 Food and Nutrition Sciences, 2013, 4, 56-66 Published Online November 2013 (http://www.scirp.org/journal/fns) http://dx.doi.org/10.4236/fns.2013.411A008 Open Access FNS Isolation and Characterization of Bacteriophages from Laban Jameed* Murad Mohammad Ishnaiwer, Fawzi Al-Razem# Biotechnology Research Center, Palestine Polytechnic University, Hebron, Palestine. Email: #razemf@ppu.edu Received July 25th, 2013; revised August 25th, 2013; accepted September 2nd, 2013 Copyright © 2013 Murad Mohammad Ishnaiwer, Fawzi Al-Razem. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Laban jameed is a dried salty dairy product obtained by fermentation of milk using a complex population of lactic acid bacteria. Jameed is considered a traditional food product in most eastern Mediterranean countries and is usually made from sheep or cow milk. The aim of this study was to assess phage contamination of jameed dairy product. Two phages were isolated: one from sheep milk jameed (PPUDV) and the other from cow milk jameed (PPURV). Each of the two bacteriophages was partially characterized. The PPUDV phage was identified as a single stranded DNA virus with an approximately 20 kb genome that was resistant to RNase, whereas PPURV phage possessed a double stranded RNA genome of approximately 20 kb and was resistant to DNase. The phage bacterial strain hosts were identified as Lacto- bacillus h elveticus and Bacillus amyloliqu efaciens for PPUDV and PPURV, respectively. One step growth curve using a double layer plaque assay test was carried out to monitor the phage life cycle after host infection. PPUDV showed a latent period of about 36 h, burst period of 70 h and a burst size of about 600 Plaque Forming Units (PFU) per infected cell. PPURV phage showed a latent period of about 24 h, burst period of 47 h and a burst size of about 700 PFU per infected cell. SDS-PAGE analysis of total viral proteins showed at least three major bands (27, 40, and 45 kDa) for PPUDV. This is the first study to report the isolation of both DNA and RNA bacteriophages from laban jameed. This study adds new insights into the complexity of dairy contamination and fermentation microbiology of the jameed re- vealing the existence of two viral genomes in this highly dried and salty dairy product. Keywords: Lactic Acid Bacteria; Bacteriophages; Laban Jameed; DNA Viruses; RNA Viruses 1. Introduction Laban jameed is a dried and salty dairy product that forms part of an ancient traditional diet that is common in the Middle East, particularly in Syria, Lebanon, Jordan, Saudi Arabia and Palestine [1-3]. It is favored by Bedouin communities because of the ease of storing this dairy product for a long period of time and due to its high stability and resistance to pathogens. Jameed is tradi- tionally made from sheep and/or cow fermented milk and is used in many Arab food dishes, such as Mansaf [1,2]. Milk fermentation occurs mostly through the action of a highly complex microflora of lactic acid bacteria (LAB) through continuous shaking, leading to a highly acidic product, formation of butter (curd) and expulsion of the milk serum liquid (whey). The curd is then slightly heat- ed to accelerate the onset of the fermentation process and to further ensure the full separation of whey. The whey is decanted and the curd is added into a cheesecloth container to remove the excess water. When it becomes a thick paste, called labaneh in Palestine, it is kneaded or