 Vol.3, No.4, 334-342 (2013) Open Journal of Animal Sciences http://dx.doi.org/10.4236/ojas.2013.34050 Diabetic mouse models Yoshiaki Katsuda1, Takeshi Ohta1* , Masami Shinohara2, Tong Bin3, Takahisa Yamada3 1Japan Tobacco Inc., Central Pharmaceutical Research Institute, Takatsuki, Japan; *Corresponding Author: takeshi.ota@jt.com 2Planning and Development Section, CLEA Japan Inc., Tokyo, Japan 3Laboratory of Animal Genetics, Graduate School of Science and Technology, Niigata University, Niigata, Japan Received 11 September 2013; revised 16 October 2013; accepted 26 October 2013 Copyright © 2013 Yoshiaki Katsuda et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The number of patients with lifestyle-related dis- eases, such as cardiovascular disease, diabetes mellitus, hypertension, atherosclerosis, and can- cer, is increasing all over the world, and that of diabetics is increasing especially rapidly. Dia- betic animal models have played a key role in elucidating the etiology of diabetes and devel- oping anti-diabetic drugs. In this review, we overviewed characteristics of diabetic mouse models and pharmacological evaluation using the diabetic models. Keywords: Diabetes; Diabet ic Complica tion; Mouse Model; Obesity 1. INTRODUCTION Diabetes has become a global health problem, and the incidence of diabetes is rapidly increasing in all regions of the world. The prevalence of diabetes across the world is forecast to increase from 171 million in 2000 to 366 million in 2030 [1]. Diabetes is classified into two categories: type 1 and type 2. Type 1 diabetes is characterized by a loss of insu- lin secretion due to pancreatic ß-cell degeneration, lead- ing to autoimmune attack. Type 2 diabetes is a metabolic disorder that is caused by insufficient insulin secretion and/or insulin resistance in peripheral and liver tissues [2]. To help develop new diabetic therapies, it is important to reveal the complex mechanisms of diabetes mellitus. In particular, investigations using diabetic animal models are essential to clarify the pathogenesis and progression in human disease course [3]. We reviewed nonobese dia- betic (NOD) mouse and nonobese C57BL/6 mutant (Aki- ta) mouse as type 1 diabetic models, and Lepob mutant (ob/ob) mouse, Lepdb mutant (db/db) mouse, KKAy mouse, and Tsumura Suzuki obese diabetes (TSOD) mouse as type 2 diabetic models, with respect to charac- teristic features and pharmacological evaluations using the diabetic models. 2. TYPE 1 DIABETIC MOUSE MODELS It is known that type 1 diabetes is caused by auto- immune destruction of ß cells of pancreas in genetically susceptible individuals. Understanding of the genetics and mechanisms of the disease has been facilitated by the use of nonobese diabetic (NOD) mouse. Another mouse model, nonobese C57BL/6 mutant (Akita) mouse, de- velops early age-onset diabetes, characterized by an auto- somal dominant mode of inheritance. 2.1. NOD Mouse 2.1.1. Background and Characteristic s NOD mouse was established as an inbred strain of mouse with spontaneous development of autoimmune type 1 diabetes by Makino et al. in Shionogi laboratory [4]. The origin of the NOD traces back to a mouse with cataract among Jcl:ICR mice in 1966. Later, an inbred strain of mouse with cataracts and small eyes, the CTG mouse, was established by Ohtori et al. [5]. After selec- tive breeding for hyperglycemia and euglycemia over about 10 generations, two sublines were transferred to Makino’s group, and strict brother-sister mating was started. At the 20th generation in selective breeding, a mouse with polyuria, polydipsia, and weight loss was found in the line with normal fasting blood glucose lev- els (approximately 100 mg/dl). Inbreeding was continued with this mouse to establish an inbred strain with spon- taneous development of diabetes, which culminated in a strain currently known as the NOD mouse. In NOD mice, infiltration of monocular cells into pan- creatic islets, insulitis, was observed at about 4 weeks of age [6]. The infiltrating monocular cells were mostly T cells (CD4+ and CD8+), and macrophages were also observed. ß cell destruction became aggressive after 15 weeks of age and overt diabetes developed. After the Copyright © 2013 SciRes. OPEN ACCESS  Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 335 onset of diabetes, NOD mice showed decreased body weight and died within one to two months unless treated with insulin injection [4]. Sex differences in incidence of diabetes existed, and the cumulative incidence of diabe- tes in Shionogi laboratory was approximately 80% in females and less than 20% in males at 30 weeks of age [4]. Moreover, diet, room temperature, and pathogens have been suggested as factors influencing the incidence of diabetes [7-9], but the exact causes are still unknown. 