Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 97-104 doi:10.4236/msa.2011.22013 Published Online February 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 97 Potential Energy Curves & Material Properties Devarakonda Annapurna Padmavathi Chemistry Department, Post Graduate College of Science, Saifabad Osmania University, Hyderabad, India. Email: dapadma@rediffmail.com Received August 6th, 2010; revised December 16th, 2010; accepted January 19th, 2011. ABSTRACT Potential energy curves govern the properties of materials. A critical analysis of the potential energy curve helps better understand the properties of the material. Potential energy curve and in turn the properties of any material depend on the composition, bonding, crystal structure, their mechanical processing and microstructure. The type, strength, and directionality of atomic bonding controls the structure and material properties viz., melting temperature, thermal ex- pansion, elastic stiffness, electrical properties, ductility and toughness etc. This paper attempts to bring out the correla- tion between the potential energy curves with the properties of materials. Keywords: Potential Energy Curves, Material Properties 1. Introduction Properties are the way the material responds to the envi- ronment and external forces. Mechanical properties re- spond to mechanical forces, strength, etc. Electrical and magnetic properties deal with their response to electrical and magnetic fields, conductivity etc. Thermal properties are related to transmission of heat and heat capacity. With the aid of potential energy curve(s), this paper intends to see the response of the material, from the atomic and subatomic particle arrangement, towards ex- ternal forces. Interatomic forces present in atomic bond- ing is reflected in the potential energy curves which in turn help predict many physical properties namely melt- ing temperature, elasticity, thermal expansion, and strength of materials. Choice of a material for a specific purpose can be made from the materials performance under differ- ent conditions and is reflected in potential energy curves. Content in the paper is organized with the description of atomic structure and bonding, first, followed by analy- sis of how it gets reflected in the potential energy curve and subsequently proceeds to bring the relation of mate- rial properties with potential energy curves. 2. Atomic Structure and Bonding All the elements that exist are classified according to electronic configuration in the periodic table. Electrons in atoms have discrete energy states and tend to occupy lowest available energy levels. The electronic structure of atoms governs their interaction with other atoms. Filled outer shells result in a stable configuration as in noble inert gases. Atoms with incomplete outer shells strive to reach this noble gas configuration by sharing or transferring