 World Journal of Cardiovascular Diseases, 2013, 3, 493-498 WJCD http://dx.doi.org/10.4236/wjcd.2013.38078 Published Online December 2013 (http://www.scirp.org/journal/wjcd/) Left atrial voltage remodeling after pulmonary venous isolation with multipolar radiofrequency ablation* Francesco Laurenzi#, Piergiuseppe De Girolamo, Augusto Pappalardo, Andrea Avella Department of Cardiology, Cardiac Arrhythmia Center, St. Camillo-Forlanini Hospital, Rome, Italy Email: #laurenzi.f@tiscali.it Received 30 August 2013; revised 29 September 2013; accepted 18 October 18, 2013 Copyright © 2013 Francesco Laurenzi et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Purpose: Pulmonary vein isolation (PVI) is the ac- cepted primary endpoint for catheter ablation of atrial fibrillation (AF). The aim of this study was to evaluate the level of PVI by PVAC, a multipolar cir- cular catheter utilizing bipolar/unipolar radiofre- quency (RF) energy. Methods: Twenty patients with paroxysmal AF underwent PVAC ablation. PVI was validated by voltage reduction and pacing tests. Be- fore and after RF ablation, left atrium (LA) and PV electroanatomic mapping (EAM) were performed by EnSite NavX system. Voltage abatement was consid- ered for potentials < 0.5 mV. RF lesion setting was compared to the PVs anatomy previously acquired by a cardiac CT scan. Results: Seventeen patients had four veins and three had a left common PV. All 77 PVs were isolated by PVAC. After RF, EAM showed low voltages areas at th e prox ima l PV ostium an d LA. Segmental voltage abatement slightly distal to the anatomic PV ostia was achieved in 20/77 (26%) PVs, more frequently in veins > 24mm: 9/20 (45%) vs 11/57 (19%), p < 0.05. Antral lesions were evident in 38/77 PVs (49%), limited to a part of the antrum in 29/38 (76%) veins, with larger occurrence in round than in oval PVs ostia: 25/36 (69%) vs 13/41 (32%), p < 0.001. Conclusions: Electrophysiological PVI with PVAC is achieved in all the veins with low voltages areas at the proximal PV ostium. A low voltage circumferential lesion at the anatomic PV ostia is more challenging in larger veins. Antral lesions, fre- quently affecting part of the antra, were more fre- quent in round PV ostia. Keywords: Atrial Fibrillation; Pulmonary Vein Isolation; Multipolar Circular Ablation Catheter; Electroanatomic Mapping 1. INTRODUCTION Different strategies are currently used for atrial fibrilla- tion (AF) catheter ablation, but there is a general con- sensus on pulmonary vein isolation (PVI) with lesions proximal to the ostia [1]. A debate is still ongoing about the amount of left atrium (LA) substrate proximal to the ostia that should be included in the targeting lesion, as antra can be involved in initiation and maintenance of the arrhythmia. The vast majority of AF catheter ablation procedures rely on point-by-point RF applications and they need a double transseptal puncture and additional techniques such as tree-dimensional (3D) mapping sys- tems or intracardiac echocardiography [1]. To simplify the procedure, different technologies and tools have been designed in order to perform a “one-shot” circular lesion at the PVs ostia. One of these tools is an over-the-wire circular decapolar