2.1.2. Pharmacological Evaluation It was reported that thiazolidinediones (TZDs) reduced diabetes incidence in NOD mice [10,11]. Rosiglitazone and troglitazone both significantly reduced the diabetic incidence in NOD mice as compared with litter-matched control mice. The effect is considered to have been in- duced by anti-inflammatory properties of TZDs. Com- bined treatment with lisofyline to inhibit autoimmunity and exendin-4 to enhance ß cell proliferation showed therapeutic effects in NOD mice [12]. Since NOD mice develop autoimmune-mediated inflammation of pancre- atic islets, anti-inflammatory drugs are reported to be useful for prevention or treatment of diabetes in the mice [13,14]. 2.2. Akit a Mouse 2.2.1. Background and Characteristic s A nonobese C57BL/6 mutant mouse found in Akita colony, which spontaneously develops early age-onset diabetes, is characterized by an autosomal dominant mode of inheritance. In Akita mice, a diabetic locus named MODY4, was mapped to chromosome 7 in the region distal to D7Mit189 [15]. Akita mice develop diabetic symptoms, such as hy- perglycemia, polydipsia, and polyuria, soon after wean- ing. The diabetic symptoms progress continuously in males, but the females exhibit mild symptoms. Survival rate in the male mice decreases gradually after about 25 weeks of age, and the rate at 52 weeks of age is about 20%. Neither infiltration of lymphocyte nor any signs of inflammatory reactions were detectable in pa the ncreas, but selective decreases in densities of active ß cells oc- curred in Akita mice [15]. In Akita mice, furthermore, it was reported that the Ins2 mutation resulted in a single amino acid substitution in the insulin 2 gene, which causes misfolding of insulin protein. For this mutation, Akita mice showed progressive loss of ß cell function, ß cell mass reduction, and overt hyperglycemia, as early as 4 weeks of age. There are some reports of kidney injury in Akita mice. At 6 months of age, albumin excretion and glomerular filtration rate (GFR) were increased and pathological changes such as glomerular hypertrophy and increases in mesangial matrix were observed [16,17]. It was consid- ered that hyperglycemia in Akita mice induced oxidative stress and the kidney injury was caused possibly by the oxidation stress [18]. In examination of the diabetic pe- ripheral neuropathy, Akita mice presented decreased motor nerve conduction velocity (MNCV), sensory nerve conduction velocity (SNCV), and slower reaction time to heat [19]. Moreover, there are some reports of retinal complications in diabetic Akita mouse model. Akita mice showed decreased retinal blood flow after several weeks of hyperglycemia [20], and the retinal ganglion cells were lost from the peripheral retina within 12 weeks of diabe- tes onset [21]. Furthermore, the mice showed increased retinal vascular permeability, increased acellular capil- laries, and alterations in morphology of astrocyte and microglia from 12 to 36 weeks of hyperglycemia [22,23]. 2.2.2. Pharmacological Evaluation Recently, drug therapies for diabetic complications in Akita mice have been reported. Koshizaka et al. reported that telmisartan, an angiotensin II type 1 receptor blocker, prevented diabetic nephropathy through the inhibition of Notch pathway [24]. Furthermore, Chen et al. reported that fenofibrate, a peroxisome proliferator-activated re- ceptor α (PPARα) agonist, showed therapeutic effects on diabetic retinopathy in Akita mice [25]. Fenofibrate at- tenuated overexpression of intercellular adhesion mole- cule (ICAM)-1, monocyte chemoattractant protein (MCP)-1, and vascular endotherial growth factor (VEGF), and inhibited activation of hypoxia-inducible factor (HIF)-1 and nuclear factor (NF)-κB in retina of Akita mice. 3. TYPE 2 DIABETIC MOUSE MODELS It is known that 90% - 95% of diabetes is diagnosed as type 2 diabetes [2]. Type 2 diabetes is a heterogeneous disease with multiple etiologies. The incidence and progression of diabetes characterized by insulin re- sistance and/or impaired insulin secretion are caused by genetic and environmental factors. The etiology is con- sidered to be multigenetic rather than monogenic. The development of diabetic animal models and patho- physiological analyses of the models are very important to aid in clarification of the pathogenesis and the patterns of progression in the human disease course. Diabetic mouse models, such as ob/ob mouse, db/db mouse, and KKAy mouse, are most commonly used in such studies. 