electrons among each other for maximal sta- bility. There are two main types of bonding: 1) Primary bon- ding 2) Secondary bonding 1) Primary bonding results from the electron sharing or transfer. There are three types of primary bonding viz., ionic, covalent, and metallic (24-240 kcal/mol) [1]. In ionic bonding, atoms behave like either positive or negative ions, and are bound by Coulomb forces. A large difference in electronegativity is required for an ionic bond to be formed. The ionic bonding is nondirectional, i.e., the magnitude of the bond is equal in all directions. In ceramics, bonding is predominantly ionic. They are usually combinations of metals or semiconductors with oxygen, nitrogen or carbon (oxides, nitrides, and car- bides). Ionic materials are hard and brittle due to electri- cally charged nature of component ions. And, further- more they are electrical and thermal insulators due to absence of large number of free electrons. (Examples: glass, porcelain, examples of other ceramic materials range from household items to high performance com- bustion engines which utilize both metals and ceramics.) Covalent Bonding: In covalent bonding, electrons are shared between the molecules, to saturate the valency. Covalent bonds are highly directional. The simplest ex- ample is the SiO2 molecule. Their electrical properties depend strongly on minute proportions of contaminants 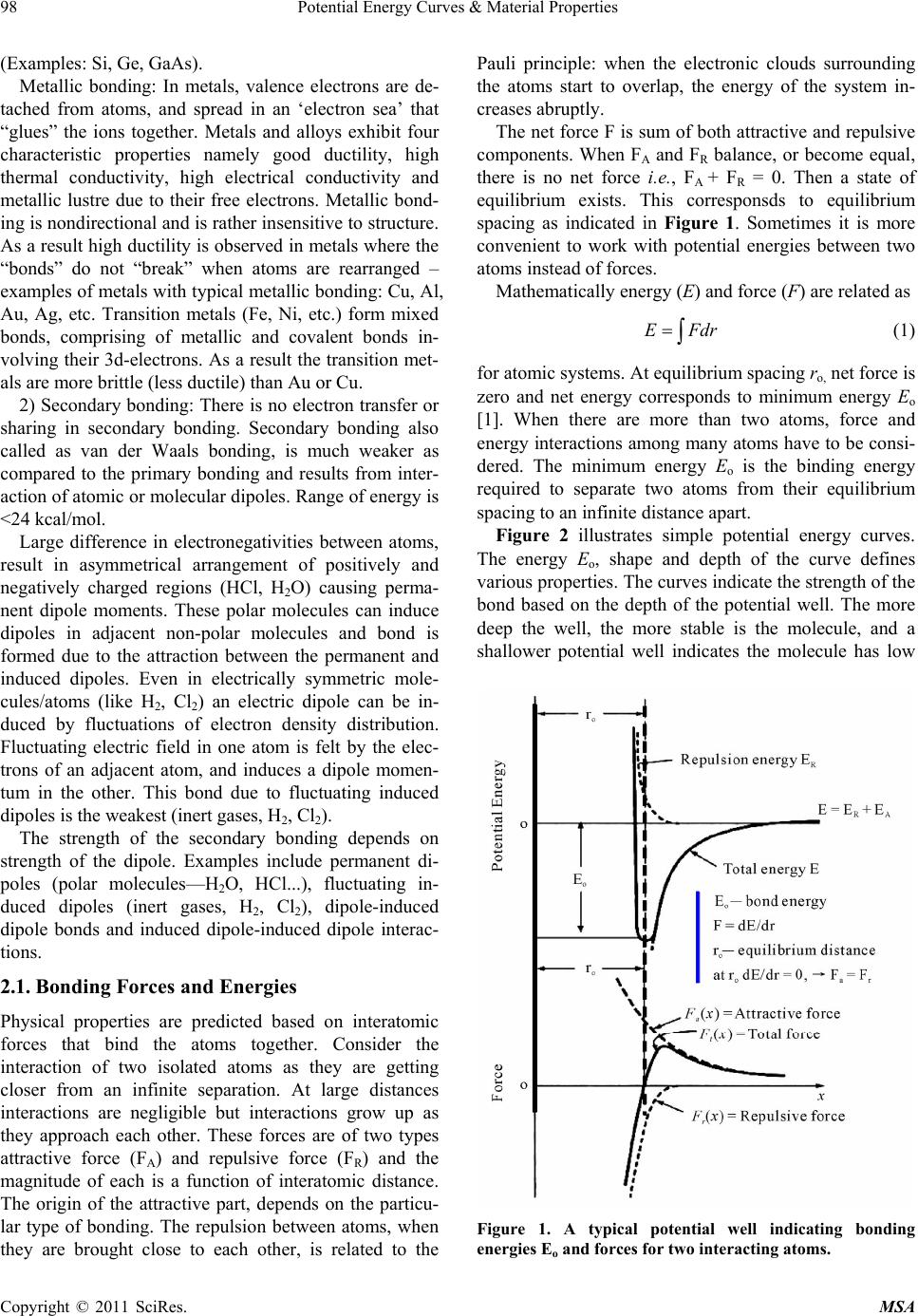 Potential Energy Curves & Material Properties 98 (Examples: Si, Ge, GaAs). Metallic bonding: In metals, valence electrons are de- tached from atoms, and spread in an ‘electron sea’ that “glues” the ions together. Metals and alloys exhibit four characteristic properties namely good ductility, high thermal conductivity, high electrical conductivity and metallic lustre due to their free electrons. Metallic bond- ing is nondirectional and is rather insensitive to structure. As a result high ductility is observed in metals where the “bonds” do not “break” when atoms are rearranged – examples of metals with typical metallic bonding: Cu, Al, Au, Ag, etc. Transition metals (Fe, Ni, etc.) form mixed bonds, comprising of metallic and covalent bonds in- volving their 3d-electrons. As a result the transition met- als are more brittle (less ductile) than Au or Cu. 2) Secondary bonding: There is no electron transfer or sharing in secondary bonding. Secondary bonding also called as van der Waals bonding, is much weaker as compared to the primary bonding and results from inter- action of atomic or molecular dipoles. Range of energy is <24 kcal/mol. Large difference in electronegativities between atoms, result in asymmetrical arrangement of positively and negatively charged regions (HCl, H2O) causing perma- nent dipole moments. These polar molecules can induce dipoles in adjacent non-polar molecules and bond is formed due to the attraction between the