catheter (PVAC, Medtronic, Minnea- polis, MN, USA), capable of mapping and ablating using alternating bipolar-unipolar radiofrequency (RF) energy. The feasibility and efficacy of PVAC-based procedures have been already described with results comparable with point-by-point strategies, shorter procedure time and reduced Rx exposure [2-8]. The aim of this study was to analyse the PVAC lesion setting through a 3D impedance-based electroanatomic mapping (EAM), to assess how proximal the lesion extension is with respect to the PV-LA junction and how it is influenced by ana- tomical variants. 2. METHODS 2.1. Patients The study group included patients with symptomatic paroxysmal AF refractory to antiarrhythmic drugs, who underwent bipolar-unipolar RF ablation and EAM, be- *Disclosures: None. #Corresponding author. OPEN ACCESS  F. Laurenzi et al. / World Journal of Cardiovascular Diseases 3 (2013) 493-498 494 tween January and November 2012. Exclusion criteria were the presence of a large left atrium (long axis di- ameter >50 mm), left appendage thrombus, left ventricu- lar ejection fraction (LVEF) <45%, severe mitral valve disease, severe renal or pulmonary comorbidity, contra- indication to oral coagulation and pregnancy. 2.2. Electrophysiological Study and 3D Voltage Mapping All patients received oral anticoagulation therapy for at least 4 weeks up to 3 days before the procedure, then replaced by a low molecular weight heparin. A computed tomography (CT) scan of the heart and a transoesophag- eal echocardiogram were performed within 48 hours be- fore the procedure. Surface and bipolar electrocardio- grams (ECGs) were continuously monitored and re- corded. LA access was gained through a single transsep- tal puncture to position a steerable 9.5 F sheath (Channel, Bard EP, Lowell, MA, USA), continuously perfused with 0.9% saline solution. After the transseptal puncture, in- travenous heparin was administered as a bolus (100 IU/kg) followed by infusion, to achieve and maintain a target activated clotting time of 350 s. A 3D reconstruction of the LA and PVs was performed using the NavX system (EnSite NavX, St Jude Medical, St Paul, MN, USA). A circular multipolar mapping catheter (Inquiry A-Focus II, St Jude Medical) was used for the acquisition of the 3D geometry, which was then compared side by side to the CT scan-derived anatomy. PVs ostia were tagged at the intersection of the tangential lines to PVs and LA walls [9]. A common PV was de- fined as a unique take-off of contiguous PVs, bifurcating > 5 mm distal to the PV-LA junction. The proximal bor- der of the antra has not been standardized yet, but arbi- trarily we judged the antral extension for lesions occur- ring > 5 mm from the PVs ostia. PVs ostia were ana- tomically characterized by measurement of the maxi- mum and minimum diameter and the relative ratio, de- fining round shaped ostia for a value ≤ 1.2 and oval shaped ostia for a ratio > 1.2 [10]. Bipolar voltage maps were acquired before and after PVs isolation in sinus rhythm. The following settings were used: interpolation of 10 mm, internal and external projections of 5 mm. Normal ECGs were evaluated for voltage recordings greater than 0.5 mV and were