sprinkled with sodium chloride salt (approximately 10% salt contents) and placed to dry for a few days in the sun to ensure that no dampness occurs, which could spoil the product [1-3]. In most processed dairy products, the fermentation of milk is facilitated by using a mixture of LAB, such as Lactobacillus acidophilus, L. brevis, L. casei, L. fermen- tum, L. kefir, L. parakefir, L. plantarum, and L. helviticus [4,5]. Lactic acid bacteria are generally gram positive and acid tolerant and producing lactic acid as a final product of carbohydrate fermentation [5,6]. Similar to *Authors’ contribution: M.M—I carried out the experimental work and rovided the first draft of the manuscript. F. Al-R designed and super- vised the work. Both authors have read and approved the final manu- script. #Corresponding author.  Isolation and Characterization of Bacteriophages from Laban Jameed 57 many other bacterial species, the LAB are susceptible to several types of bacteriophages and some of these phage particles have been isolated and characterized [5-8]. Most LAB known phages are tailed and are members of the Caudovirales order [8]. With the expanding dairy product industries, phage contamination is a growing problem. This contamination is believed to affect the fermentation process and the quality of dairy product [6,9]. Contamination could ori- ginate from different sources, such as water, soil, air, cattle feces, cattle udder and milk equipment [1,9]. The phage contamination in dairy products becomes proble- matic to dairy industries even with a minute amount of phage particles due to phage ability to rapidly increase its numbers once a bacterial host is available. To add to this problem, phages usually tolerate high acidity and high temperature values during the pasteurization process. Phages can cause a collapse of the lactose-lactic acid pathway and decrease the overall efficiency of the fermentation process. These problems are detrimental to dairy product quality and often make the food products susceptible to spoilage by additional bacterial invasion, which can further hurt the fermentation process [1,6]. Lactic acid bacteria adapted and modified a variety of anti-phage defense mechanisms to escape phage infec- tions. Such mechanisms were mostly observed against dsDNA phages [8,10,11]. Phages, however, utilize several tricky circumventions that were reported to sup- press those mechanisms [8,9,11]. The most common bacterial anti-phage defense mechanisms are developed to suppress phage adsorption, DNA injection and recrui- tment of restriction modification systems. Accordingly, bacteria use specific proteins that mask the phage reco- gnition site receptor located at bacterial cell surface or even invoke a conformational change, which causes pha- ges to mistake their attachment point. In addition, bacteria can alternatively mask their receptors through secreting specific sugar residues called exopolysaccha- rides, which help in inhibiting phage adsorption. Phages, however, can predominantly secrete specific polysaccha- ride degrading enzymes called lyases that degrade those bacterial residues [8,10,11]. Moreover, some LAB generate bacteriophage-insen- sitive mutants (BIMs) in which a cascade of phage mutations results in the alteration of recognition sites that inhibit bacteriophage adsorption. Such point mutations have been reported in chromosomal genes coding for Lactococcus cell receptors [8,10,11]. Mutant