permanent and induced dipoles. Even in electrically symmetric mole- cules/atoms (like H2, Cl2) an electric dipole can be in- duced by fluctuations of electron density distribution. Fluctuating electric field in one atom is felt by the elec- trons of an adjacent atom, and induces a dipole momen- tum in the other. This bond due to fluctuating induced dipoles is the weakest (inert gases, H2, Cl2). The strength of the secondary bonding depends on strength of the dipole. Examples include permanent di- poles (polar molecules—H2O, HCl...), fluctuating in- duced dipoles (inert gases, H2, Cl2), dipole-induced dipole bonds and induced dipole-induced dipole interac- tions. 2.1. Bonding Forces and Energies Physical properties are predicted based on interatomic forces that bind the atoms together. Consider the interaction of two isolated atoms as they are getting closer from an infinite separation. At large distances interactions are negligible but interactions grow up as they approach each other. These forces are of two types attractive force (FA) and repulsive force (FR) and the magnitude of each is a function of interatomic distance. The origin of the attractive part, depends on the particu- lar type of bonding. The repulsion between atoms, when they are brought close to each other, is related to the Pauli principle: when the electronic clouds surrounding the atoms start to overlap, the energy of the system in- creases abruptly. The net force F is sum of both attractive and repulsive components. When FA and FR balance, or become equal, there is no net force i.e., FA + FR = 0. Then a state of equilibrium exists. This corresponsds to equilibrium spacing as indicated in Figure 1. Sometimes it is more convenient to work with potential energies between two atoms instead of forces. Mathematically energy (E) and force (F) are related as EFd=r ∫ (1) for atomic systems. At equilibrium spacing ro, net force is zero and net energy corresponds to minimum energy Eo [1]. When there are more than two atoms, force and energy interactions among many atoms have to be consi- dered. The minimum energy Eo is the binding energy required to separate two atoms from their equilibrium spacing to an infinite distance apart. Figure 2 illustrates simple potential energy curves. The energy Eo, shape and depth of the curve defines various properties. The curves indicate the strength of the bond based on the depth of the potential well. The more deep the well, the more stable is the molecule, and a shallower potential well indicates the molecule has low Figure 1. A typical potential well indicating bonding energies Eo and forces for two interacting atoms. Copyright © 2011 SciRes. MSA  Potential Energy Curves & Material Properties99 Figure 2. Calculated quantum mechanical potential energy curves of He2 and H2. dissociation energy. The shallow potential energy curve of helium states that the forces