3.1. Ob/Ob Mouse 3.1.1. Background and Characteristic s NOD Lepob mutation on chromosome 6 was discov- ered at the Jackson laboratory in a multiple recessive stock in 1949 [26], and the Lepob mutation was subse- quently transferred to B6 inbred strain background [27]. Lepob mutation on the B6 background (ob/ob) mice Copyright © 2013 SciRes. OPEN ACCESS 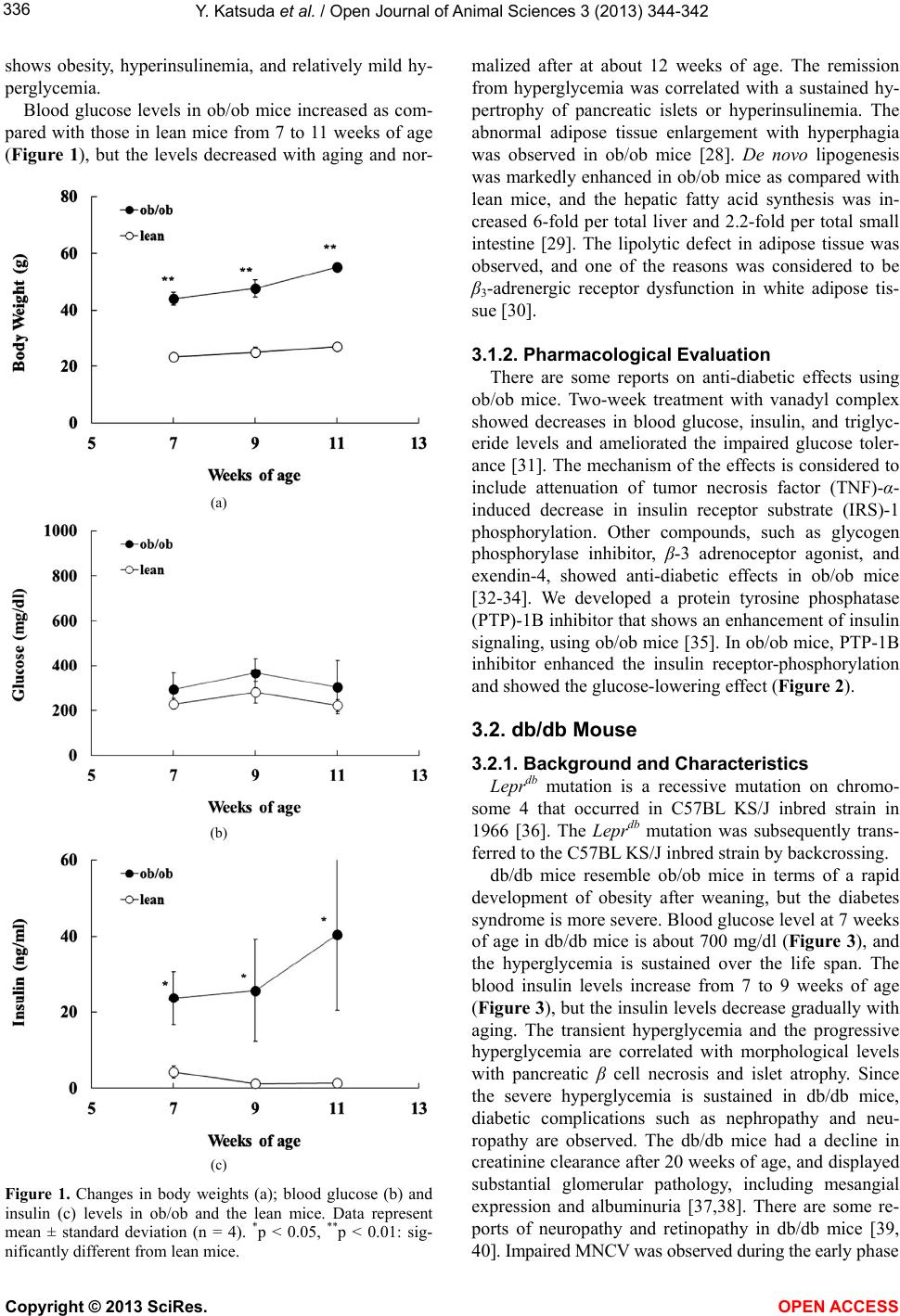 Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 336 shows obesity, hyperinsulinemia, and relatively mild hy- perglycemia. Blood glucose levels in ob/ob mice increased as com- pared with those in lean mice from 7 to 11 weeks of age (Figure 1), but the levels decreased with aging and nor- (a) (b) (c) Figure 1. Changes in body weights (a); blood glucose (b) and insulin (c) levels in ob/ob and the lean mice. Data represent mean ± standard deviation (n = 4). *p < 0.05, **p < 0.01: sig- nificantly different from lean mice. malized after at about 12 weeks of age. The remission from hyperglycemia was correlated with a sustained hy- pertrophy of pancreatic islets or hyperinsulinemia. The abnormal adipose tissue enlargement with hyperphagia was observed in ob/ob mice [28]. De novo lipogenesis was markedly enhanced in ob/ob mice as compared with lean mice, and the hepatic fatty acid synthesis was in- creased 6-fold per total liver and 2.2-fold per total small intestine [29]. The lipolytic defect in adipose tissue was observed, and one of the reasons was considered to be β3-adrenergic receptor dysfunction in white adipose tis- sue [30]. 3.1.2. Pharmacological Evaluation There are some reports on anti-diabetic effects using ob/ob mice. Two-week treatment with vanadyl complex showed decreases in blood glucose, insulin, and triglyc- eride levels and ameliorated the impaired glucose toler- ance [31]. The mechanism of the effects is considered to include attenuation of tumor necrosis factor (TNF)-α- induced decrease in insulin receptor substrate (IRS)-1 phosphorylation. Other compounds, such as glycogen phosphorylase inhibitor, β-3 adrenoceptor agonist, and exendin-4, showed anti-diabetic effects in ob/ob mice [32-34]. We developed a protein tyrosine phosphatase (PTP)-1B inhibitor that shows an enhancement of insulin signaling, using ob/ob mice [35]. In ob/ob mice, PTP-1B inhibitor enhanced the insulin receptor-phosphorylation and showed the glucose-lowering effect (Figure 2). 3.2. db/db Mouse 3.2.1. Background and Characteristic s Leprdb mutation is a recessive mutation on chromo- some 4 that occurred in C57BL KS/J inbred strain in 1966 [36]. The Leprdb mutation was subsequently trans- ferred to the C57BL KS/J inbred strain by backcrossing. db/db mice resemble ob/ob mice in terms of a rapid development of obesity after weaning, but the diabetes syndrome is more severe. Blood glucose level at 7 weeks of age in db/db mice is about 700 mg/dl (Figure 3), and the hyperglycemia is sustained over the life span. The blood insulin levels increase from 7 to 9 weeks of age (Figure 3), but the insulin levels decrease gradually with aging. The transient hyperglycemia and the progressive hyperglycemia are correlated with morphological levels with pancreatic β cell necrosis and islet atrophy. Since the severe hyperglycemia is sustained in db/db mice, diabetic complications such as nephropathy and neu- ropathy are observed. The db/db mice had a decline in creatinine clearance after 20 weeks of age, and displayed substantial glomerular pathology, including mesangial expression and albuminuria [37,38]. There are some re- ports of neuropathy and retinopathy in db/db mice [39, 40]. Impaired MNCV was observed during the early phase Copyright © 2013 SciRes. OPEN ACCESS  Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 337 (a) (b) Figure 2. Effects on PTP-1B inhibitor (JTT-551) in ob/ob mice. (a) Enhancement effect of JTT-551 (10 mg/kg) on the insulin receptor (IR) phosphorylation in liver of ob/ob mice. The intensity of the IR phosphorylation was calculated as the ratio of the density of phosphorylation-IRβ to the density of IRβ; (b) Effect of JTT-551 (10 mg/kg) on blood glucose in ob/ob mice. JTT-551 was administered to the mice for 7 days. Blood samples were collected at 3 h after dosing. Data repre- sent mean + standard deviation (n = 5). **p < 0.01: signifi- cantly different from the control (ob/ob mice). of the diabetic syndrome. In morphological study, db/db mice showed loss or shrinkage of myelinated fibers in sural nerve and ventral root, and axonal atrophy [41,42]. In the retina, pathological changes such as loss of peri- cytes and acellular capillaries were observed [40]. 