phages could, however, overcome this modification and infect those resistant bacteria. In some cases, if bacteria couldn’t inhibit phage adsorption, they instead secret other specific proteins that affect genome translocations to the cytoplasm through changing the injection site and blocking the cell wall degradation [8,10,11]. Otherwise, if phage genomes are able to adapt to these challenges and successfully pass through the cytoplasm, bacteria recruit other further defense mechanisms termed as res- triction-modification systems to degrade unmethylated genomes at specific sites. Due to multiple antibiotic-resistant bacteria, the use of bacterial viruses, i.e., bacteriophage therapy as alterna- tive to conventional antibiotics is rapidly increasing. Bacteriophages can be more specific than antibiotics. One advantage of phage therapy is the specificity of targeting only the host bacterial cells, while antibiotics could also kill a wide range of bacteria in addition to the targeted harmful one [9]. In addition, there are no repor- ted cases of side effects following the use of LAB phages, unlike most antibiotics that may enhance the side effects [9]. The aim of this study was to isolate and characterize jameed milk bacteriophages and identify their host cells. To our knowledge this study represents the first isolation of ssDNA and dsRNA bacteriophages from dairy sour- ces. 2. Materials and Methods 2.1. Laban Jameed Bacterial Culture Samples of laban jameed used in this study were obtained from the cities of Tulkaram (north) and Dahriya (south) of Palestine. The Tulkaram jameed was made of cow fermented milk, whereas the Dahriya jameed was made of sheep fermented milk. The isolated bacterio- phages were named after the Palestine Polytechnic Uni- versity (PPU) and its nucleic acid composition: PPUDV (PPU DNA Virus) for phage isolated from Dahriya jameed and PPURV (PPU RNA Virus) for phage isolated from jameed obtained from the city of Tulkaram. Jameed samples were stored at −80˚C until they were processed. From each jameed sample, a small piece of approxi- mately 1.0 gram was incubated for 2 days at 37˚C with continuous shaking at 200 rpm in 100 ml skim milk broth (5 g skim milk (SM) powder in 100 ml ddH2O) until the O.D at 600 nm reached 0.2. This was followed by plating 200 μl from each culture on SM agar plates (100 ml SM broth added to it 1 g agar). Individual colo- nies were then picked and re-cultured separately in new flasks containing 100 ml SM broth to examine the lysis of the bacteriophages. All bacterial stock cultures were stored at −80˚C in SM containing 16% (v/v) glycerol. When needed, frozen culture stock was allowed to thaw and used to prepare an overnight culture in SM broth before plating 200 μl from the overnight culture on SM agar plates. 2.2. Phage Isolation Fifty ml of two cultures of SM broth inoculated with a Open Access FNS  Isolation and Characterization of Bacteriophages from Laban Jameed 58 piece of jameed milk were tested for the presence of phages. To ensure purified phage filtration from bacteria, 5 drops of chloroform were added to each sample, stored for 15 min at room temperature before centrifugation at 13,000 rpm for 5 min. This step was repeated twice to ensure that sufficient phage particles were purified. The supernatant was then transferred to a new microfuge tube and re-centrifuged again. It was finally filtered through 0.45 μm sterile filters and filtrates were