that bind are very weak. Almost a zero Eo value indicates the instability of the molecule. Very small bonding energy Eo of hydrogen states that gaseous state is favored. Table 1 gives list of bonding energies and melting tenperatures for various bond types. Increase in depth of the potential well, increases the melting temperature Tm (Figure 3(b)). Molecules with large bonding energies have high melting temperatures generally these exist as solids. As the depth of the potential well decreases. the molecules move from solid state to gaseous state [3]. 3. Analysis of Potential Energy Curves 3.1. Packing of Crystal Structures and Their Influence on Bonding Energies Different atoms based on their nature, arrange them- selves in different crystalline forms. The order in which atoms associate with neighbors, determine the bonding energy, the shape and depth of the potential well. Table 1. Bonding energies and melting temperatures for various substances. Bonding Type Substance Bonding Energy (kcal/mol) Melting Temperature (˚C) NaCl 153 801 Ionic MgO 239 1000 Si 108 1410 C 170 > 3550 Covalent Hg 16 –39 Al 77 660 Fe 97 1538 Metallic W 203 3410 Ar 1.8 –189 vander Waals Cl2 7.4 –101 (a) (b) Figure 3. (a) A typical potential energy curve; (b) Change in shape of the well with temperature. Copyright © 2011 SciRes. MSA  Potential Energy Curves & Material Properties Copyright © 2011 SciRes. MSA 100 te, compress, twist) or break as a function of applied load, time, temperature, and other conditions is described by mechanical proper- ties Non-crystalline solids lack a systematic and regular arrangement of atoms over relatively large atomic dis- tances. This disordered or random packing of atoms changes the force that binds the neighbouring atoms, and hence the bonding energy Eo of neighbouring atoms is different (Figure 4(a)). Due to the variation in neigh- bouring bond lengths, materials density decreases which has an influence on material properties. In crystal structures with long range order atoms are positioned in a repititive 3-dimensional The standard language to discuss mechanical proper- ties of materials is in terms of Hooke’s law. In this law, stress ‘σ’ and strain ‘ε’ are related to each other by the equation E σ ε = (2) where E is the modulus of Elasticity or Young’s Modulus (Figure 6(a)). pattern in which each atom is bonded to its nearest neighbouring atoms with similar force. As a result, equilibrium bond length ro and bond energy Eo remains same between any two neigbouring atoms (Figure 4(b)). And hence materials with high ordered packing have good density and hence good strength [2]. Different ordered packing results in different crystal- line patterns. The Hooke’s law allows one to compare specimens of dif- ferent cross sectional area A0 and different length L0. This equation can also be written in terms of force F as 0 o F L E A L Δ = (3) type of packing, nearest neighbour bonding and crystal structure decide the properties of substances. For ex., pure Mg hexagonal closely packed crystal is more brittle than Al a face centered cubic crys- tal due to less number of slip planes and hence undergoes fracture at lower degrees of deformation (Figure 5). 