 Journal of Cancer Therapy, 2013, 4, 1435-1442 Published Online November 2013 (http://www.scirp.org/journal/jct) http://dx.doi.org/10.4236/jct.2013.49171 Open Access JCT 1435 Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt Emad Moussa1, Mohamed Fawzy2*, Alaa Younis3, Maged El Shafei3, Mohamed Saad Zaghloul4, Naglaa El Kinaai5, Amal Refaat6, Noha Atta7, Alaa El Haddad2 1Department of Pediatric Oncology, Children’s Cancer Hospital-Egypt, Cairo, and Clinical Oncology Department, Faculty of Medi- cine, Menufeya University, Menufeya, Egypt; 2Department of Pediatric Oncology, National Cancer Institute, Cairo University, and Children’s Cancer Hospital-Egypt, Cairo, Egypt; 3Department of Surgical Oncology, National Cancer Institute, Cairo University, and Children’s Cancer Hospital-Egypt, Cairo, Egypt; 4Department of Radiotherapy, National Cancer Institute, Cairo University, and Children’s Cancer Hospital-Egypt, Cairo, Egypt; 5Department of Pathology, National Cancer Institute, Cairo University, and Chil- dren’s Cancer Hospital Egypt, Cairo, Egypt; 6Department of Radiodiagnosis, National Cancer Institute, Cairo University, and Chil- dren’s Cancer Hospital-Egypt, Cairo, Egypt; 7Department of Research, Children’s Cancer Hospital-Egypt, Cairo, Egypt. Email: *mohammed.fawzy@57357.com Received September 13th, 2013; revised October 12th, 2013; accepted October 20th, 2013 Copyright © 2013 Emad Moussa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Neuroblastoma (NB) is remarkable for its wide spectrum of clinical behavior and biological characteris- tics in relation to outcome. The use of aggressive therapy, including autologous hematopoietic stem cell transplantation (HSCT) and the addition of isoretionin (cis-Retinoic Acid/cis-RA), has increased survival rates of patients with ad- vanced disease. Methods: Pediatric 271 newly diagnosed high risk NB patients were prospectively enrolled into the study. Patients received neoadjuvant chemotherapy of alternating cycles: [cyclophosphamide, doxorubicin, vincristine (CAdO)] and [etoposide, carboplatin]. Intensification courses of “ICE” (ifosfamide, carboplatin, and etoposide) regimen were administered to patients with bone marrow (BM) residual infiltration. Whenever safely feasible, complete surgical resection or debulking of the primary tumor was attempted for patients achieving partial response. Eligible patients un- derwent HSCT, while radiation therapy to the primary and metastatic sites, as well as maintenance with cis-RA was given for 6 months. Results: The median age of our patients was 2.8 years with male to female ratio of 1.65:1. At 4 years, the overall and event free survivals were 33.7% and 23.3% for the entire group under study, with significantly higher rates (42.7% and 35.6%, respectively) for HSCT patients (n = 94; p < 0.001). The outcome was also significantly correlated with response to induction therapy, pathological subtype, as well as other variables. Conclusion: Myeloabla- tive therapy followed by stem cell rescue is regarded as the most important goal of high risk NB treatment to improve survival till present. Each of consolidation HSCT, post induction disease status, as well as international neuroblastoma pathology classification (INPC) subtype was an independent predictive variable of survival. A collaborative effort with an emphasis on biologic characteristics of aggressive disease and tailored therapy needs to be strengthened to further our understanding of this disease. Keywords: High-Risk Neuroblastoma; Treatment; Outcome 1. Introduction Neuroblastoma (NB) accounts for more than 8% of malignancies in patients younger than 15 years of age, and is responsible for 15% of all pediatric oncology deaths [1]. The disease is remarkable for its wide spec- trum of clinical behavior. Although substantial improve- ment in outcome occurred in lower risk categories during the past few decades, the outcome for children with a high-risk (HR) disease has been improved only modestly having less than 40% long-term survival rates [2]. It is generally believed that biological characteristics are more relevant to the outcome in advanced NB than the type of chemotherapy or extent of resection [3]. However, the use of aggressive chemotherapy increased the survi- *Corresponding author.  Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt 1436 val rates of patients with advanced NB [4]. Myeloab- lative therapy and autologous hematopoietic stem cell rescue can significantly result in a better 5-year Overall Survival (OS) and Event Free Survival (EFS) than non- myeloablative chemotherapy. While adding isoretionin (cis-Retinoic Acid/cis-RA) to consolidation therapy inde- pendently resulted in a significantly improved OS; analy- sis of consecutive trials from a single center demons- trated that combining cis-RA with monoclonal antibody (MoAb 3F8), and Granulocyte-Macrophage Colony Sti- mulating Factor (GM-CSF) has also improved survival significantly in HR-NB [5,6]. Chemotherapy dose-esca- lation strategy using tandem autologous Hematopoietic Stem Cell Transplantation (HSCT) was also encouraging for survival [7]. Other new chemotherapeutic agents such as irinotecan and paclitaxel are also evaluated in the treatment of advanced and refractory NB by ongoing clinical trials, however, further studies incorporating new- er modalities are still required as well to reduce late ad- verse effects without jeopardizing survival outcome [8]. The purpose of this study was to evaluate the outcome of HR-NB in Egyptian children treated with aggressive multimodality approach that included HSCT and cis-RA. 2. Patients and Methods 2.1. Patients Newly diagnosed and treated pediatric NB patients at The Children’s Cancer Hospital-Egypt (CCHE/57357) were prospectively enrolled onto study from July 2007 till December 2011. The international criteria were used for risk stratification (International Neuroblastoma Stag- ing System/INSS), and assessment of disease response defined as; complete response (CR), very good partial response (VGPR), partial response (PR), no response (NR), progressive disease (PD) or mixed response (MR); according to the International Neuroblastoma Response Criteria (INRC) [9]. Data was timely updated and ana- lyzed at different checkpoints throughout study period. 2.2. Workup Tissue samples were processed and routinely stained with hematoxylin and eosin stain. Tissues were diag- nosed as neuroblastoma and were classified histologi- cally as poorly differentiated, undifferentiated or di- fferentiating while ganglioneuroblastoma (GNB) were classified as nodular or intermixed. Tumor was classified according to the international neuroblas- toma pathology classification (INPC/modified Shi- mada) into favorable or unfavorable; based on his- tology, mitosis/karyorrhexis index, and age [10]. Im- munostains using BenchMark XT Ventana automated slide staining system were used either for confir- mation; synaptophysin (most consistent in our lab), chromogranin, neuron specific enolase (NSE), CD56 or for differential diagnosis especially in undifferen- tiated and/or extra adrenal location (WT1, CD99, myogenin, LCA, MPO). Tissue samples were further studied for NMYC gene amplification in most of our patients. According to tissue feasibility, NMYC gene status was assessed by fluorescence in situ hybridization (FISH) on paraffin embedded tissue sections using Vysis LSI NMYC spectrum orange probe 2p 24.1 (Abott molecular) according to manufacturer instructions [11]. Cases were categorized as having normal diploid pattern or amplified pattern in the form of homogenous staining region or double minutes (more than 10 copies). Other laboratory work included; serum ferritin, LDH, NSE, and urinary valinylmandelic acid (VMA) as ba- seline, prior to chemotherapy cycles, surgery, HSCT, and during follow up. Computed tomography (CT) was routinely done for tumor assessment at different checkpoints, while ma- gnetic resonance imaging (MRI) was the preferred study for paraspinal and intracranial lesions. 