displayed in purple. Volt- age abatement was considered for potentials ranging from 0.5 to 0.05 mV displayed in a spectrum of colours ranging from red to blue, and for signals lower than 0.05 mV marked in grey and conventionally considered as electrically silent [11]. Off-line quantitative voltage analysis was performed collecting voltage values of points mapped at each PV-LA junction and relative proximal segment of the vein, listed as below: LSPV for the left superior PV, LIPV for the left inferior PV, LCPV for the left common PV, RSPV for the right superior PV and RIPV for the right inferior PV. 2.3. Multipolar Radiofrequency Catheter Ablation Ablation of PVs was performed using the PVAC, a de- capolar circular over-the-wire catheter capable of map- ping and ablation. The distal circular loop, measuring 25 mm in diameter, can be steered in a bidirectional fashion and extended in a spiral configuration. The ablation re- quires a specifically designed RF generator (GENius, Ablation Frontiers, Medtronic, USA), that allows RF delivery in various settings of bipolar/unipolar energy output and, through continuous temperature monitoring in each electrode, restrict the power output to a maxi- mum of 10 watts. The location of the decapolar ring was checked both using fluoroscopy and the 3D PVs-LA re- construction. RF energy was delivered for 60 s with a temperature limit of 60˚C, with a bipolar/uniratio of 2:1 when the catheter was in close proximity to the PV an- trum and 4:1 if the position was more ostial. Cavotricus- pid isthmus ablation was also performed using an 8-mm tip RF ablation catheter if a typical right atrial flutter was observed during the procedure or previously docu- mented. 2.4. Confirmation of Pulmonary Vein Isolation Before NavX remapping, PVI was always checked using the PVAC and then a standard circular multipolar cathe- ter (Inquiry A-Focus II, St Jude Medical). Electrical de- connection was assessed by abatement of local potentials in sinus rhythm and during atrial pacing and by achie- vement of PVs-LA conduction block. Entry conduction block was demonstrated by the absence or dissociation of PVs potentials. Exit block was assessed by pacing at high output (10 V at 2 ms) from all electrode pairs of the catheter, positioned in the proximal PVs and at the ostia. Persistence of PVs isolation was assessed again in all the PVs at the end of the study. No pharmacological tests were performed. 2.5. Statistical Analysis Statistical analysis was performed using SPSS 12.0 for Windows (SPSS, Inc., Chicago, IL, USA) Continuous data are expressed as mean values ± standard deviation. Categorical variables are expressed as a percentage. Dif- ferences between distributions were compared with a paired t-test for paired data and unpaired t-test for inde- pendent values. Differences in proportions were com- pared with a Fisher’s exact test. A P value < 0.05 was considered statistically significant. Copyright © 2013 SciRes. OPEN ACCESS 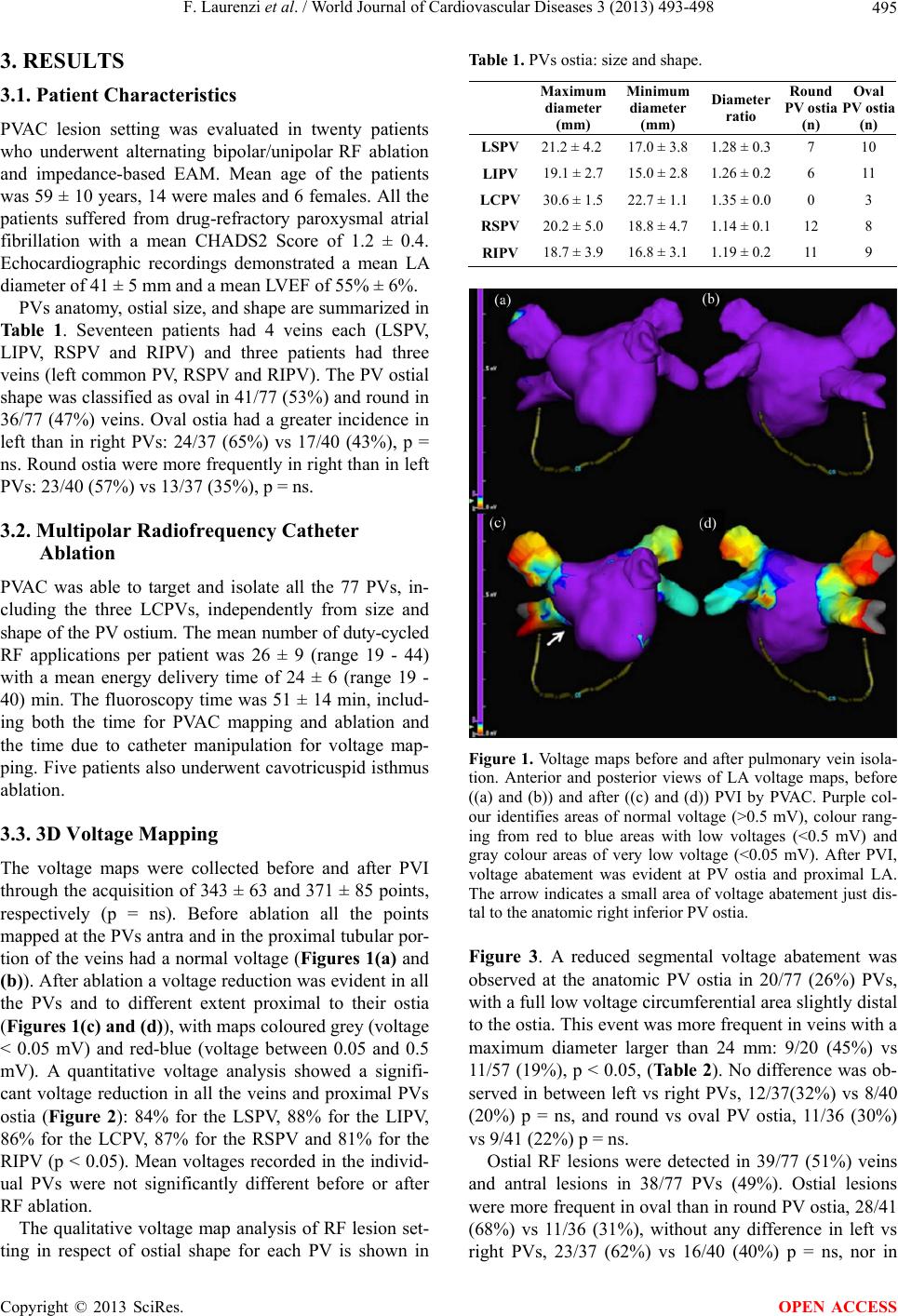 F. Laurenzi et al. / World Journal of Cardiovascular Diseases 3 (2013) 493-498 495 3. RESULTS 3.1. Patient Characteristics PVAC lesion setting was evaluated in twenty patients who underwent alternating