3.2. Mechanical Properties In the elastic limit Modulus of elasticity E is the slope of the stress (F/A0) versus strain (ΔL/L0) curve (Figure 6(b)). Higher the modulus of elasticity higher is the stiffness of the bond. After the stress is removed, if the material returns to the dimension it had before the load- ing, it is elastic deformation. If the material does not re- turn to its previous dimension it is referred as plastic de- formation. On an atomic scale, macroscopic elastic strain is mani- How materials deform (elonga (a) (b) Figure 4. (a) Random packing of atoms and the corresping potential energy curve; (b) Dense orderedacking of atoms and the corresponding potential energy curve. ond p  Potential Energy Curves & Material Properties101 (a) (b) (c) Figure 5. Packinracture. g of atoms in (a) Al, (b) Mg and (c) Relation between packing and f (a) (b) Figure 6. (a) Variation of strwith strain; (b) Linearity of stress strain relation. ges in the interatomic spacing and Modulus of eleasticity E is proportional to slope of the (Figure 7(a)) at equilibrium spacing. Slope of the curve at r = r es n solids the principle mode of s through increase in vibra- ess fested as small chan the stretching of interatomic bonds. As a consequence, the magnitude of modulus of elasticity is a measure of the resistance to separation of adjacent atoms i.e., intera- tomic bonding forces. o position is steep for very stiff materials and shallower for flexible materials [2,3]. force versus interatomic separation curve The energy interatomic distance curve, Figure 7(b) illustrates that as modulus of elasticity E decreases, energy minima decreases and hence the strength of the bond. Values of modulus of eleasticity E are highest for cera- mics, higher for metals and lower for polymers which is a direct consequence of the different types of atomic bonding. Another measured mechanical property is yield strength. This is the level of stress above which a material begins to show permanent deformation. From an atomic pers- pective, plastic deformation corresponds to the breaking of bonds with original atom neighbors and then for- mation of bonds with new ones as large number of molecules move relative to one another. In plastic defor- mation, upon removal of stress the atoms do not return to their original positions. Low yield strength corresponds to the inability of the molecule to regain its initial state, which corresponds to low elastic modulus and hence low bonding energy in the potential energy curve. Metals have high yield strength but for ceramics yield strength is hard to measure, as since in tension fracture occurs before it yields. 