99Tcm diphosphonate bone scan and Metaiodoben- zylguanidine (MIBG) scintigraphy were performed at presentation and by the end of induction therapy for evaluation of; bone, BM, and soft tissue disease. Bone marrow disease was assessed in all patients via bilateral bone marrow aspirates and biopsies. 2.3. Inclusion Criteria No prior systemic therapy except for localized emer- gency radiation to sites of life threatening or func- tion-threatening disease and/or no more than one cy- cle of chemotherapy. Age: patients must be ≤ 18 years of age at initial di- agnosis. Established unequivocal diagnosis of NB or GNB morphology verified by histology and/or demonstra- tion of clumps of tumor cells in BM with elevated urinary VMA. Presence of high risk defined features [12]: INSS Stage 2a/2b patients ≥ 12 months of age with NMYC amplification, and unfavorable pa- thology. INSS Stage 3 patients ≥ 12 months of age with NMYC amplification or unfavorable pathology. INSS Stage 4 with any of the following: a) Age ≥ 18 months regardless of biologic features, or, b) Age 12 - 18 months with any unfavorable or indeter- minant/unsatisfactory/unknown biologic features. INSS Stage 3, 4, 4S patients < 12 months of age with NMYC amplification. Patients ≥ 12 months of age; initially diagnosed Open Access JCT  Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt 1437 with INSS stage 1, 2, 4S who progressed to a stage 4 without interval chemotherapy. Adequate liver function: bilirubin ≤ 1.5 mg/dL and ALT ≤ 300. Adequate renal function: 24 hours urine collection for creatinine clearance ≥ 60 mL/min/1.73 m² and a se- rum creatinine ≤ 1.5 mg/dl. Normal cardiac function: ejection fraction ≥55% and fractional shortening ≥28% documented by echocar- gram. Informed consent: the patient’s legally authorized guar- dian must acknowledge in writing that consent to re- ceive chemotherapy, radiotherapy and surgery has been obtained, in accordance with local policies for CCHE. 2.4. Off Protocol Criteria Progressive disease/no response on protocol therapy. Treatment limiting organ dysfunction. 2.5. Treatment Plan Patients were started on upfront induction chemotherapy with “cyclophosphamide, doxorubicin, vincristine (CAdO)” alternating with “carboplatin and etoposide” for 6 cycles [13]. Patients failed to have their BM cleared of metas- tatic disease by end of upfront regimen or had less than PR after 4 cycles, were upgraded to receive additional 2 - 4 intensification courses of “ICE” [ifosfamide: 1800 mg/m2/d (d1 - d5), carboplatin: 560 mg/m2 (d1), and etopo- side: 100 mg/m2/d (d1 - d5)]; guided with response. When safely feasible, surgical resection or debulking of the primary tumor was attempted between 5th and 6th chemo- therapy cycles for patients achieved PR. By end of induc- tion, consolidation with autologous HSCT was offered to eligible patients with at least PR and had their BM clear- ed of disease unless denied by parents. Radiation therapy was consistently delivered to the primary as well as post- induction MIBG avid metastatic bony sites. Maintenance cis-RA was given for 14 days q monthly at 160 mg/m2/day for 6 months following HSCT or post induction for non transplant patients attained CR/VGPR/PR [14]. 2.6. Statistical Analysis All data were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Correlations were analyzed using χ2 test. OS was calculated from date of diagnosis to the date of death for any cause, and EFS was defined as the time from diagnosis to the time of first occurrence of relapse, progression, or death. The Kaplan-Meier curves were plotted to calculate 5-year survival curves, and log-rank test was used to es- timate the differences. Factors known to be associated with prognosis were tested in univariate analysis. Varia- bles that were found to be significant in univariate ana- lysis were then entered in a multivariate Cox proportion- al hazards regression model to identify those with inde- pendent prognostic information for EFS and OS. The “P” value was defined as statistically significant if < 0.05. 