bipolar/unipolar RF ablation and impedance-based EAM. Mean age of the patients was 59 ± 10 years, 14 were males and 6 females. All the patients suffered from drug-refractory paroxysmal atrial fibrillation with a mean CHADS2 Score of 1.2 ± 0.4. Echocardiographic recordings demonstrated a mean LA diameter of 41 ± 5 mm and a mean LVEF of 55% ± 6%. PVs anatomy, ostial size, and shape are summarized in Table 1. Seventeen patients had 4 veins each (LSPV, LIPV, RSPV and RIPV) and three patients had three veins (left common PV, RSPV and RIPV). The PV ostial shape was classified as oval in 41/77 (53%) and round in 36/77 (47%) veins. Oval ostia had a greater incidence in left than in right PVs: 24/37 (65%) vs 17/40 (43%), p = ns. Round ostia were more frequently in right than in left PVs: 23/40 (57%) vs 13/37 (35%), p = ns. 3.2. Multipolar Radiofrequency Catheter Ablation PVAC was able to target and isolate all the 77 PVs, in- cluding the three LCPVs, independently from size and shape of the PV ostium. The mean number of duty-cycled RF applications per patient was 26 ± 9 (range 19 - 44) with a mean energy delivery time of 24 ± 6 (range 19 - 40) min. The fluoroscopy time was 51 ± 14 min, includ- ing both the time for PVAC mapping and ablation and the time due to catheter manipulation for voltage map- ping. Five patients also underwent cavotricuspid isthmus ablation. 3.3. 3D Voltage Mapping The voltage maps were collected before and after PVI through the acquisition of 343 ± 63 and 371 ± 85 points, respectively (p = ns). Before ablation all the points mapped at the PVs antra and in the proximal tubular por- tion of the veins had a normal voltage (Figures 1(a) and (b)). After ablation a voltage reduction was evident in all the PVs and to different extent proximal to their ostia (Figures 1(c) and (d)), with maps coloured grey (voltage < 0.05 mV) and red-blue (voltage between 0.05 and 0.5 mV). A quantitative voltage analysis showed a signifi- cant voltage reduction in all the veins and proximal PVs ostia (Figure 2): 84% for the LSPV, 88% for the LIPV, 86% for the LCPV, 87% for the RSPV and 81% for the RIPV (p < 0.05). Mean voltages recorded in the individ- ual PVs were not significantly different before or after RF ablation. The qualitative voltage map analysis of RF lesion set- ting in respect of ostial shape for each PV is shown in Table 1. PVs ostia: size and shape. Maximum diameter (mm) Minimum diameter (mm) Diameter ratio Round PV ost ia (n) Oval PV ost ia (n) LSPV 21.2 ± 4.217.0 ± 3.8 1.28 ± 0.3 7 10 LIPV 19.1 ± 2.715.0 ± 2.8 1.26 ± 0.2 6 11 LCPV 30.6 ± 1.522.7 ± 1.1 1.35 ± 0.0 0 3 RSPV 20.2 ± 5.018.8 ± 4.7 1.14 ± 0.1 12 8 RIPV 18.7 ± 3.916.8 ± 3.1 1.19 ± 0.2 11 9 Figure 1. Voltage maps before and after pulmonary vein isola- tion. Anterior and posterior views of LA voltage maps, before ((a) and (b)) and after ((c) and (d)) PVI by PVAC. Purple col- our identifies areas of normal voltage (>0.5 mV), colour rang- ing from red to blue areas with low voltages (<0.5 mV) and gray colour areas of very low voltage (<0.05 mV). After PVI, voltage abatement was evident at PV ostia and proximal LA. The arrow indicates a small area of voltage abatement just dis- tal to the anatomic