toward S1P,” Cel- lular & Molecular Immunology, Vol. 3, No. 6, 2006, pp. 429-437. [31] C. Wu, S. Y. Leong, C. S. Moore, Q. L. Cui, P. Gris, L. P. Bernier, T. A. Johnson, P. Seguela, T. E. Kennedy, A. Bar-Or and J. P. Antel, “Dual Effects of Daily FTY720 on Human Astrocytes in Vitro: Relevance for Neuroin- flammation,” Journal of Neuroinflammation, Vol. 10, Open Access PP  Role of Sphingosine 1-Phosphate (S1P) Receptor 1 in Experimental Autoimmune Encephalomyelitis Open Access PP 646 2013, p. 41. http://dx.doi.org/10.1186/1742-2094-10-41 [32] L. Izikson, R. S. Klein, I. F. Charo, H. L. Weiner and A. D. Luster, “Resistance to Experimental Autoimmune En- cephalomyelitis in Mice Lacking the CC Chemokine Re- ceptor (CCR)2,” Journal of Experimental Medicine, Vol. 192, No. 7, 2000, pp. 1075-1080. http://dx.doi.org/10.1084/jem.192.7.1075 [33] E. B. Samoilova, J. L. Horton, B. Hilliard, T. S. Liu and Y. Chen, “IL-6-Deficient Mice Are Resistant to Experi- mental Autoimmune Encephalomyelitis: Roles of IL-6 in the Activation and Differentiation of Autoreactive T Cells,” Journal of Immunology, Vol. 161, No. 12, 1998, pp. 6480-6486. [34] Y. Okuda, S. Sakoda, C. C. Bernard, H. Fujimura, Y. Saeki, T. Kishimoto and T. Yanagihara, “IL-6-Deficient Mice Are Resistant to the Induction of Experimental Autoimmune Encephalomyelitis Provoked by Myelin Oli- godendrocyte Glycoprotein,” International Immunology, Vol. 10, No. 5, 1998, pp. 703-708. http://dx.doi.org/10.1093/intimm/10.5.703 [35] R. S. Klein, L. Izikson, T. Means, H. D. Gibson, E. Lin, R. A. Sobel, H. L. Weiner and A. D. Luster, “IFN-Inducible Protein 10/CXC Chemokine Ligand 10-Independent In- duction of Experimental Autoimmune Encephalomye- litis,” Journal of Immunology, Vol. 172, No. 1, 2004, pp. 550-559. [36] M. Giralt, R. Ramos, A. Quintana, B. Ferrer, M. Erta, M. Castro-Freire, G. Comes, E. Sanz, M. Unzeta, P. Pifarre, A. Garcia, I. L. Campbell and J. Hidalgo, “Induction of Atypical EAE Mediated by Transgenic Production of IL- 6 in Astrocytes in the Absence of Systemic IL-6,” Glia, Vol. 61, No. 4, pp. 587-600. http://dx.doi.org/10.1002/glia.22457 [37] S. L. Carter, M. Muller, P. M. Manders and I. L. Camp- bell, “Induction of the Genes for Cxcl9 and Cxcl10 Is Dependent on IFN-Gamma but Shows Differential Cellu- lar Expression in Experimental Autoimmune Encephalo- myelitis and by Astrocytes and Microglia in Vitro,” Glia, Vol. 55, No. 16, 2007, pp. 1728-1739. http://dx.doi.org/10.1002/glia.20587 [38] R. Van Doorn, J. Van Horssen, D. Verzijl, M. Witte, E. Ronken, B. Van Het Hof, K. Lakeman, C. D. Dijkstra, P. Van Der Valk, A. Reijerkerk, A. E. Alewijnse, S. L. Pe- ters and H. E. De Vries, “Sphingosine 1-Phosphate Re- ceptor 1 and 3 Are Upregulated in Multiple Sclerosis Le- sions,” Glia, Vol. 58, No. 12, 2010, pp. 1465-1476. [39] A. Kimura, T. Ohmori, R. Ohkawa, S. Madoiwa, J. Mi- muro, T. Murakami, E. Kobayashi, Y. Hoshino, Y. Ya- tomi and Y. Sakata, “Essential Roles of Sphingosine 1-Phosphate/S1P1 Receptor Axis in the Migration of Neural Stem Cells toward a Site of Spinal Cord Injury,” Stem Cells, Vol. 25, No. 1, 2007, pp. 115-124. http://dx.doi.org/10.1634/stemcells.2006-0223 [40] K. Lieb, C. Kaltschmidt, B. Kaltschmidt, P. A. Baeuerle, M. Berger, J. Bauer and B. L. Fiebich, “Interleukin-1 Beta Uses Common and Distinct Signaling Pathways for Induction of the Interleukin-6 and Tumor Necrosis Factor Alpha Genes in the Human Astrocytoma Cell Line U373,” Journal of Neurochemistry, Vol. 66, No. 4, 1996, pp. 1496-1503. http://dx.doi.org/10.1046/j.1471-4159.1996.66041496.x [41] H. L. Hsieh, C. C. Sun, C. B. Wu, C. Y. Wu, W. H. Tung, H. H. Wang and C. M. Yang, “Sphingosine 1-Phosphate Induces EGFR Expression via Akt/NF-kappaB and ERK/ AP-1 Pathways in Rat Vascular Smooth Muscle Cells,” Journal of Cellular Biochemistry, Vol. 103, No. 6, 2008, pp. 1732-1746. http://dx.doi.org/10.1002/jcb.21563
|