3. Results Two hundreds and seventy one patients were enrolled during our study period. Patients age ranged between 2 months and 12.7 years (Median: 2.8 years) at time of diagnosis; 169 males (62.4%) and 102 females (37.6%) with a ratio of 1.65:1. Histologically, NB was confirmed in 251 patients (92.6%); 200 cases were unfavorable according to INPC with poorly differentiated morphology, while GNB was seen in 14 patients (5.2%). Diagnosis was established in another 6 patients (2.2%) by infiltrated BM biopsy and elevated urinary VMA. As regards NMYC gene status, 82 of studied cases showed an amplified gene (Figure 1); 2/82 showed mosaic pattern. Abdominal tumor was the most common primary site of disease found in 245 of the study patients (90.4%), with adrenal origin seen in 190 patients (70.1%) while 55 (20.3%) had extra-adrenal locations. BM was the most common site of metastasis which seen in 53.5% of pa- tients. Clinicopathological data are presented in Table 1. Local surgical control (complete/partial) was feasible in 157 patients. Due to early death or lost follow up, 23 patients were not evaluable for induction response whereas disease status for 248/271 evaluable patients showed: 4 CR (1.6%), 14 VGPR (5.6%), 177 PR (71.4%), 25 NR (10%), 28 PD (11.3%). Collectively, objective response (OR) was 78.6% (CR + VGPR + PR). Five of the 271 study patients died before receiving any treat- ment and thus were excluded from survival analysis. The 4 years OS for all valid patients was 33.7% (median sur- vival: 31.1 months; 95% CI: 24.3 - 37.9), while EFS was Figure 1. Amplifie d NMYC gene by fluorescence in situ hy - bridization (FISH) shows formation of fluorescent signal clusters (×1000). Open Access JCT 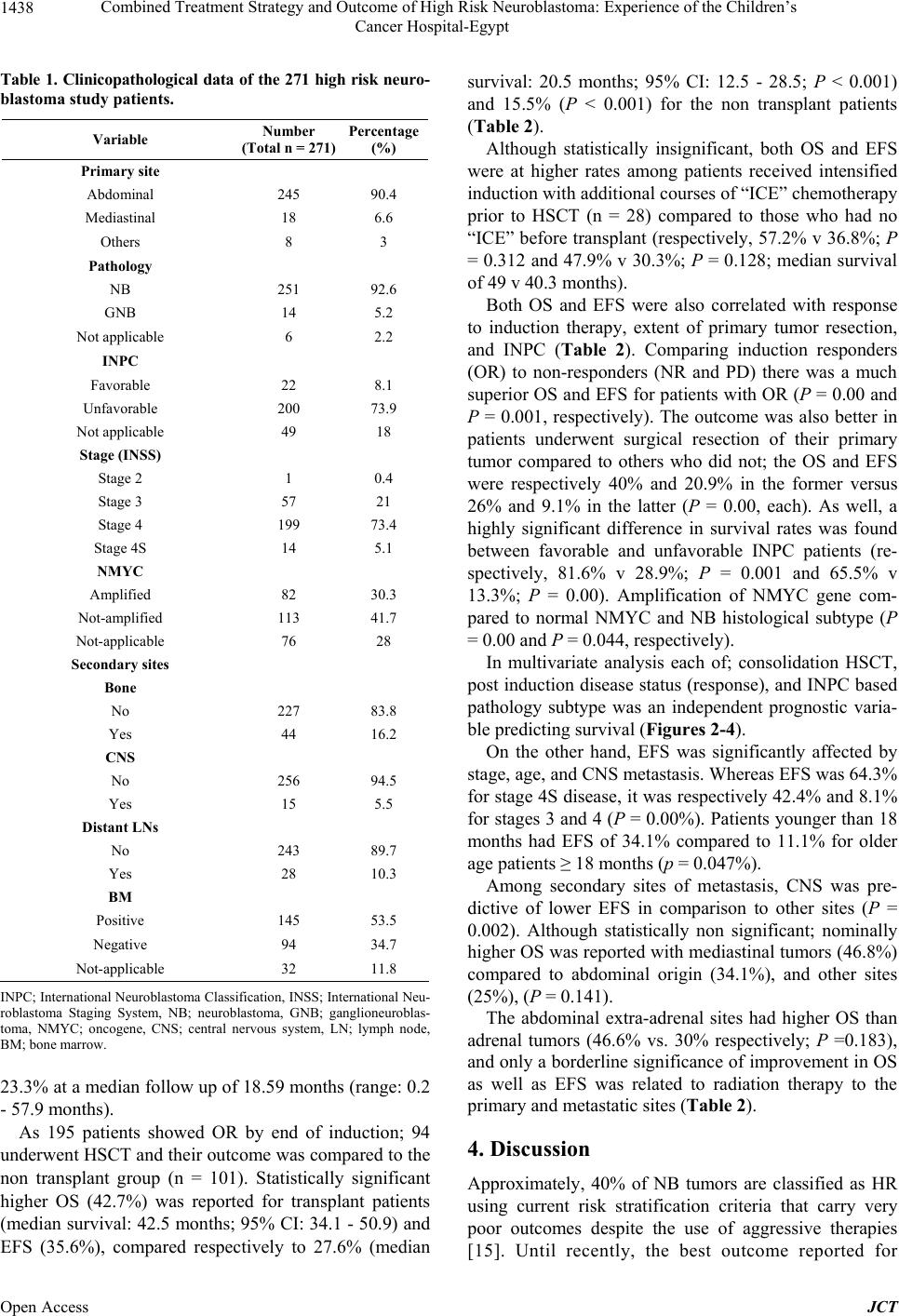 Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt 1438 Table 1. Clinicopathological data of the 271 high risk neuro- blastoma study patients. Variable Number (Total n = 271) Percentage (%) Primary site Abdominal 245 90.4 Mediastinal 18 6.6 Others 8 3 Pathology NB 251 92.6 GNB 14 5.2 Not applicable 6 2.2 INPC Favorable 22 8.1 Unfavorable 200 73.9 Not applicable 49 18 Stage (INSS) Stage 2 1 0.4 Stage 3 57 21 Stage 4 199 73.4 Stage 4S 14 5.1 NMYC Amplified 82 30.3 Not-amplified 113 41.7 Not-applicable 76 28 Secondary sites Bone No 227 83.8 Yes 44 16.2 CNS No 256 94.5 Yes 15 5.5 Distant LNs No 243 89.7 Yes 28 10.3 BM Positive 145 53.5 Negative 94 34.7 Not-applicable 32 11.8 INPC; International Neuroblastoma Classification, INSS; International Neu- roblastoma Staging System, NB; neuroblastoma, GNB; ganglioneuroblas- toma, NMYC; oncogene, CNS; central nervous system, LN; lymph node, BM; bone marrow. 23.3% at a median follow up of 18.59 months (range: 0.2 - 57.9 months). As 195 patients showed OR by end of induction; 94 underwent HSCT and their outcome was compared to the non transplant group (n = 101). Statistically significant higher OS (42.7%) was reported for transplant patients (median survival: 42.5 months; 95% CI: 34.1 - 50.9) and EFS (35.6%), compared respectively to 27.6% (median survival: 20.5 months; 95% CI: 12.5 - 28.5; P < 0.001) and 15.5% (P < 0.001) for the non transplant patients (Table 2). Although statistically insignificant, both OS and EFS were at higher rates among patients received intensified induction with additional courses of “ICE” chemotherapy prior to HSCT (n = 28) compared to those who had no “ICE” before transplant (respectively, 57.2% v 36.8%; P = 0.312 and 47.9% v 30.3%; P = 0.128; median survival of 49 v 40.3 months). Both OS and EFS were also correlated with response to induction therapy, extent of primary tumor resection, and INPC (Table 2). Comparing induction responders (OR) to non-responders (NR and PD) there was a much superior OS and EFS for patients with OR (P = 0.00 and P = 0.001, respectively). The outcome was also better in patients underwent surgical resection of their primary tumor compared to others who did not; the OS and EFS were respectively 40% and 20.9% in the former versus 26% and 9.1% in the latter (P = 0.00, each). As well, a highly significant difference in survival rates was found between favorable and unfavorable INPC patients (re- spectively, 81.6% v 28.9%; P = 0.001 and 65.5% v 13.3%; P = 0.00). Amplification of NMYC gene com- pared to normal NMYC and NB histological subtype (P = 0.00 and P = 0.044, respectively). In multivariate analysis each of; consolidation HSCT, post induction disease status (response), and INPC based pathology subtype was an independent prognostic varia- ble predicting survival (Figures 2-4). On the other hand, EFS was significantly affected by stage, age, and CNS metastasis. Whereas EFS was 64.3% for stage 4S disease, it was respectively 42.4% and 8.1% for stages 3 and 4 (P = 0.00%). Patients younger than 18 months had EFS of 34.1% compared to 11.1% for older age patients ≥ 18 months (p = 0.047%). Among secondary sites of metastasis, CNS was pre- dictive of lower EFS in comparison to other sites (P = 0.002). Although statistically non significant; nominally higher OS was reported with mediastinal tumors (46.8%) compared to abdominal origin (34.1%), and other sites (25%), (P = 0.141). The abdominal extra-adrenal sites had higher OS than adrenal tumors (46.6% vs. 30% respectively; P =0.183), and only a borderline significance of improvement in OS as well as EFS was related to radiation therapy to the primary and metastatic sites (Table 2). 4. Discussion Approximately, 40% of NB tumors are classified as HR using current risk stratification criteria that carry very poor outcomes despite the use of aggressive therapies 15]. Until recently, the best outcome reported for [ Open Access JCT 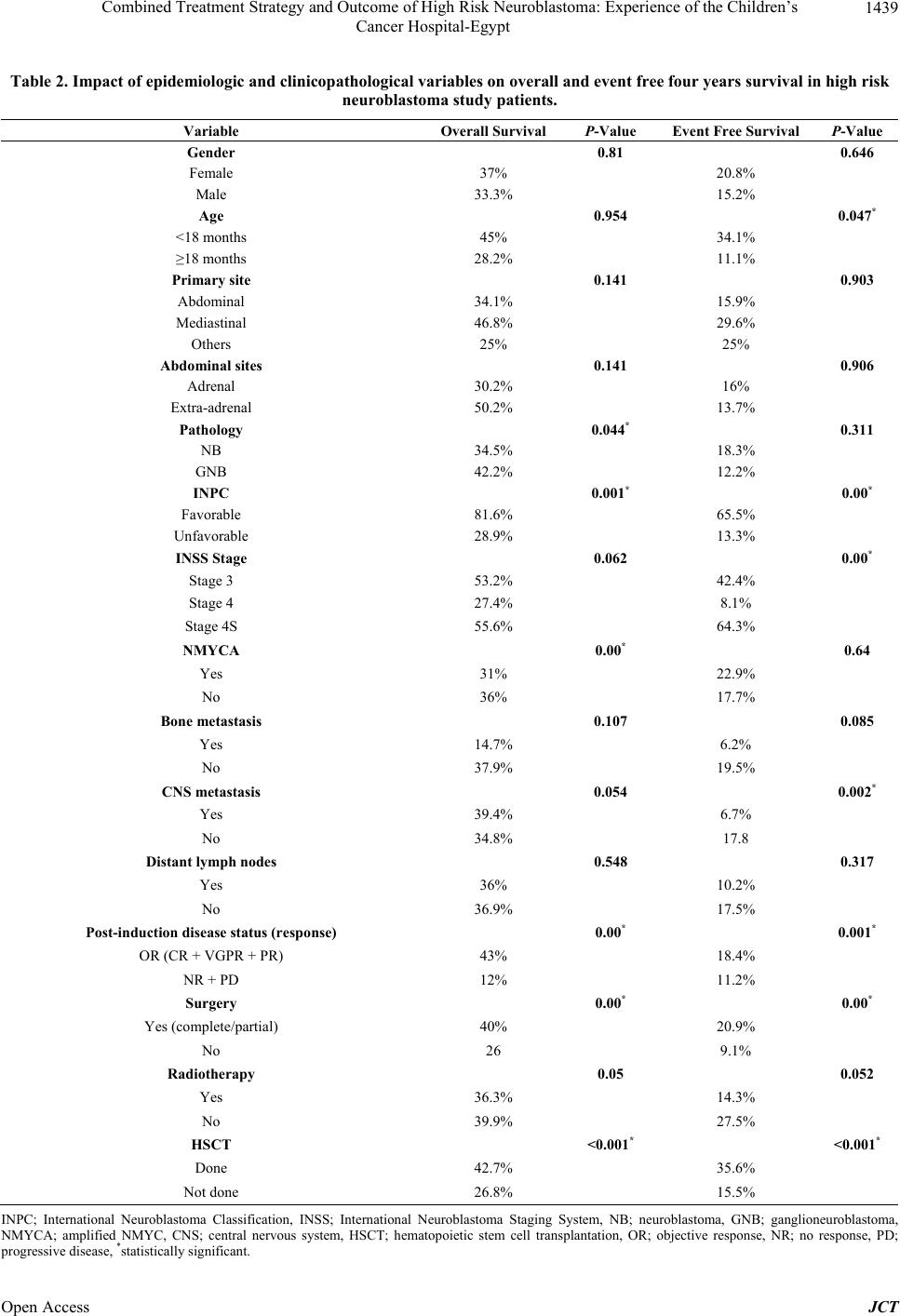 Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt Open Access JCT 1439 Table 2. Impact of epidemiologic and clinicopathological variables on overall and event free four years survival in high risk neuroblastoma study patients. Variable Overall Survival P-Value Event Free Survival P-Value Gender 0.81 0.646 Female 37% 20.8% Male 33.3% 15.2% Age 0.954 0.047* <18 months 45% 34.1% ≥18 months 28.2% 11.1% Primary site 0.141 0.903 Abdominal 34.1% 15.9% Mediastinal 46.8% 29.6% Others 25% 25% Abdominal sites 0.141 0.906 Adrenal 30.2% 16% Extra-adrenal 50.2% 13.7% Pathology 0.044* 0.311 NB 34.5% 18.3% GNB 42.2% 12.2% INPC 0.001* 0.00* Favorable 81.6% 65.5% Unfavorable 28.9% 13.3% INSS Stage 0.062 0.00* Stage 3 53.2% 42.4% Stage 4 27.4% 8.1% Stage 4S 55.6% 64.3% NMYCA 0.00* 0.64 Yes 31% 22.9% No 36% 17.7% Bone metastasis 0.107 0.085 Yes 14.7% 6.2% No 37.9% 19.5% CNS metastasis 0.054 0.002* Yes 39.4% 6.7% No 34.8% 17.8 Distant lymph nodes 0.548 0.317 Yes 36% 10.2% No 36.9% 17.5% Post-induction disease status (response) 0.00* 0.001* OR (CR + VGPR + PR) 43% 18.4% NR + PD 12% 11.2% Surgery 0.00* 0.00* Yes (complete/partial) 40% 20.9% No 26 9.1% Radiotherapy 0.05 0.052 Yes 36.3% 14.3% No 39.9% 27.5% HSCT <0.001* <0.001* Done 42.7% 35.6% Not done 26.8% 15.5% INPC; International Neuroblastoma Classification, INSS; International Neuroblastoma Staging System, NB; neuroblastoma, GNB; ganglioneuroblastoma, NMYCA; amplified NMYC, CNS; central nervous system, HSCT; hematopoietic stem cell transplantation, OR; objective response, NR; no response, PD; rogressive disease, *statistically significant. p 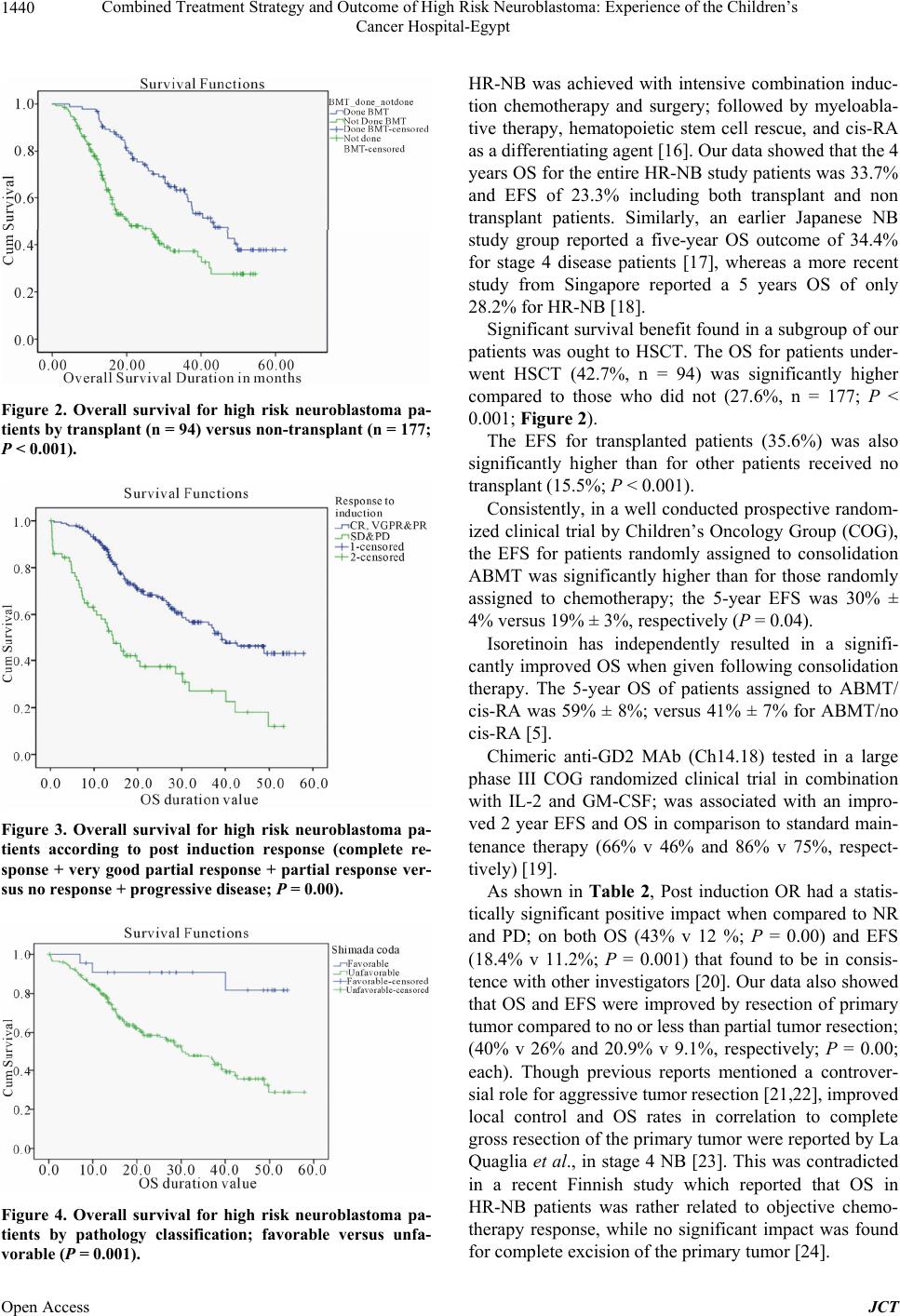 Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt 1440 Figure 2. Overall survival for high risk neuroblastoma pa- tients by transplant (n = 94) versus non-transplant (n = 177; P < 0.001). Figure 3. Overall survival for high risk neuroblastoma pa- tients according to post induction response (complete re- sponse + very good partial response + partial response ver- sus no response + progr essive disease; P = 0.00). Figure 4. Overall survival for high risk neuroblastoma pa- tients by pathology classification; favorable versus unfa- vorable (P = 0.001). HR-NB was achieved with intensive combination induc- tion chemotherapy and surgery; followed by myeloabla- tive therapy, hematopoietic stem cell rescue, and cis-RA as a differentiating agent [16]. Our data showed that the 4 years OS for the entire HR-NB study patients was 33.7% and EFS of 23.3% including both transplant and non