right inferior PV ostia. Figure 3. A reduced segmental voltage abatement was observed at the anatomic PV ostia in 20/77 (26%) PVs, with a full low voltage circumferential area slightly distal to the ostia. This event was more frequent in veins with a maximum diameter larger than 24 mm: 9/20 (45%) vs 11/57 (19%), p < 0.05, (Ta ble 2). No difference was ob- served in between left vs right PVs, 12/37(32%) vs 8/40 (20%) p = ns, and round vs oval PV ostia, 11/36 (30%) vs 9/41 (22%) p = ns. Ostial RF lesions were detected in 39/77 (51%) veins and antral lesions in 38/77 PVs (49%). Ostial lesions were more frequent in oval than in round PV ostia, 28/41 (68%) vs 11/36 (31%), without any difference in left vs right PVs, 23/37 (62%) vs 16/40 (40%) p = ns, nor in Copyright © 2013 SciRes. OPEN ACCESS  F. Laurenzi et al. / World Journal of Cardiovascular Diseases 3 (2013) 493-498 496 Figure 2. Voltage before and after pulmonary vein isolation. Mean voltage values (mV) before (light bars) and after (dark bars) PVI. LSPV: left superior PV; LIPV: left inferior PV; LCPV: left common PV; RSPV: right superior PV; RIPV: right inferior PV. Figure 3. Lesion setting and ostial shape in each PV. Abbrevia- tions as in Ta ble 1 . In brackets are indicated the veins with a segmental voltage abatement slightly distal to the anatomic PV ostia. veins with a diameter larger than 24 mm, 23/37 (62%) vs 16/40 (40%), p = ns. Antral lesions had a greater occurrence in round than in oval PV ostia: 25/36 (69%) vs 13/41(32%), p < 0.001. No any difference was observed in left vs right PVs, 14/37(38%) vs 24/40 (60%) p = ns, nor in veins larger than 24 mm, 8/20 (40%) vs 30/57 (53%), p = ns. Antral extension of RF lesions were evident in the whole antra in 9/38 veins and limited to a part of the PV antrum in 29/38 (76%) PVs: the posterior wall in 24 and the anterior wall in 5 PV antra. 4. DISCUSSION Our data confirm the results of a previous study [12] about the ability of PVAC to perform PVI at the proximal ostium, even in more challenging anatomical variants as three left common PVs, in which ostial lesions were car- ried out by insertion of the guide in different venous branches. After RF ablation, the impedance-based EAM showed a significant reduction in the bipolar voltage at the PV ostia, proximal to the tubular part of the PVs. However about one in four veins, frequently the larger ones, show- ed a segment of reduced voltage abatement at the anat- omic PV ostium with slightly distal low voltage encir- cling. Far-field signal recording, particularly frequent in left-sided veins because of their proximity to the left atrial appendage, was ruled out by remapping with stand- ard catheters and pacing maneuvers. On the other hand, a partial sided placement of the PVAC at the ostia may be the cause of the distal segmental voltage abatement: before RF erogation in any new catheter position, the location of the PVAC was displayed on the 3D mapping system, but a tiny displacement may have occurred as a consequence of the pressure exerted during RF delivery in order to achieve a better contact and the target tem- perature. In our experience antral extension of RF lesions was achieved more frequently in round