3.3. Thermal Properti Response of a material to the application of heat is often studied in terms of heat capacity, thermal expansion and thermal conductivity. I thermal energy assimilation i tion energy of atoms. The vibrations of adjacent atoms are coupled based on the nature of atomic bonding lead- ing to lattice waves termed phonons which transfer en- ergy through material. Most solids expand on heating and contract on cooling. Thermal expansion results in an increase in the average distance between atoms. When the temperature changes, Copyright © 2011 SciRes. MSA 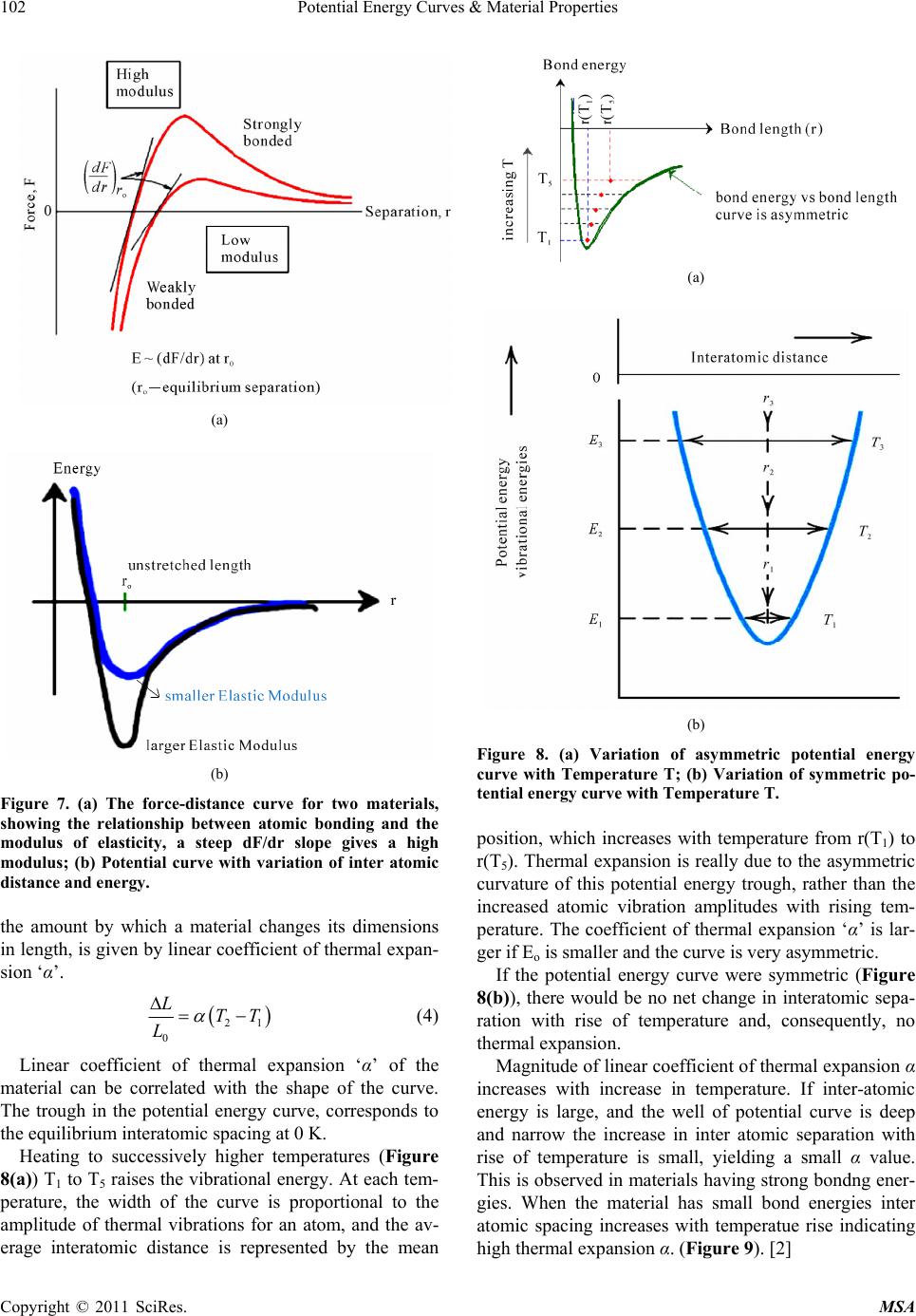 Potential Energy Curves & Material Properties 102 (a) (b) Figure 7. (a) The force-distance curve for two materials, showing the relationship between atomic bonding and the modulus of elasticity, a steep dF/dr slope gives a high modulus; (b) Potential curve with variation of inter atomic distance and energy. the amount by which a material changes its dimensions in length, is given by linear coefficient of thermal expan- sion ‘α’. () 21 0 L Linear coefficient of thermal expansion ‘α’ of the material ca LTT α =− (4) n be correlated with the shape of the curve. The trough in the potential energy curve, corresponds to the equilibrium interatomic spacing Heating to successively higher temperatures (Figure 8( by the mean Δ at 0 K. a)) T1 to T5 raises the vibrational energy. At each tem- perature, the width of the curve is proportional to the amplitude of thermal vibrations for an atom, and the av- erage interatomic distance is represented (a) (b) Figure 8. (a) Variation of asymmetric potential energy curve with Temperature T; (b) Variation of symmetric po- tential energy curve with Temperature T. position, which increases w temperature from r(T1) to ith rising tem- erature. The coefficient of thermal expansion ‘α’ is lar- ith r(T5). Thermal expansion is really due to the asymmetric curvature of this potential energy trough, rather than the increased atomic vibration amplitudes w p ger if Eo is smaller and the curve is very asymmetric. If the potential energy curve were symmetric (Figure 8(b)) n α in is small, yielding a small α value. Th , there would be no net change in interatomic sepa- ration with rise of temperature and, consequently, no thermal expansion. Magnitude of linear coefficient of thermal expansio creases with increase in temperature. If inter-atomic energy is large, and the well of potential curve is deep and narrow the increase in inter atomic separation with rise of temperature is is observed in materials having strong bondng ener- gies. When the material has small bond energies inter atomic spacing increases with temperatue rise indicating high thermal expansion α. (Figure 9). [2] Copyright © 2011 SciRes. MSA 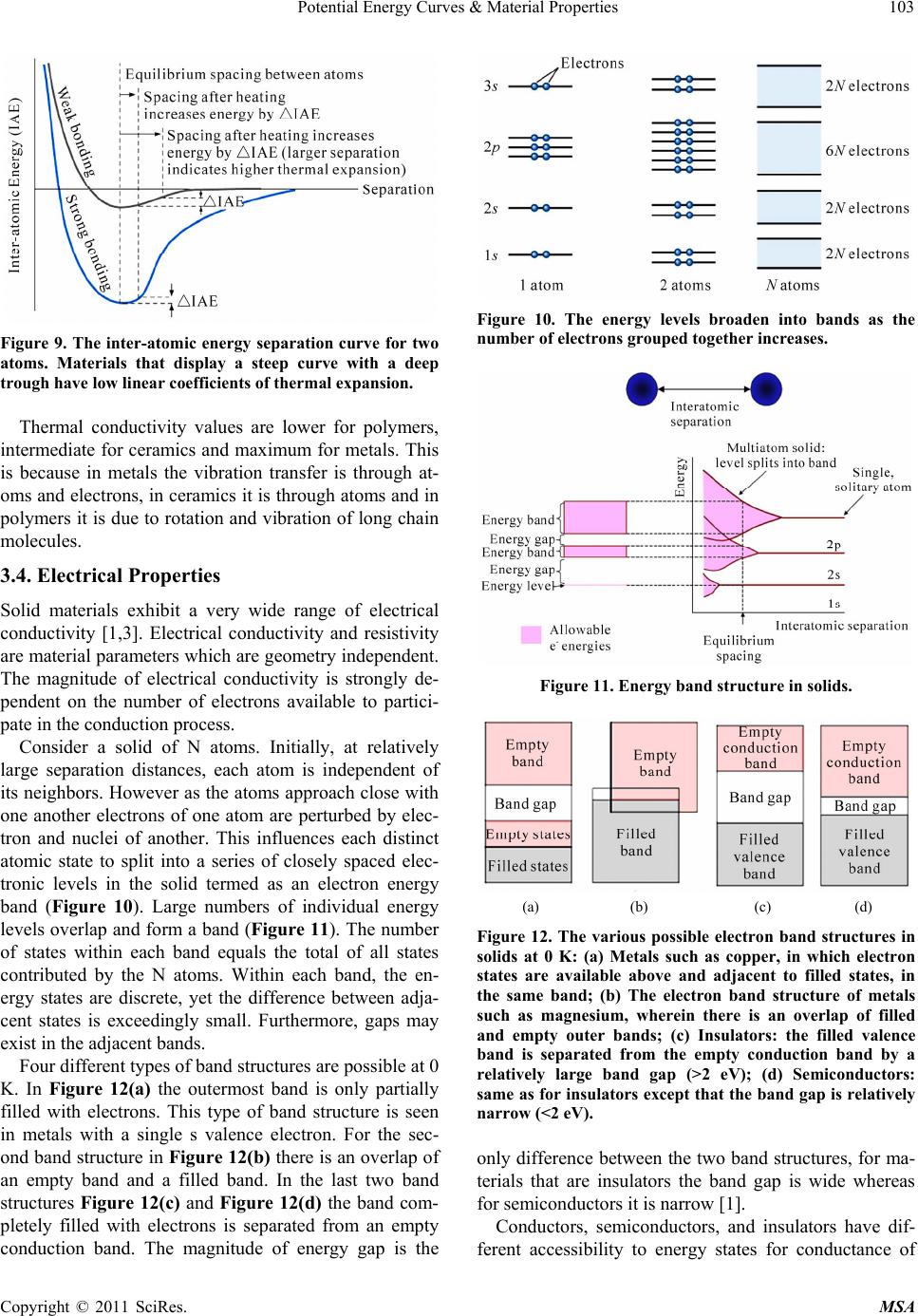 Potential Energy Curves & Material