transplant patients. Similarly, an earlier Japanese NB study group reported a five-year OS outcome of 34.4% for stage 4 disease patients [17], whereas a more recent study from Singapore reported a 5 years OS of only 28.2% for HR-NB [18]. Significant survival benefit found in a subgroup of our patients was ought to HSCT. The OS for patients under- went HSCT (42.7%, n = 94) was significantly higher compared to those who did not (27.6%, n = 177; P < 0.001; Figure 2). The EFS for transplanted patients (35.6%) was also significantly higher than for other patients received no transplant (15.5%; P < 0.001). Consistently, in a well conducted prospective random- ized clinical trial by Children’s Oncology Group (COG), the EFS for patients randomly assigned to consolidation ABMT was significantly higher than for those randomly assigned to chemotherapy; the 5-year EFS was 30% ± 4% versus 19% ± 3%, respectively (P = 0.04). Isoretinoin has independently resulted in a signifi- cantly improved OS when given following consolidation therapy. The 5-year OS of patients assigned to ABMT/ cis-RA was 59% ± 8%; versus 41% ± 7% for ABMT/no cis-RA [5]. Chimeric anti-GD2 MAb (Ch14.18) tested in a large phase III COG randomized clinical trial in combination with IL-2 and GM-CSF; was associated with an impro- ved 2 year EFS and OS in comparison to standard main- tenance therapy (66% v 46% and 86% v 75%, respect- tively) [19]. As shown in Table 2, Post induction OR had a statis- tically significant positive impact when compared to NR and PD; on both OS (43% v 12 %; P = 0.00) and EFS (18.4% v 11.2%; P = 0.001) that found to be in consis- tence with other investigators [20]. Our data also showed that OS and EFS were improved by resection of primary tumor compared to no or less than partial tumor resection; (40% v 26% and 20.9% v 9.1%, respectively; P = 0.00; each). Though previous reports mentioned a controver- sial role for aggressive tumor resection [21,22], improved local control and OS rates in correlation to complete gross resection of the primary tumor were reported by La Quaglia et al., in stage 4 NB [23]. This was contradicted in a recent Finnish study which reported that OS in HR-NB patients was rather related to objective chemo- therapy response, while no significant impact was found for complete excision of the primary tumor [24]. Open Access JCT  Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt 1441 Unfavorable histological differentiation, amplified NMYC gene, CNS metastasis, and older age at diagnosis were all shown to have a significantly negative impact on the survival of our patients (Table 2). While unfavorable INPC histology was associated with poorer OS and EFS than favorable subtype, CNS found to be the only metastatic site to show significant correlation to patients outcome as regards EFS (P = 0.002). Similarly, EFS was significantly lower among patients ≥ 18 months of age (P = 0.047) that was sup- ported by other reports stated that age at diagnosis was one of the single most important indicators of survival in NB [3,25]. Unfavorable clinical variables such as; age above18 months and advanced stage were found to be closely associated with poor biologic risk factors includ- ing unfavorable histopathology, MYCN amplification, 1p and 11q loss of heterozygosity, as well as other partial chromosomal deletions [26]. 5. Conclusion In spite of aggressive therapy, HR-NB carried a discour- aging survival outcome. The prognosis for high risk NB remained poor but myeloablative therapy followed by stem cell rescue is regarded as the most important goal of high risk NB treatment to improve survival till present. Each of consolidation HSCT, post induction disease sta- tus, as well as INPC-based pathological subtype was an independent predictive variable of survival. A collabora- tive effort with an emphasis on biologic characteristics of aggressive disease and tailored therapy needs to be stren- gthened to further our understanding of this disease. REFERENCES [1] S. L. Cohn, “Surveillance, Epidemiology, and End Re- sults (SEER) Database,” 2007, National Cancer Institute. http://seer.cancer.gov [2] S. L. Cohn, A. D. J. Pearson, W. B. London, T. Monclair, P. F. Ambros, G. M. Brodeur, A. Faldum, B. Hero, T. Ie- hara, D. Machin, V. Mosseri, T. Simon, A. Garaventa, V. Castel and K. K. Matthay, “The International Neuroblas- toma Risk Group (INRG) Classification System: An INRG Task force Report,” Journal of Clinical Oncology, Vol. 27, No. 2, 2009, pp. 289-297. http://dx.doi.org/10.1200/JCO.2008.16.6785 [3] M. Kubota, M. Yagi, S. Kanada, N. Okuyama, Y. Ki- noshita, S. Yamazaki, K. Asami, A. Ogawa and T. Wata- nabe, “Long-Term Follow-Up Status of Patients With Neuroblastoma after Undergoing Either Aggressive Sur- gery or Chemotherapy—A Single Institutional Study,” Journal of Pediatric Surgery, Vol. 39, No. 9, 2004, pp. 1328-1332. http://dx.doi.org/10.1016/j.jpedsurg.2004.05.012 [4] M. Kaneko, Y. Tsuchida, H. Muqishima, N. Ohnuma, K. Yamamoto, K. Kawa, M. Iwafuchi, T. Sawada and S. Suita, “Intensified Chemotherapy Increases the Survival Rates in Patients with Stage 4 Neuroblastoma with MYCN Amplification,” Journal of Pediatric Hematol- ogy/Oncology, Vol. 24, No. 8, 2002, pp. 613-621. http://dx.doi.org/10.1097/00043426-200211000-00004 [5] K. K. Matthay, C. P. Reynolds, R. C. Seeger, H. Shimada, E. S. Adkins, D. Haas-Kogan, R. B. Gerbing, W. B. London and J. G. Villablanca, “Long-Term Results for Children with High-Risk Neuroblastoma Treated on a Randomized Trial of Myeloablative Therapy Followed by 13-cis-Retinoic Acid: A Children’s Oncology Group Stu- dy,” Journal of Clinical Oncology, Vol. 27, No. 7, 2009, pp. 1007-1013. http://dx.doi.org/10.1200/JCO.2007.13.8925 [6] B. Kushner, K. Kramer, S. Modak and N. K. Cheung, “Anti-GD2 Monoclonal Antibody 3F8 Plus Granulocyte Macrophage Colony Stimulating Factor for Primary Re- fractory Neuroblastoma in Bone Marrow,” Proceedings of ASCO, Vol. 25, 2007, p. 9502. [7] K. W. Sung, M. H. Son, S. H. Lee, K. H. Yoo, H. H. Koo, J. Y. Kim, E. J. Cho, S. K. Lee, Y. S. Choi, D. H. Lim, J. S. Kim and D. W. Kim, “Tandem High-Dose Chemo- therapy and Autologous Stem Cell Transplantation in Pa- tients with High-Risk Neuroblastoma: Results of SMC NB-2004 Study,” Bone Marrow Transplant, Vol. 48, No. 1, 2013, pp. 68-73. http://dx.doi.org/10.1038/bmt.2012.86 [8] G. Surico, P. Muggeo, F. De Leonardis and N. Rigillo, “New Paclitaxel Cisplatin Based Chemotherapy Regimen for Advanced Stage, Recurrent, or Refractory Neuroblas- toma—Preliminary Report,” Medical and Pediatric On- cology, Vol. 40, No. 2, 2003, pp. 130-132. http://dx.doi.org/10.1002/mpo.10106 [9] G. M. Brodeur, J. Pritchard, F. Berthold, N. L. Carlsen, V. Castel, R. P. Castelberry, B. De Bernardi, A. E. Evans, M. Favrot, F. Hedborg, et al., “Revisions of the International Criteria for Neuroblastoma Diagnosis, Staging and Re- sponse to Treatment,” Journal of Clinical Oncology, Vol. 11, 1993, pp. 1466-1477. [10] O. Burques, S. Navarro, R. Noquera, A. Pellín, A. Ruiz, V. Castel and A. Llombart-Bosch, “Prognostic Value of the International Neuroblastoma Pathology Classification in Neuroblastoma (Schwannian Stroma-Poor) and Com- parison with Other Prognostic Factors: A Study of 182 Cases from the Spanish Neuroblastoma Registry,” Vir- chows Archives, Vol. 449, No. 4, 2006, pp. 410-420. http://dx.doi.org/10.1007/s00428-006-0253-y [11] Y. Hachitanda, M. Saito, I. Mori and M. Hamazaki, “Ap- plication of Fluorescent in Situ Hybridization to Detect N-myc Gene Amplification on Paraffin-Embedded Tissue Sections of Neuroblastoma,” Medical and Pediatric On- cology, Vol. 29, 1997, pp. 135-138. [12] G. M. Brodeur, M. D. Hogarty, Y. P. Mosse and J. M. Maris, “Neuroblastoma,” In: P. A. Pizzo, P. C. Adamson and D. G. Poplack, Eds., Principles and Practice of Pedi- atric Oncology. 