veins, as counter- clockwise catheter rotation allows a wider sweep more easily around the circumference of round PV ostia. More often the antra were partially affected by RF lesions, probably as a result of different causes. Firstly, the PV’s short axis is oriented approximately in the anteroposte- rior direction. Secondly, the transseptal access naturally directs the catheter posteriorly into the LA, so that its circular ending is obliquely oriented with the anterior part located at the PV ostia and the posterior part more atrial. Finally, a greater antral contact was more easily achieved posteriorly by bending and rotating the catheter around the ostial circumference. On the contrary, the small distance between the left PVs and the LA append- age [10] can limit the placement of the circular catheter in the anterior LA walls more than that of a single-tip catheter. In our study the fluoroscopic and procedural mean times were longer than those previously described [2-8], because the procedure required an additional EAM and probably a different learning curve, since the mean num- ber of RF applications was similar. The PVAC is an over-the-wire catheter designed to be managed under fluoroscopic control, with more detailed definition of the PV-LA junction usually achieved by angiography. A small risk of PV stenosis is described in some studies [13,14]. In our experience the 3D-geometry reconstruction eased the positioning of the PVAC at the ostia and the parallel orientation to the PV’s walls, but was not useful for catheter navigation as it is conditioned by the over-the-wire design. Regarding the voltage map- ing, the low voltage signals recorded are not accurate enough to indicate PVI, which in fact was validated with electrophysiological entry and exit blocks. Moreover, maps can be affected by far-field potentials, particularly from the left atrial appendage, thus requiring accurate characteristics analysis and pacing maneuvers. As the PVAC-EAM combined strategy is more expensive and Copyright © 2013 SciRes. OPEN ACCESS  F. Laurenzi et al. / World Journal of Cardiovascular Diseases 3 (2013) 493-498 Copyright © 2013 SciRes. 497 Table 2. Qualitative voltage map analysis. Segmental low voltage area distal to the a natomic PV ostia n. 20/77 p value Left vs right PVs 12/37 (32%) vs 8/40 (20%) 0.30 Round vs oval PV ostia 11/36 (30%) vs 9/41 (22%) 0.44 PV diameter > 24 vs < 24 mm 9/20 (45%) vs 11/57 (19%) 0.037 Ostial lesions n . 39/77 Left vs right PVs 23/37 (62%) vs 16/40 (40%) 0.069 Round vs oval PV ostia 11/36 (31%) vs 28/41 (68%) 0.001 PV diameter > 24 vs < 24 mm 12/20 (60%) vs 27/57 (47%) 0.44 Antral lesion n. 38/77 Left vs right PVs 14/37 (38%) vs 24/40 (60%) 0.069 Round vs oval PV ostia 25/36 (69%) vs 13/41(32%) 0.001 PV diameter > 24 vs < 24 mm 8/20 (40%) vs 30/57 (53%) 0.44 time consuming, other techniques such as angiography or 3D CT/MRI cardiac scan reconstruction are to be pre- ferred for PV-LA junction definition. OPEN ACCESS This study outlines the significance of two factors in order to choose the more effective ablation tool: the knowledge of the individual anatomy that is useful to perform a right positioning of the circular mapping-ab- lation catheter, and