Properties103 Figure 9. The inter-atomic energy separation curve for two atoms. Materials that display a steep curve with a deep trough have low linear coefficients of thermal expansion. Thermal conductivity values are lower for polymers, in olymers it is due to rotation and vibration of long chain m arameters which are geometry independent. nductivity is strongly de- ctrons available to partici- nces each distinct at e 12(b) there is an overlap of an intermediate for ceramics and maximum for metals. This is because in metals the vibration transfer is through at- oms and electrons, in ceramics it is through atoms and p olecules. 3.4. Electrical Properties Solid materials exhibit a very wide range of electrical conductivity [1,3]. Electrical conductivity and resistivity are material p The magnitude of electrical co pendent on the number of ele pate in the conduction process. Consider a solid of N atoms. Initially, at relatively large separation distances, each atom is independent of its neighbors. However as the atoms approach close with one another electrons of one atom are perturbed by elec- tron and nuclei of another. This influe omic state to split into a series of closely spaced elec- tronic levels in the solid termed as an electron energy band (Figure 10). Large numbers of individual energy levels overlap and form a band (Figure 11). The number of states within each band equals the total of all states contributed by the N atoms. Within each band, the en- ergy states are discrete, yet the difference between adja- cent states is exceedingly small. Furthermore, gaps may exist in the adjacent bands. Four different types of band structures are possible at 0 K. In Figure 12(a) the outermost band is only partially filled with electrons. This type of band structure is seen in metals with a single s valence electron. For the sec- ond band structure in Figur empty band and a filled band. In the last two band structures Figure 12(c) and Figure 12(d) the band com- pletely filled with electrons is separated from an empty conduction band. The magnitude of energy gap is the Figure 10. The energy levels broaden into bands as the number of electrons grouped together increases. Figure 11. Energy band structure in solids. (a) (b) (c) (d) Figure 12. The various possible electron band structures in solids at 0 K: (a) Metals such as copper, in which electron states are available above and adjacent to filled states, in the same band; (b) The electron band structure of metal such illed and empty outer bands; (c) Insulators: the filled va emiconductors, and insulators have dif- rent accessibility to energy states for conductance of s as magnesium, wherein there is an overlap of f lence band is separated from the empty conduction band by a relatively large band gap (>2 eV); (d) Semiconductors: same as for insulators except that the band gap is relatively narrow (<2 eV). only