3.2.2. Pharmacological Evaluation There are many reports in which db/db mice showed anti-diabetic potency. Recently, it was reported that novel compounds, such as GPR119 agonists and PTP-1B in- hibitors, show anti-diabetic effects in db/db mice [43,44]. A novel PTP-1B inhibitor that we developed also showed good glycemic control in db/db mice (Figure 4) [35]. Furthermore, combination therapy with pioglitazone, a PPARγ agonist, and alogliptin, a dipeptidyl peptidase (a) (b) (c) Figure 3. Changes in body weights (a); blood glucose (b) and insulin (c) levels in db/db and the lean mice. Data represent mean ± standard deviation (n = 4). *p < 0.05, **p < 0.01: sig- nificantly different from lean mice. (DPP)IV inhibitor, completely normalized β cell func- tions in db/db mice [45]. Recently, also, there have been many reports about pharmacological effects on diabetic complications in db/db mice. It was reported that treat- ment with various compounds, such as fibroblast growth factor (FGF) 21, erythropoietin, C-C chemokine receptor Copyright © 2013 SciRes. OPEN ACCESS  Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 338 (a) (b) Figure 4. Effect of JTT-551 (30 mg/kg) on blood glucose (a) and HbA1c (b) levels in db/db mice. JTT-551 was administered to the mice for 4 weeks. *p < 0.05, **p < 0.01: significantly different from the control. type 2 (CCR2) inhibitor, and nuclear factor of activated T cells (NFAT) inhibitor, ameliorated diabetic nephropathy in db/db mice [46-49]. Interestingly, in erythropoietin or NFAT inhibitor treatment, one of the therapeutic targets is a protection effect against podocyte injury [47,48]. Tofogliflozin, a novel sodium/glucose cotransporter (SGLT)2 inhibitor, or celastrol, an NF-κB inhibitor, also improved renal injury in db/db mice [50,51]. 3.3. KKAy Mouse 3.3.1. Background and Characteristic s Kondo et al. selected and established many mouse strains from Japanese native mice [52]. Among these inbred strains, Nakamura et al. found that the KK mouse is spontaneously diabetic [52,53]. Since diabetes and obesity in KK mice were relatively moderate, Nishimura et al. transferred the yellow obese gene (Ay) into KK mice by crossing yellow obese mice with KK mice [54]. The Ay allele was associated phenotypically with yellow fur, hyperphagia, and obesity [55,56]. This congenic strain of KK mice has been named KK Ay mouse. In KK Ay mice, diabetic characteristics, such as obe- sity, hyperinsulinemia, and hyperglycemia, were ob- served from young ages (6 - 8 weeks of age), but re- verted apparently to normal after 40 weeks of age [57, 58]. Insulin resistance in KK Ay mouse is considered to be caused by various physiological changes, such as re- duction in serum adiponectin levels, high activities of gluconeogenesis-enzymes in liver, and elevated produc- tion of TNF-α or other cytokines [59,60]. In pancreas of KK Ay mice, pathohistological changes, such as de- granulation, glycogen deposition, and hypertrophy β cells were observed at 5 - 10 weeks of age, suggesting that synthesis and release of insulin were increased with hyperinsulinemia [61]. In KK Ay mice, renal lesions, such as diffuse glomerulosclerosis, nodular changes, and peripheral glomerular basement membrane (GBM) thickening were observed [53,57]. 3.3.2. Pharmacological Evaluation Anti-diabetic drugs that reduce insulin resistance or increase insulin sensitivity have been developed using KK Ay mice. The first in the class of the TZD group of drugs, ciglitazone, was discovered in an in vivo screening system using KK Ay mice [62]. Moreover, pioglitazone was selected by Ikeda et al. [63]. These compounds de- creased hyperinsulinemia, hyperglycemia, and hyperlip- idemia, accompanied by improvement of insulin resis- tance. TZDs are considered to exert insulin-sensitizing action by binding to PPARγ [64]. Treatment with vana- dium complex showed an amelioration in diabetes, obe- sity, and hypertension in KK Ay mice [65]. 3.4. Tsumura Suzuki Obese Diabetics (TSOD) Mouse 3.4.1. Background and Characteristic s By the selective breeding of obese male mice of ddy strain and using indices of heavy body weight and ap- pearance of urinary glucose, Suzuki et al. established two inbred strains in 1992: one with obesity and urinary glucose (TSOD) and the other without them (Tsumura Suzuki non obese: TSNO) [66,67]. The male TSOD mice showed diabetic symptoms, such as hyperphagia, polydipsia, obesity, hyperglycemia, hyperlipidemia, and hypeinsulinemia. Pancreatic islets of the TSOD mice showed hypertrophy with the increase in the number of β cells and complete or partial degranula- tion of β cells [66]. Iizuka et al. reported diabetic com- plications in TSOD mice in 2005 [68]. In the kidney, histological changes, such as thickening of the basement membrane in glomeruli and increase of the mesangial area were observed after 18 months of age. The motor neuropathy showed after 14 months of age, and the mice at 17 months of age showed weakness of front and hind Copyright © 2013 SciRes. OPEN ACCESS  Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 