the a priori preference of a wide en- circling PVI strategy that is easier to achieve with a sin- gle-point RF catheter. Limitations This study analyzed the primary lesion setting after PVAC ablation in a relatively small but representative group of patients, referred for paroxysmal AF catheter ablation. PVI was assessed by standard electrophysiological criteria as there is no absolute agreement between myo- cardial viability and low voltages and also because the maps can be affected by LA far-field potentials. As re- peated mapping procedures were not performed, the study does not clarify to what extent the ablation lesions remain permanent. 5. CONCLUSION Electrical PVI with PVAC is not affected by the size and shape of the PV ostium and results in low voltage areas mainly at the PV ostium and proximal LA. However, voltage reduction in the whole circumference of the anatomic PV ostia is more challenging in larger veins, and antral lesions are variable in occurrence and exten- sion, so knowledge of the individual anatomy of the PVs and LA can be useful in order to choose the more effec- tive ablation tool. 6. ACKNOWLEDGEMENTS The authors wish to thank Simona Benedetti, Roberta Annibali and Sergio Orsini for technical assistance and data collection and Tiziana De Santo for statistics. REFERENCES [1] Calkins, H., Kuck, K.H., Cappato, R., Brugada, J., Camm, J., Chen, S.A., Crijns, H.J., Damiano, R.J., Davies, W., Di Marco, J., Edgerton, J., Ellenbogen, K., Ezekowitz, M.D., Haines, D.E., Haissaguerre, M., Hindricks, G., Ie- saka, Y., Jackman, W., Jalife, J., Jais, P., Kalman, J., Keane, D., Kim, Y.H., Kirchhof, P., Klein, G., Kottkamp, H., Kumagai, K., Lindsay, B.D., Mansour, M., March- linski, F.E., McCarthy, P.M., Mont, L., Morady, F., Na- demanee, K., Nakagawa, H., Natale, A., Nattel, S., Packer, D.L., Pappone, C., Prystowsky, E., Raviele, A., Reddy, V., Ruskin, J.N., Shemin, R.J., Tsao, H.M. and Wilber, D. (2012) HRS/EHRA/ECAS Expert consensus statement on catheter and surgical ablation of atrial fib- rillation: Recommendations for patient selection, proce- dural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm, 9, 632-696. [2] Boersma, L., Wijffels, M., Oral, H., Wever, E. and Mo- rady, F. (2008) Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a mul- tielectrode ablation catheter. Heart Rhythm, 5, 1635- 1642. [3] Fredersdorf, S., Weber, S., Jilek, C., Heinicke, N., Von Bary, C., Jungbauer, C., Riegger, G.A., Hamer, O. and Jeron, A. (2009) Safe and rapid isolation of pulmonary veins using a novel circular ablation catheter and duty- cycled RF generator. Journal of Cardiovascular Electro- physiology, 20, 1097-1101. http://dx.doi.org/10.1111/j.1540-8167.2009.01501.x [4] Wiekzorek, M., Hoeltgen, R., Akin, E., Salili, A.R., Oral, H. and Morady, F. (2010) Results of short-term and long-term pulmonary vein isolation for paroxysmal atrial fibrillation using duty-cycled bipolar and unipolar ra- diofrequency energy. Journal of Cardiovascular Elec- trophysiology, 21, 399-405. http://dx.doi.org/10.1111/j.1540-8167.2009.01640.x [5] Duytschaever, M., Anne, W., Papiashvili, G., Vande- kerckhove, Y. and Tavernier, R. (2010) Mapping and isolation of the pulmonary veins using the PVAC catheter.  