named according to the sample origin. Phage filtrates were added upon overnight bacterial culture plated on SM agar plates to monitor phage sensitivity (lysis) or resistance (no lysis). Phage filtrates from Tulkaram region were tested on both Tulkaram and Dahriya bacterial colonies and the same was done to the Dahriya filtrate. As a control, each time a tube containing only bacteria without phage filtrates was used in each manipulation, plates were kept for 15 min in a laminar flow hood to dry, before incubation at 37˚C up to 3 days until phage lysis was detected. Each bacteriophage lysis was carried out for at least three subsequent times until a pure phage was obtained. A small piece of each lysis was stored in a small 1.5 ml Eppendorf tube in −80˚C freezer as a stab culture. 2.3. Characterization of the Host Bacteria and Marker Analysis Genomic DNA for each bacterial host that showed sensitivity to phage lysis was extracted using EZ-DNA Kit (Biological Industries, Cat# 20-600-50). The primer sequences for the two markers used to identify the host strains isolated from the Jameed are shown in (Table 1). The two markers were the recA gene, which is considered specific for LAB and the gene encoding the 16S rRNA. For both recA and 16S rRNA genes, the PCR reaction contained 1 μl of template DNA for each host colony, 2.5 μl of 10 x PCR reaction buffer, 0.5 μM of each primer (10 pmol concentration), 2.5 μl of 20 mM MgSO4, 0.5 μl of 20 mM dNTPs and 1.25 U of thermostable Taq polymerase. The mixture volume was completed with ultrapure water Table 1. Primer sequences used to amplify the marker genes, recA and 16S rDNA partial sequences. Information in relative to their sequences and size of amplified fragment are included. Name Sequence Length recA reverse 5’-T TY ATHGAY GCN GAR CAY GC-3’ recA forward 5’-CCW CCW GKWGTHGTYTCNGG-3’340 bp 16S rDNA reverse 5’-GGACTACCAGGGTATCTAAT-3’ 16S rDNA forward 5’-AGTTTGATCCTGGCTC AG-3 780 bp Y for (C or T), H for (A or C or T), R for (A or G), W for (A or T), N for (A or C or G or T), M for (A or C), K for (G or T). to a final volume of 25 μl. Amplification of the recA gene was conducted using the following conditions: initial denaturation was performed at 94˚C for 3 min and for 30 sec for the subsequent 30 cycles, followed by 30 sec for primer annealing at 54˚C, elongation of the target gene with taq polymerase at 72˚C for 30 sec. A final extension of 5 min at 72˚C was followed by cooling down to 4˚C. The 16S rRNA PCR reaction conditions were as follows: 95˚C for 5 min for the initial denaturation, 1 min dena- turation for the subsequent 30 cycles, primer annealing at 51˚C for 1 min, target elongation at 72˚C for 1.30 min. A final extension of 10 min at 72˚C was followed by cooling down to 4˚C. For gene sequencing, PCR product puri- fication was achieved by following instructions provided in the AccuPrep PCR Purification Kit (Bioneer K-3035). DNA sequences of the isolated DNA markers were aligned manually, then similarity trees were constructed using UPGMA method with MEGA V.5 software. 