339 paws caused by neuron degeneration. Moreover, in sen- sory neuropathy, the threshold in tail pressure test de- creased at 12 months of age. In histopathological analy- sis at sciatic nerves at 18 and 22 months of age, TSOD mice showed a decrease in the density of nerve fibers by endoneural fibrosis and loss of these fibers. Furthermore, degenerative changes of myelinated fibers, separation of myelin sheaths with intralamellar edema and remyelina- tion were observed. Kondo et al. 4. CONCLUSION In this review, we overviewed the characteristic fea- tures of type 1 and type 2 diabetic mouse models and pharmacological evaluations using diabetic models. Va- rious diabetic mouse models have been developed, and the models have played a key role in elucidating the pathogenesis of human diabetes and its complications. Moreover, diabetic mouse models are essential for de- veloping novel drugs for diabetes and its complications. The importance of the mouse models will be a constant in the future, and establishment of novel mouse models is also necessary for further understanding of diabetic etiology and development of new therapies. REFERENCES [1] Wild, S., Roglic, G., Green, A., Sicree, R. and King, H. (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care, 27, 1047-1053. http://dx.doi.org/10.2337/diacare.27.5.1047 [2] Inzucchi, S.E. and Sherwin, R.S. (2005) The prevention of type 2 diabetes mellitus. Endocrinology Metabolism Clinics of North America, 34, 199-219. http://dx.doi.org/10.1016/j.ecl.2004.11.008 [3] Kemmochi, Y., Fukui, K., Maki, M., Kimura, S., Ishii, Y., Sasase, T., Miyajima, K. and Ohta, T. (2013) Metabolic disorders and diabetic complications in Spontaneously Diabetic Torii Leprfa (SDT fatty) Rat, a new obese type 2 diabetic model. Journal of Diabetes Research, Article ID: 948257, 9 pages. [4] Makino, S., Kunimoto, K., Muraoka, Y., Mizushima, Y., Katagiri, K. and Tochino, Y. (1980) Breeding of nonobese diabetic strain of mice. Experimental Animals, 29, 1-13. [5] Ohtori, H., Yoshida, T. and Inuma, T. (1968) Small eye and cataract, a new dominant mutation in the mouse. Ex- perimental Animals, 17, 91-96. [6] Makino, S., Muraoka, Y., Kishimoto, Y. and Hayashi, Y. (1985) Genetic analysis for insulitis in NOD mice. Ex- perimental Animals, 34, 425-432. [7] Elliott, R.B., Reddy, S.N., Bibby, N.J. and Kida, K. (1988) Dietary prevention of diabetes in the nonobese diabetic mouse. Diabetologia, 31, 62-64. [8] Williams, A.J., Krug, J., Lampeter, E.F., Mansfield, K., Beales, P.E., Signore, A., Gale, E.A. and Pozzilli, P. (1990) Raised temperature reduces the incidence of diabetes in the NOD mouse. Diabetologia, 33, 635-637. http://dx.doi.org/10.1007/BF00400211 [9] Ohsugi, T. and Kurosawa, T. (1994) Increased incidence of diabetes mellitus in specific pathogen-eliminated off- spring produced by embryo transfer in NOD mice with low incidence of the disease. Laboratory Animal Science, 44, 386-388. [10] Beales P.E. and Pozzilli, P. (2002) Thiazolidinediones for the prevention of diabetes in the non-obese diabetic (NOD) mouse: Implications for human type 1 diabetes. Diabetes/Metabolism Research and Reviews, 18, 114-117. http://dx.doi.org/10.1002/dmrr.262 [11] Beales, P.E., Liddi, R., Giorgini, A.E., Signore, A., Pro- caccini, E., Batchelor, K. and Pozzilli, P. (1998) Troglita- zone prevents insulin dependent diabetes in the non-obese diabetic mouse. European Journal of Pharmacology, 357, 221-225. http://dx.doi.org/10.1016/S0014-2999(98)00574-3 [12] Yang, Z., Chen, M., Carter, J.D., Nunemaker, C.S., Gar- mey, J.C., Kimble, S.D. and Nadler, J.L. (2006) Com- bined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochemical and Biophysical Re- search Communications, 344, 1017-1022. http://dx.doi.org/10.1016/j.bbrc.2006.03.177 [13] Johnson, C.G., Mikulowska, A., Butcher, E.C., McEvoy, L.M. and Michie, S.A. (1999) Anti-CD43 monoclonal an- tibody L11 blocks migration of T cells to inflamed pan- creatic islets and prevents development of diabetes in nonobese diabetic mice. The Journal of Immunology, 163, 5678-5685. [14] Yokono, K., Amano, K., Suenaga, K., Hari, J., Shii, K., Yaso, S., Yonezawa, K., Imamura, Y. and Baba, S. (1985- 1986) Effect of antiserum to monoclonal anti-islet cell surface antibody on pancreatic insulitis in non-obese dia- betic mice. Diabetes Research and Clinical Practice, 1, 315-321. http://dx.doi.org/10.1016/S0168-8227(86)80043-2 [15] Yoshioka, M., Kayo, T., Ikeda, T. and Koizumi, A. (1997) A novel locus, Mody4, distal to D7Mit189 on chromo- some 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes, 46, 887-894. http://dx.doi.org/10.2337/diab.46.5.887 [16] Gurley, S.B., Mach, C.L., Stegbauer, J., Yang, J., Snow, K.P., Hu, A., Meyer, T.W. and