F. Laurenzi et al. / World Journal of Cardiovascular Diseases 3 (2013) 493-498 498 Pacing and Clinical Electrophysiology, 33, 168-178. http://dx.doi.org/10.1111/j.1540-8159.2009.02609.x [6] Bulava, A., Hanis, J., Sitek, D., Osmera, O., Karpianus, D., Snorek, M., Rehouskova, K., Tousek, F. and Pesl, L. (2010) Catheter ablation for paroxysmal atrial fibrillation: A randomized comparison between multielectrode cathe- ter and point-by-point ablation. Pacing and Clinical Electrophysiology, 33, 1039-1046. http://dx.doi.org/10.1111/j.1540-8159.2010.02807.x [7] Bittner, A., Mönnig, G., Zellerhoff, S., Pott, C., Köbe, J., Dechering, D., Millberg, P., Wasmer, K. and Eckardt, L. (2011) Randomized study comparing duty-cycled bipolar and unipolar radiofrequency with point by point ablation in pulmonary vein isolation. Heart Rhythm, 8, 1383-1390. http://dx.doi.org/10.1016/j.hrthm.2011.03.051 [8] Mulder, A.A., Wijffels, M.C., Wever, E.F. and Boersma, L.V. (2012) Freedom from paroxysmal atrial fibrillation after successful pulmonary vein isolation with pulmonary vein ablation catheter-phased radiofrequency energy: 2- year follow-up and predictors of failure. EP Europace, 14, 818-825. http://dx.doi.org/10.1093/europace/eus010 [9] Ho, S.Y., Cabrera, J.A., Tran, V.H., Farré, J., Anderson, R.H. and Sánchez-Quintana, D. (2001) Architecture of the pulmonary veins: relevance to radiofrequency abla- tion. Heart, 86, 265-270. http://dx.doi.org/10.1136/heart.86.3.265 [10] Schmidt, B., Ernst, S., Ouyang, F., Chun, J., Broemel, T., Bänsch, D., Kuck, K.H. and Antz, M. (2006) External and endoluminal analysis of left atrial anatomy and the pulmonary veins in three-dimensional reconstructions of magnetic resonance angiography: The full insight from inside. Journal of Cardiovascular Electrophysiology, 17, 957-964. http://dx.doi.org/10.1111/j.1540-8167.2006.00548.x [11] Chang, S.L., Tai, C.T., Lin, Y.J., Wongcharoen, W., Lo, L.W., Tuan, T.C,, Udyavar A.R., Chang, S.H., Tsao, H.M., Hsieh, M.H., Hu, Y.F., Chen, Y.J. and Chen, A.S. (2007) Biatrial substrate properties in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology, 18, 1134-1139. http://dx.doi.org/10.1111/j.1540-8167.2007.00941.x [12] Raffa, S., Groβe, A., Brunelli, M., Wauters, K. and Geller, J.C. (2010) Voltage mapping and pacing to assess the level of pulmonary isolation achieved with a novel circu- lar multielectrode ablation catheter. EP Europace, 12, 933-940. [13] Von Bary, C., Weber, S., Dornia, C., Eissnert, C., Fellner, C., Latzin, P., Fredersdorf, S., Stadler, S. and Hamer, O.W. (2011) Evaluation of pulmonary vein stenosis after pulmonary vein isolation using a novel circular mapping and ablation catheter (PVAC). Circulation: Arrhythmia and Electrophysiology, 4, 630-636. http://dx.doi.org/10.1161/CIRCEP.111.963397 [14] De Greef, Y., Tavernier, R., Raeymaeckers, S., Schwag- ten, B., Desurgeloose, D., De Keulenaer, G., Stockman, D., De Buyzere, M. and Duytschaever, M. (2012) Preva- lence, characteristics and predictors of pulmonary vein narrowing after PVAC ablation. Circulation: Arrhythmia and Electrophysiology, 5, 52-60. http://dx.doi.org/10.1161/CIRCEP.111.961888 Copyright © 2013 SciRes. OPEN ACCESS
|