2.4. Bacteriophage Genome Isolation Bacteriophage genomes were extracted according to a protocol developed by Manasra and Barghouthi [12] with minor modifications as follows. Two volumes (1000 μl) of saturated ammonium sulfate containing 0.1% of 2- mercaptoethanol were mixed with the phage filtrate (500 μl) for 5 min. Supernatant was removed after centri- fuging at 13,000 rpm for 5 min at 4˚C. The pellet was then dissolved in 0.2 ml 1% SDS and 0.2 ml 0.5 N NaOH and centrifuged at 13,000 rpm for 5 min. To the clear supernatant, 0.4 ml of 3 N sodium acetate was added in addition to 0.6 volume of isopropanol to precipitate the genome, and then held for 15 min at room temp. The mixture was then centrifuged at 13,000 rpm for 10 min and the resulting pellet was incubated with 100 μg/μl of proteinase K at 37˚C for 30 min. Finally, the phage ge- nome was precipitated using 70% ethanol and the pellet was collected in 0.2 ml TE buffer after 2 min centri- fugation at 8000 rpm. 2.5. Bacteriophage Genome Characterization The entity of nucleic acids was determined via the treat- ment of phage genome with DNase and RNase. Ten μl of each genome sample was incubated with 3 μl of DNase and the same with RNase for 35 min at 37˚C. The mix- ture was then loaded on 0.7% agarose gel using undi- gested genome as a control, and lambda phage ge nome- treated with HindIII restriction enzyme was used as a high molecular weight ladder. The RNase A treatment at low and high concentrations was used to determine ss/ds RNA genomes according to the following references [13-17]. Briefly, 10 μl of PPURV genome were incubated with 3 μl RNase A ei- ther with low (0.1 M) or high (0.4 M) NaCl concen- Open Access FNS  Isolation and Characterization of Bacteriophages from Laban Jameed 59 tration for 1 h at 37˚C. Following treatment, samples were mixed with 6× loading dye and loaded on 0.7% aga- rose gel as above. To determine whether phage genomes belong to dsDNA or ssDNA, 10 μl of genome was boiled for 6 min, placed on ice, before rapidly being loaded on a 0.7% gel electrophoresis with un-boiled genome as a control and lambda phage genome was used as a ladder. Furthermore, isolated genomes were treated with a set of specific dsDNA restriction enzymes including, MluI, BamHI, EcoRI, HindIII and PvuI following protocols provided by the manufacture. Briefly, the same reaction mixture of 20 μl was prepared for all restriction enzymes at which 16 μl nuclease-free water was mixed with 2 μl 10× buffer, 1 μl from each restriction enzyme and 1 μl of DNA template. Samples were then incubated at 37˚C for 8 and 16 h periods before the reaction mixtures were loaded on a 1% agarose gel. The SDS-PAGE was carried out according to [18,19]. Total phage proteins were separated on a 10% SDS- PAGE, stained with 0.1% (w/v) Coomassie Brilliant Blue (Applichem/ A3480,0010) before documentation using a digital camera. 2.6. One Step Growth Curve The double layer Plaque assay method was used accord- ing to published work [20,21] as follows: Previously prepared high titer filtrates were used, a single plaque was picked up from an agar plate, mixed with log phase bacteria O.D600 nm of 0.2, and then incubated for 3 hours. Samples were then purified as above and used for one step growth curve. 10-fold serial dilutions were performed with each dilution prepared by mixing 0.1 ml of stock phage suspension in 0.9 ml water (tube labeled as tube 1). The 0.1 ml from tube 1 sample was trans- ferred into tube 2, filled with 0.9 ml water; the same was done for the other remaining dilutions. From each titer, 0.1 ml bacteriophage suspension dilution was inoculated to 0.5 ml of O.D600 nm = 0.2 bacterial culture, incubated at 37˚C for 40 min, and then added to a tube containing 3 ml of 0.7% soft agar heated at 49˚C and gently mixed. Finally it was poured onto a separated prepared mono- layer SM plate. Plates were then incubated upside down at 37˚C. Plaque formation was monitored and data were recorded. 