Coffman, T.M. (2010) In- fluence of genetic background on albuminuria and kidney injury in Ins2+/C96Y (Akita) mice. American Journal of Physiology-Renal Physiology, 298, F788-F795. http://dx.doi.org/10.1152/ajprenal.90515.2008 [17] Chang J.H. and Gurley, S.B. (2012) Assessment of dia- betic nephropathy in the Akita mice. Methods in Molecu- lar Biology, 933, 17-29. [18] Ueno, Y., Horio, F., Uchida, K., Naito, M., Nomura, H., Kato, Y., Tsuda, T., Toyokuni, S. and Osawa, T. (2002) Increase in oxidative stress in kidneys of diabetic Akita mice. Bioscience, Biotechnology, and Biochemistry, 66, 869-872. http://dx.doi.org/10.1271/bbb.66.869 [19] de Preux Charles, A.S., Verdier, V., Zenker, J., Peter, B., Médard, J.J., Kuntzer, T., Beckmann, J.S., Bergmann, S. and Chrast, R. (2010) Global transcriptional programs in Copyright © 2013 SciRes. OPEN ACCESS  Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 340 peripheral nerve endoneurium and DRG are resistant to the onset of type 1 diabetic neuropathy in Ins2 mice. PLoS One, 5, e10832. http://dx.doi.org/10.1371/journal.pone.0010832 [20] Wright, W.S., Yadav, A.S., McElhatten, R.M. and Harris, N.R. (2012) Retinal blood flow abnormalities following six months of hyperglycemia in the Ins2 (Akita) mouse. Experimental Eye Research, 98, 9-15. http://dx.doi.org/10.1016/j.exer.2012.03.003 [21] Gastinger, M.J., Kunselman, A.R., Conboy, E.E., Bronson, S.K. and Barber, A.J. (2008) Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Investigative Opthalmology & Visual Science, 49, 2635-2642. http://dx.doi.org/10.1167/iovs.07-0683 [22] Barber, A.J., Antonetti, D.A., Kern, T.S., Reiter, C.E., Soans, R.S., Krady, J.K., Levision, S.W., Gardner, T.W. and Bronson, S.K. (2005) The Ins2Akita mouse as a model of early retinal complications in diabetes. Investi- gative Opthalmology & Visual Science, 46, 2210-2218. http://dx.doi.org/10.1167/iovs.04-1340 [23] Han, Z., Guo, J., Conley, S.M. and Naash, M.I. (2013) Retinal angiogenesis in the Ins2 (Akita) mouse model of diabetic retinopathy. Investigative Opthalmology & Visual Science, 54, 574-584. http://dx.doi.org/10.1167/iovs.12-10959 [24] Koshizaka, M., Takemoto, M., Sato, S., Tokuyama, H., Fujimoto, M., Okabe, E., Ishibashi, R., Ishikawa, T., Tsu- rutani, Y., Onishi, S., Mezawa, M., He, P., Honjo, S., Ueda, S., Saito, Y. and Yokote, K. (2012) An angiotensin II type 1 receptor blocker prevents renal injury via in- hibition of the Notch pathway in Ins2 Akita diabetic mice. Experimental Diabetes Research, 2012, 159874. http://dx.doi.org/10.1155/2012/159874 [25] Chen, Y., Hu, Y., Lin, M., Jenkins, A.J., Keech, A.C., Mott, R., Lyons, T.J. and Ma, J.X. (2013) Therapeutic ef- fects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes, 62, 261-272. http://dx.doi.org/10.2337/db11-0413 [26] Ingalls, A.M., Dickie, M.M. and Snell, G.D. (1950) Obese, a new mutation in the house mouse. Journal of Heredity, 41, 317-318. [27] Drasher, M.L., Dickie, M.M. and Lane, W.D. (1955) Phy- siological differences in uteri of obese stock mice. A com- parison between obese mice and their thin sibs. Journal of Heredity, 46, 209-212. [28] Contaldo, F., Gerber, H., Coward, W.A. and Trayhurn, P. (1981) Milk intake in pre-weanling genetically obese (ob/ ob) mice. In: Enzi, G., Crepaldi, G., Pozza, G. and Renold, A.E., Eds., Obesity: Pathogenesis and Treatment, Aca- demic Press, London/New York, 319-322. [29] Memon, R.A., Grunfeld, C., Moser, A.H. and Feingold, K.R. (1194) Fatty acid synthesis in obese insulin resistant diabetic mice. Hormone and Metabolic Research, 26, 85- 87. http://dx.doi.org/10.1055/s-2007-1000778 [30] Begin-Heick, N. (1996) Beta-adrenergic receptors and G- protein in the ob/ob mouse. International Journal of Obe- sity, 20, S32-S35. [31] Takeshita, S., Kawamura, I., Yasuno, T., Kimura, C., Ya- mamoto, T., Seki, J., Tamura, A., Sakurai, H. and Goto, T. (2001) Amelioration of insulin resistance in diabetic ob/ob mice by a new type of orally avtive insulin-mimetic vanadyl complex: Bis(1-oxy-2-piridinethiolato)oxovana- dium(IV) with VO(S(2)O(2)) coordination mode. Journal of Inorganic Biochemistry, 85, 179-186. http://dx.doi.org/10.1016/S0162-0134(01)00192-1 [32] Mackay, P., Ynddal, L., Andersen, J.V. and McCormack, J.G. (2003) Pharmacokinetics and anti-hyperglycaemic efficacy of a novel inhibitor of glycogen phosphorylase, 1,4-dideoxy-1,4-imino-d-arabinitol, in glucagon-challenged rats and dogs and in diabetic ob/ob mice. Diabetes, Obe- sity, and Metabolism, 5, 397-407. http://dx.doi.org/10.1046/j.1463-1326.2003.00293.x [33] Schaeffer, P., Bernat, A., Arnone, M., Manara, L., Gallas, J.F., Dol-Gleizes, F., Millet, L., Grosset, A. and Herbert, J.M. (2206) Effect of SR58611A, a potent beta-3 adreno- ceptor agonist, on