difference between the two band structures, for ma- terials that are insulators the band gap is wide whereas for semiconductors it is narrow [1]. Conductors, s fe Copyright © 2011 SciRes. MSA  Potential Energy Curves & Material Properties Copyright © 2011 SciRes. MSA 104 ve very low conduc- tiv curve indicates large bond emperature, large elastic modulus t of thermal expansion. different m nd small coefficient of linear expansion fo tial energy curves of metals possess variable bo an pansion. Polymers possessing directional prop- ert ion exists for solid materials as in covalent and ionic in semi- co gineering: An Introduction,” & Sons, Inc., Ho- boken, 2008. rove, 2003. electrons. Metallic conductivity is of the order of 107 (Ω-m)–1, semiconductors have intermediate conductivities 10–6 to 104 (Ω-m)–1 and insulators ha ities 10–10 to 10–20 (Ω-m)–1. 4. Conclusions The magnitude of bonding energy and shape of the po- tential energy curve varies from material to material. A deep and narrow trough in the energy, high melting t and small coefficien Generally substances with large bonding energy Eo are solids, intermediate energies are liquids and small ener- gies are gases. The width and asymmetry of the well in the potential energy curve represents varying properties of aterials. Large bond energies, high melting temperature, large elastic modulus a und in potential energy curve(s) of ceramics represent very good strength, characteristically hard nature. The poten nd energy, reasonably high melting point, high elastic modulus, and moderate thermal expansion. The ductility of metals is implicitly related to the characteristics of the metallic bond. The grouping of energy levels as bands [3] W. F. Smith, “Principles of Materials Science and Engi- neering,” 3rd Edition, McGraw-Hill, Columbus, 1996. d their overlap with availability of large number of free electrons is responsible for electrical conductivity of metals. The potential curve(s) of polymers possess low melt- ing point low elastic modulus and large coefficient of linear ex ies due to covalent bonding have dominating second- dary forces of interactions and influence the physical properties of materials. This paper focused on an ideal situation involving only two atoms to understand some of the properties, a similar yet more complex condit teractions among many atoms need to be addressed. However, the energy versus interatomic separation curve defines the basic property. In many materials more than one type of bonding is involved viz., ionic and covalent in ceramics, covalent and secondary in polymers, nductors. These are to be considered while deriving the properties from the potential energy curves. REFERENCES [1] W. D. Callister, “Materials Science & En 7th Edition, John Wiley [2] D. R. Askeland and P. P. Phule. “The Science and Engi- neering of Materials,” 4th Edition, Thomas Book Com- pany, Pacific G |