6th Edition, Lippincott Williams and Wilkins, Philadelphia, 2011, pp. 886-922. [13] B. H. Kushner, R. J. O’Reilly, M. LaQuaglia and N. K. Cheung, “Dose-Intensive Use of Cyclophosphamide in Ablation of Neuroblastoma,” Cancer, Vol. 66, 1990, pp. 1095-1100. Open Access JCT  Combined Treatment Strategy and Outcome of High Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt Open Access JCT 1442 http://dx.doi.org/10.1002/1097-0142(19900915)66:6<109 5::AID-CNCR2820660603>3.0.CO;2-0 [14] G. J. Veal, M. Cole, J. Errington, A. D. Pearson, A. B. Foot, G. Whyman and A. V. Boddy, “UKCCSG Pharma- cology Working Group. Pharmacokinetics and Metabo- lism of 13-Cis-Retinoic Acid (Isotretinoin) in Children with High-Risk Neuroblastoma—A Study of the United Kingdom Children’s Cancer Study Group,” British Jour- nal of Cancer, Vol. 96, No. 3, 2007, pp. 424-431. http://dx.doi.org/10.1038/sj.bjc.6603554 [15] J. M. Maris, M. D. Hogarty, R. Baqatell and S. L. Cohn, “Neuroblastoma,” Lancet, Vol. 369, No. 9579, 2007, pp. 2106-2120. http://dx.doi.org/10.1016/S0140-6736(07)60983-0 [16] K. K. Matthay, J. G. Villablanca, R. C. Seeger, D. O. Stram, R. E. Harris, N. K. Ramsay, P. Swift, H. Shimada, C. T. Black, G. M. Brodeur, R. B. Gerbing and C. P. Rey- nolds, “Treatment of High-Risk Neuroblastoma with In- tensive Chemotherapy, Radiotherapy, Autologous Bone Marrow Transplantation, and 13-cis-Retinoic Acid. Chil- dren’s Cancer Group,” The New England Journal of Me- dicine, Vol. 341, 1999, pp. 1165-1173. http://dx.doi.org/10.1056/NEJM199910143411601 [17] M. Kaneko, Y. Tsuchida, J. Uchino, T. Takeda, M. Iwa- fuchi, N. Ohnuma, H. Mugishima, J. Yokoyama, H. Ni- shihira, K. Nakada, S. Sasaki, T. Sawada, K. Kawa, N. Nagahara, S. Suita and S. Sawaguchi, “Treatment Results of Advanced Neuroblastoma with the First Japanese Study Group Protocol. Study Group of Japan for Treat- ment of Advanced Neuroblastoma,” Journal of Pediatric Hem a t o lo g y/Oncology, Vol. 21, No. 3, 1999, pp. 190-197. http://dx.doi.org/10.1097/00043426-199905000-00006 [18] C Tan, S. M. Sabai, A. S. Tin, T. C. Quah and L. Aung, “Neuroblastoma: Experience from National University Health System, Singapore (1987-2008),” Singapore Me- dical Journal, Vol. 53, 2012, pp. 19-25. [19] A. L. Yu, A. L. Gilman, M. F. Ozkaynak, W. B. London, S. G. Kreissman, H. X Chen, M. Smith, B. Anderson, J. G. Villablanca, K. K. Matthay, H. Shimada, S. A. Grupp, R. Seeger, C. P. Reynolds, A. Buxton, R. A. Reisfeld, S. D. Gillies, S. L. Cohn, J. M. Maris and P. M. Sondel, “Chil- dren’s Oncology Group. Anti-GD2 Antibody with GM- CSF, Interleukin-2, and Isotretinoin for Neuroblastoma,” The New England Journal of Medicine, Vol. 363, No. 14, 2010, pp. 1324-1334. http://dx.doi.org/10.1056/NEJMoa0911123 [20] R. C. Seeger, C. P. Reynolds, R. Gallego, D. O. Stram, R. B. Gerbing and K. K. Matthay, “Quantitative Tumor Cell Content of Bone Marrow and Blood as a Predictor of Outcome in Stage IV Neuroblastoma: A Children’s Can- cer Group Study,” Journal of Clinical Oncology, Vol. 18, 2000, pp. 4067-4076. [21] E. M. Kiely, “Radical Surgery for Abdominal Neuroblas- toma,” Seminars in Surgical Oncology, Vol. 9, No. 6, 1993, pp. 489-492. http://dx.doi.org/10.1002/ssu.2980090606 [22] D. von Schweinitz, B. Hero and F. Berthold, “The Impact of Surgical Radicality on Outcome in Childhood Neuro- blastoma,” European Journal of Pediatric Surgery, Vol. 12, No. 6, 2002, pp. 402-409. http://dx.doi.org/10.1055/s-2002-36952 [23] M. P. La Quaglia, B. H. Kushner, W. Su, G. Heller, K. Kramer, S. Abramson, N. Rosen, S. Wolden and N. K. Cheung, “The Impact of Gross Total Resection on Local Control and Survival in High-Risk Neuroblastoma,” Jour- nal of Pediatric Surgery, Vol. 39, No. 3, 2004, pp. 412- 417. http://dx.doi.org/10.1016/j.jpedsurg.2003.11.028 [24] A. L. Koivusalo, M. P. Pakarinen, R. J. Rintala and U. M. Saarinen-Pihkala, “Surgical Treatment of Neuroblastoma: Twenty-Three Years of Experience at a Single Institu- tion,” Surgery Today, 2013, Epub Ahead of Print. http://dx.doi.org/10.1007/s00595-013-0576-7 [25] A. M. Davidoff, “Neuroblastoma,” Seminars in Pediatric Surgery, Vol. 21, No. 1, 2012, pp. 2-14. http://dx.doi.org/10.1053/j.sempedsurg.2011.10.009 [26] S. Mueller and K. K. Matthay, “Neuroblastoma: Biology and Staging,” Current Oncology Reports, Vol. 11, No. 6, 2009, pp. 431-438. http://dx.doi.org/10.1007/s11912-009-0059-6
|