cutaneous wound healing in diabetic and obese mice. European Journal of Pharmacology, 529, 172-178. http://dx.doi.org/10.1016/j.ejphar.2005.11.005 [34] Irwin, N., McClean, P.L., Cassidy, R.S., O’harte, F.P., Green, B.D., Gault, V.A., Harriott, P. and Flatt, P.R. (2007) Comparison of the anti-diabetic effects of GIP- and GLP- 1-receptor activation in obese diabetic (ob/ob) mice: Stu- dies with DPP IV resistant N-AcGIP and exendin(1- 39)amide. Diabetes/Metabolism Research and Reviews, 23, 572-579. http://dx.doi.org/10.1002/dmrr.729 [35] Fukuda, S., Ohta, T., Sakata, S., Morinaga, H., Ito, M., Nakagawa, Y., Tanaka, M. and Matsushita, M. (2010) Pharmacological profiles of a novel protein tyrosine pho- sphatase1B inhibitor, JTT-551. Diabetes, Obesity, and Metabolism, 12, 299-306. http://dx.doi.org/10.1111/j.1463-1326.2009.01162.x [36] Hummel, K.P., Dickie, M.M. and Coleman, D.L. (1966) Diabetes, a new mutation in the mouse. Science, 153, 1127-1128. http://dx.doi.org/10.1126/science.153.3740.1127 [37] Allen, T.J., Cooper, M.E. and Lan, H.Y. (2004) Use of ge- netic mouse models in the study of diabetic nephropathy. Current Diabetes Reports, 4, 435-440. http://dx.doi.org/10.1007/s11892-004-0053-1 [38] Sharma, K., McCue, P. and Dunn, S.R. (2003) Diabetic kid- ney disease in the db/db mice. American Journal of Phy- siology-Renal Physiology, 284, F1138-F1144. [39] Islam, M.S. (2013) Animal models of diabetic neuropathy: Progress since 1960s. Journal of Diabetes Research, 2013, Article ID: 149452. http://dx.doi.org/10.1155/2013/149452 [40] Midena E., Segato, T., Radin, S., di Giorgio, G., Meneg- hini, F., Piermarocchi, S. and Belloni, A.S. (1989) Studies on the retina of the diabetic db/db mouse. I. Endothelial cell-pericyte ratio. Ophthalmic Research, 21, 106-111. http://dx.doi.org/10.1159/000266787 [41] Robertson, D.M. and Sima, A.A. (1980) Diabetic neuron- pathy in the mutant mouse [C57BL/ks(db/db)]: A mor- phometric study. Diabetes, 29, 60-67. http://dx.doi.org/10.2337/diab.29.1.60 [42] Sima, A.A. and Robertson, D.M. (1978) Peripheral neuro- pathy in mutant diabetic mouse [C57BL/ks (db/db)]. Acta Copyright © 2013 SciRes. OPEN ACCESS  Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 341 Neuropathologica, 41, 85-89. http://dx.doi.org/10.1007/BF00689757 [43] Oshima, H., Yoshida, S., Ohishi, T., Matsui, T., Tanaka, H., Yonetoku, Y., Shibasaki, M. and Uchiyama, Y. (2013) Novel GPR119 agonist AS1669058 potentiates insulin se- cretion from rat islets and has potent anti-diabetic effects in ICR and diabetic db/db mice. Life Science, 92, 167- 173. http://dx.doi.org/10.1016/j.lfs.2012.11.015 [44] Wang, C.D., Teng, B.S., He, Y.M., Wu, J.S., Pan, D., Pan, L.F., Zhang, D., Fan, Z.H., Yang, H.J. and Zhou, P. (2012) Effect of a novel proteoglycan PTP1B inhibitor from Ga- noderma lucidum on the amelioration of hyperglycaemia and dyslipidaemia in db/db mice. British Journal of Nu- trition, 108, 2014-2025. http://dx.doi.org/10.1017/S0007114512000153 [45] Kawashima, S., Matsuoka, T.A., Kaneto, H., Tochino, Y., Kato, K., Yamamoto, K., Yamamoto, T., Matsuhisa, M. and Shimomura, I. (2011) Effect of alogliptin, pioglitazone and glargine on pancreatic β-cells in diabetic db/db mice. Biochemical and Biophysical Research Communications, 404, 534-540. http://dx.doi.org/10.1016/j.bbrc.2010.12.021 [46] Kim, H.W., Lee, J.E., Cha, J.J., Hyun, Y.Y., Kim, J.E., Lee, M.H., Song, H.K., Nam, D.H., Han, J.Y., Han, S.Y., Han, K.H., Kang, Y.S. and Cha, D.R. (2013) Fibroblast growth factor 21 improves insulin resistance and ameliora- tes renal injury in db/db mice. Endocrinology, 154 , 3366- 3376. http://dx.doi.org/10.1210/en.2012-2276 [47] Loeffler, I., Ruester, C., Franks, S., Liebisch, M. and Wolf, G. (2013) Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mice. Ameri- can Journal of Physiology-Renal Physiology. http://dx.doi.org/10.1152/ajprenal.00643.2012 [48] Seok, S.J., Lee, E.S., Kim, G.T., Hyun, M., Lee, J.H., Chen, S., Choi, R., Kim, H.M., Lee, E.Y. and Chung, C.H. (2013) Blockade of CCL2/CCR2 signaling ameliorates diabetic nephropathy in db/db mice. Nephrology Dialysis Transplantation, 28, 1700-1710. http://dx.doi.org/10.1093/ndt/gfs555 [49] Zhang, L., Li, R., Shi, W., Liang, X., Liu, S., Ye, Z., Yu, C., Chen, Y., Zhang, B., Wang, W., Lai, Y., Ma, J., Li, Z. and Tan, X. (2013) NFAT2 inhibitor ameliorates diabetic nephropathy and podocyte injury in db/db mice. British Journal of Pharmacology, 170, 426-439. [50] Nagata, T., Fukuzawa, T., Takeda, M., Fukazawa, M., Mori, T., Nihei, T., Honda, K., Suzuki, Y. and Kawabe, Y. (2013) Tofogliflozin, a novel sodium-glucose co-transpor- ter 2 inhibitor, improves renal and pancreatic function in db/db mice. British Journal of Pharmacology, 170, 519- 531. http://dx.doi.org/10.1111/bph.12269 [51] Kim, J.E., Lee, M.H., Nam, D.H., Song, H.K., Kang, Y.S., Lee, J.E., Kim, H.W., Cha, J.J., Hyun, Y.Y., Han, S.Y., Han, K.H., Han, J.Y. and Cha, D.R. (2013) Celastrol, an NF-κB inhibitor, improves insulin resistance and attenu- ates renal injury in db/db mice. PLoS One, 8, Article ID: E62068. http://dx.doi.org/10.1371/journal.pone.0062068 [52] Kondo, K., Nozawa, K., Tomita,T. and Ezaki, K. (1957) Inbred strains resulting from Japanese mice. Bull Expe- rimental Animals, 6, 107-112. [53] Nakamura, M. (1962) A diabetic strain of the mouse. Pro- ceedings of the Japan Academy, 38, 348-352. [54] Nishimura, M. (1969) Breeding of mice strains for diabe- tes mellitus. Experimental Animals, 18, 147-157. [55] Bultman, S., Michaud, E.J. and Woychik, R.P. (1992) Mo- lecular characterization of the mouse agouti locus. Cell, 71, 1195-1204. http://dx.doi.org/10.1016/S0092-8674(05)80067-4 [56] Michaud, E.J., Bultman, S.J., Klebig, M.L., van Vugt, M.J., Stubbs, L.J., Russell, L.B. and Woychik, R.P. (1994) A molecular model for the genetic and phenotypic char- acteristics of the mouse lethal yellow (Ay) mutation. Pro- ceedings of the National Academy of Science, 91, 2562- 2566. http://dx.doi.org/10.1073/pnas.91.7.2562 [57] Iwatsuka, H. and Shino, A. (1970) Studies on diabetoge- nic action of obesity in mice: Congenital insulin resistan- ce of KK mice. Endocrine Journal, 17, 535-540. http://dx.doi.org/10.1507/endocrj1954.17.535 [58] Iwatsuka, H., Taketomi, S., Matsuo, T. and Suzuoki, Z. (1974) Congenitally impaired hormone sensitivity of the adipose tissue of spontaneously diabetic mice, KK. Validi- ty of thrifty genotype in KK mice. Diabetologia, 10, 611- 616. http://dx.doi.org/10.1007/BF01221994 [59] Taketomi, S., Tsuda, M., Matsuo, T., Iwatsuka, H. and Suzuoki, Z. (1973) Alternations of hepatic enzyme active- ties in KK and yellow KK mice with various diabetic states. Hormone and Metabolic Research, 5, 333-339. http://dx.doi.org/10.1055/s-0028-1093938 [60] Hofman, C., Lorenz, K. and Colca, J.R. (1991) Glucose transport deficiency in diabetic animals is corrected by treatment with oral antihyperglycemic agent pioglitazone. Endocrinology, 129, 1915-1925. http://dx.doi.org/10.1210/endo-129-4-1915 [61] Iwatsuka, H., Shino, A. and Suzuoki, Z. (1970) General survey of diabetic features of yellow KK mice. Endocrine Journal, 17, 23-35. http://dx.doi.org/10.1507/endocrj1954.17.23 [62] Fujita, T., Sugiyama, Y., Taketomi, S., Sohda, T., Kawa- matsu, Y., Iwatsuka, H. and Suzuoki, Z. (1983) Reduction of insulin resistance in obese and/or diabetic animals by 5-{4-(1-methylcyclohexylmethoxy)benzyl}-thiazolidine-2,4- dione (ADD-3878, U-63,287, ciglitazone) a new antidia- betic agent. Diabetes, 32, 804-810. http://dx.doi.org/10.2337/diab.32.9.804 [63] Ikeda, H., Taketomi, S., Sugiyama, Y., Shimura, Y., Sohda, T., Meguro, K. and Fujita, T. (1990) Effects on pioglita- zone on glucose and lipid metabolism in normal and insu- lin resistant animals. Arzneimittelforschung, 40, 156-162. [64] Willson, T.M., Cobb, J.E., Cowan, D.J. Wiethe, R.W., Correa, I.D., Prakash, S.R., Beck, K.D., Moore, L.B., Kliewer, S.A. and Lehmann, J.M. (1996) The structure-ac- tivity relationship between peroxisome proliferator-ac- tivated receptor gamma agonism and the antihyperglyce- mic avtivity of thiazolidinediones. Journal of Medicinal Chemistry, 39, 665-668. http://dx.doi.org/10.1021/jm950395a [65] Adachi, Y., Yoshikawa, Y., Kodera, Y., Katoh, A., Takeda, J. and Sakurai, H. (2006) Improvement in diabetes, obe- sity and hypertension in type 2 diabetic KKA(y) mice by Copyright © 2013 SciRes. OPEN ACCESS  Y. Katsuda et al. / Open Journal of Animal Sciences 3 (2013) 344-342 Copyright © 2013 SciRes. OPEN ACCESS 342 bis(allixunato)oxovanadium(IV) complex. Biochemical and Biophysical Research Communications, 345, 945-950. http://dx.doi.org/10.1016/j.bbrc.2006.05.003 [66] Suzuki, W., Iizuka, S., Tabuchi, M., Funo, S., Yanagisawa, T., Kimura, M., Sato, T., Endo, T. and Kawamura, H. (1999) A new mouse model of spontaneous diabetes derived from ddY strain. Experimental Animals, 48, 181-189. http://dx.doi.org/10.1538/expanim.48.181 [67] Hirayama, I., Yi, Z., Izumi, S., Arai, I., Suzuki, W., Naga- machi, Y., Kuwano, H., Takeuchi, T. and Izumi, T. (1999) Genetic analysis of obese diabetes in the TSOD mouse. Diabetes, 48, 1183-1191. http://dx.doi.org/10.2337/diabetes.48.5.1183 [68] Iizuka, S., Suzuki,W., Tabuchi, M., Nagata, M., Imamura, S., Kobayashi, Y., Kanitani, M., Yanagisawa, T., Kase, Y., Takeda, S., Aburada, M. and Takahashi, K.W. (2005) Diabetic complications in a new animal model (TSOD mouse) of spontaneous NIDDM with obesity. Experimen- tal Animals, 54, 71-83. http://dx.doi.org/10.1538/expanim.54.71
|