3. Results 3.1. Isolation of Bacterial Strains and Phage Filtrates Three bacterial colonies from each skimed milk agar plate representing different jameed samples (see M&M Section 2.1) were selected for further testing against phage filtrates. Each colony was labeled in reference to the bacteria (B) and colony number (1 - 6), (i.e., BC1 for bacteria colony 1, BC2 for bacteria colony 2, etc.). Thus, the bacterial colonies BC1, BC2, BC3 were selected from Dahriya samples, whereas BC4, BC5, BC6 were selected from Tulkaram samples. Phage filtrates were prepared from jameed samples as described above. Filtrates were tested against all bacterial colonies from both cities, Dahriya virus was tested on Tulkaram and Dahriya, the same was done for the other filtrate. Screening of phage filtrate effects on the bacterial lawns showed two clear round plaques, each with approximately 1.5 cm diameter (Figure 1). The viruses which could cause the plaques were named PPUDV for phage isolated from Dahriya sheep milk jameed (Figure 1(a)) and PPURV for phage isolated from Tulkaram cow milk jameed (Figure 1(b)). The PPUDV bacteriophage propagated on bacterial colony 1 (BC1), while PPURV bacteriophage lysis bacteria colony 4 (BC4) bacterial hosts, the results were confirmed by triplicate experi- ments. In each case, a control plate consisting of bacterial strain devoid of phage was prepared. Separated plates with two sides were used, for example bacterial colony 1 cultured on both sides of the plate, then drops of phage filtrates spotted on one of the sides, while keeping the other side as a control with just bacteria without virus. 3.2. Characterization of Phage Host Bacteria To identify the bacteria which were susceptible to phage lysis, two marker genes for the 16S rRNA and recA were amplified from genomic DNA isolated from the BC4 and BC1 host bacteria. The PCR amplification results show- ed clear bands matching the expected sizes of the two fragments (Figures 2(a) and (b)). The partial sequences targeted, as commonly assessed Figure 1. Bacterial cultures on skim milk plates showing bacteriophage lysis. Phage filtrate (30 μl) was added to each bacterial colony. (a) The PPUDV phage was able to lyse the bacterial host (BC1) isolated from jameed made of sheep fermented milk. Lysis appeared as a clear circle at the right side. The left side is a control with the host bacteria, but with no phage added. (b) the PPURV phage lysed the bacterial host (BC4) isolated from jameed made of cow fermented milk with clear circle at the left side. The right side represents the control host bacteria showed no lysis. Open Access FNS 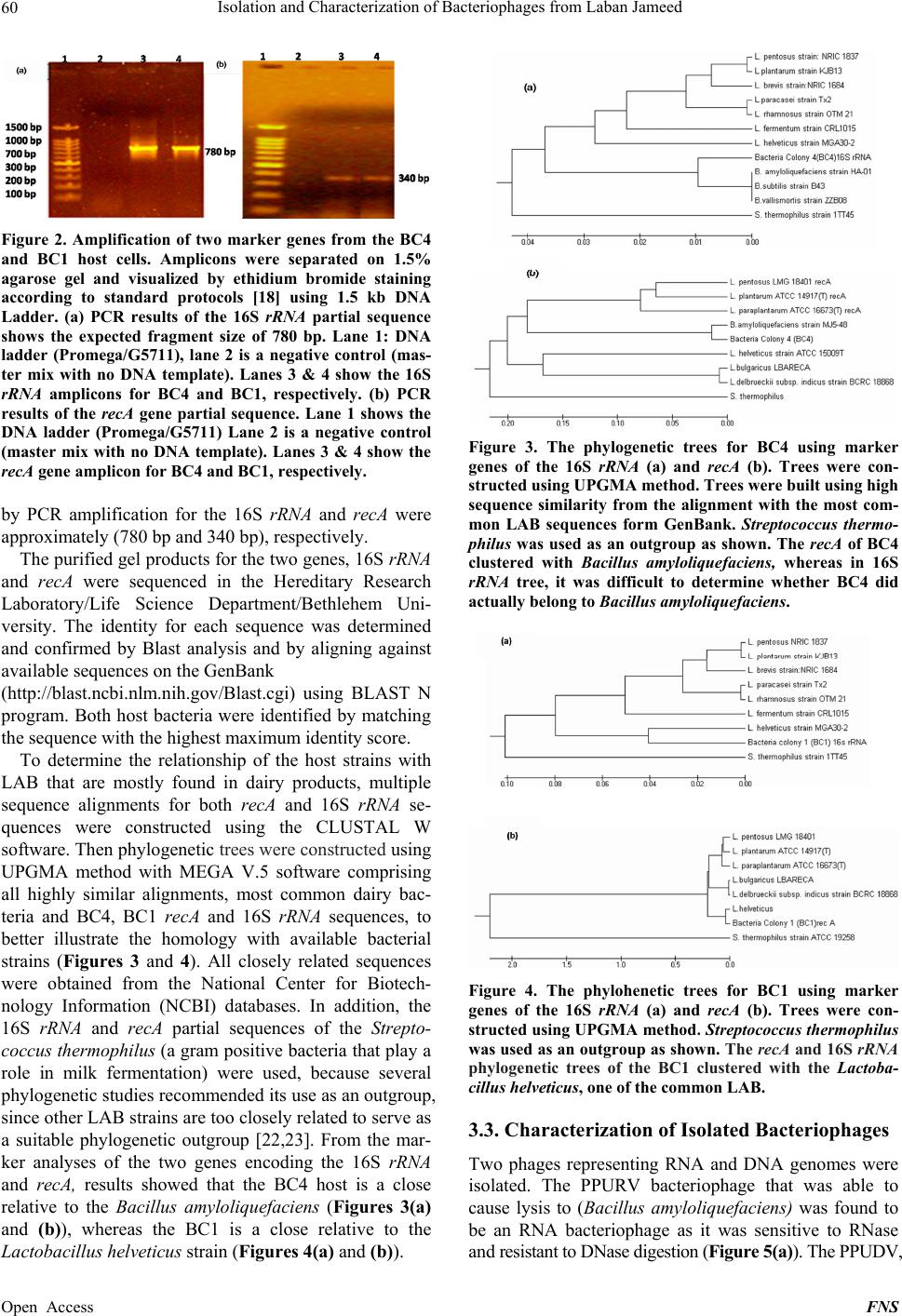 Isolation and Characterization of Bacteriophages from Laban Jameed 60 Figure 2. Amplification of two marker genes from the BC4 and BC1 host cells. Amplicons were separated on 1.5% agarose gel and visualized by ethidium bromide staining according to standard protocols [18] using 1.5 kb DNA Ladder. (a) PCR results of the 16S rRNA partial sequence shows the expected fragment size of 780 bp. Lane 1: DNA ladder (Promega/G5711), lane 2 is a negative control (mas- ter mix with no DNA template). Lanes 3 & 4 show the 16S rRNA amplicons for BC4 and BC1, respectively. (b) PCR results of the recA gene partial sequence. Lane 1 shows the DNA ladder (Promega/G5711) Lane 2 is a negative control (master mix with no DNA template). Lanes 3 & 4 show the recA gene amplicon for BC4 and BC1, respectively. by PCR amplification for the 16S rRNA and recA were approximately (780 bp and 340 bp), respectively. The purified gel products for the two genes, 16S rRNA and recA were sequenced in the Hereditary Research Laboratory/Life Science Department/Bethlehem Uni- versity. The identity for each sequence was determined and confirmed by Blast analysis and by aligning against available sequences on the GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) using BLAST N program. Both host bacteria were identified by matching the sequence with the highest maximum identity score. To determine the relationship of the host strains with LAB that are mostly found in dairy products, multiple sequence alignments for both recA and 16S rRNA se- quences were constructed using the CLUSTAL W software. Then phylogenetic trees were constructed using UPGMA method with MEGA V.5 software comprising all highly similar alignments, most common dairy bac- teria and BC4, BC1 recA and 16S rRNA sequences, to better illustrate the homology with available bacterial strains (Figures 3 and 4). All closely related sequences were obtained from the National Center for Biotech- nology Information (NCBI) databases. In addition, the 16S rRNA and recA partial sequences of the Strepto- coccus thermophilus (a gram positive bacteria that play a role in milk fermentation) were used, because several phylogenetic studies recommended its use as an outgroup, since other LAB strains are too closely related to serve as a suitable phylogenetic outgroup [22,23]. From the mar- ker analyses of the two genes encoding the 16S rRNA and recA, results showed that the BC4 host is a close relative to the Bacillus amyloliquefaciens (Figures 3(a) and (b)), whereas the BC1 is a close relative to the Lactobacillus helveticus strain (Figures 4(a) and (b)). Figure 3. The phylogenetic trees for BC4 using marker genes of the 16S rRNA (a) and recA (b). Trees were con- structed using UPGMA method. Trees were built using high sequence similarity from the alignment with the most com- mon LAB sequences form GenBank. Streptococcus thermo- philus was used as an outgroup as shown. The recA of BC4 clustered with Bacillus amyloliquefaciens, whereas in 16S rRNA tree, it was difficult to determine whether BC4 did actually belong to Bacillus amyloliquefaciens. Figure 4. The phylohenetic trees for BC1 using marker genes of the 16S rRNA (a) and recA (b). Trees were con- structed using UPGMA method. Streptococcus thermophilus was used as an outgroup as shown. The recA and 16S rRNA phylogenetic trees of the BC1 clustered with the Lactoba- cillus helveticus, one of the common LAB. 3.3. Characterization of Isolated Bacteriophages Two phages representing RNA and DNA genomes were isolated. The PPURV bacteriophage that was able to cause lysis to (Bacillus amyloliquefaciens) was found to be an RNA bacteriophage as it was sensitive to RNase and resistant to DNase digestion (Figure 5(a)). The PPUDV, Open Access FNS 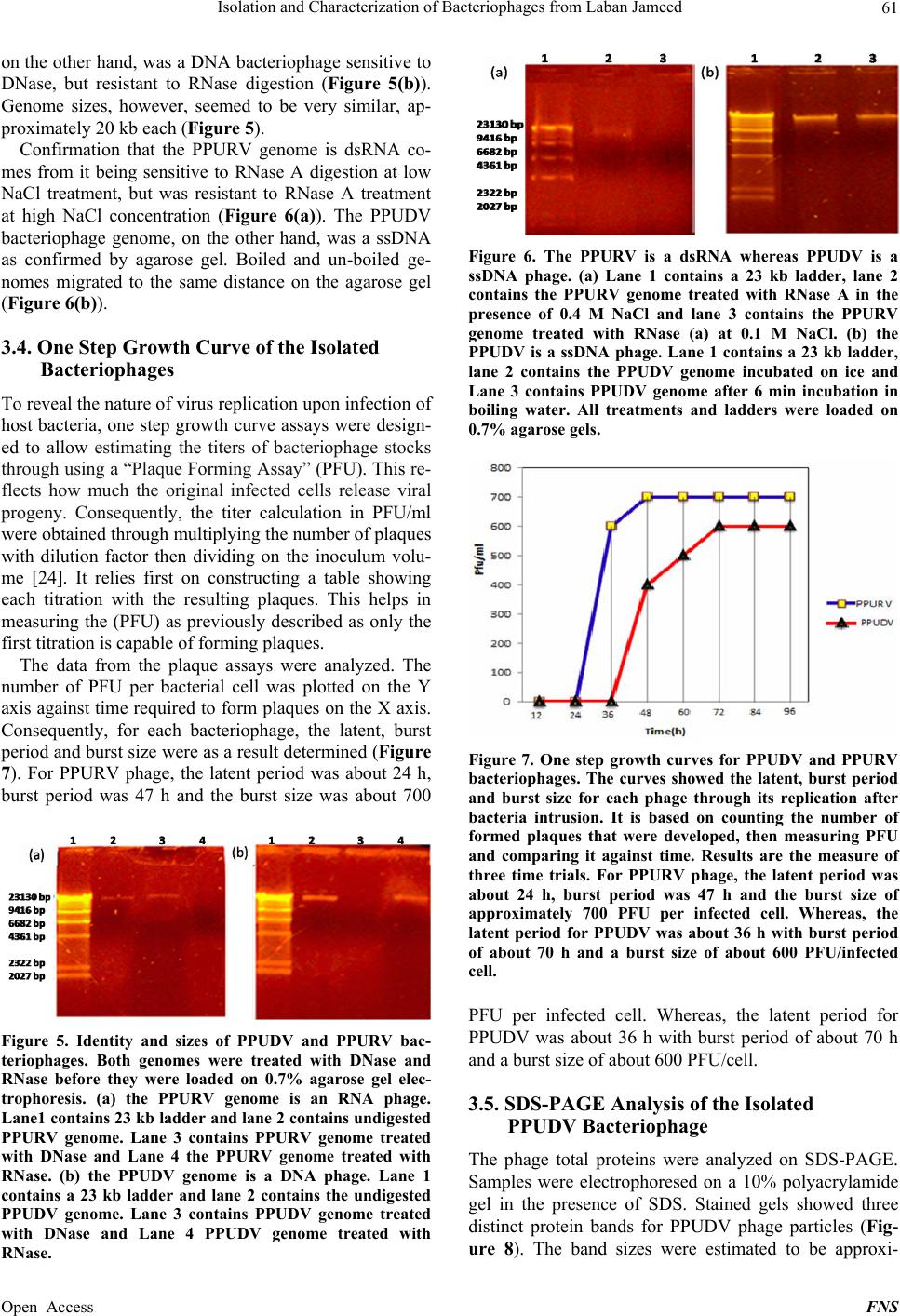 Isolation and Characterization of Bacteriophages from Laban Jameed 61 on the other hand, was a DNA bacteriophage sensitive to DNase, but resistant to RNase digestion (Figure 5(b)). Genome sizes, however, seemed to be very similar, ap- proximately 20 kb each (Figure 5). Confirmation that the PPURV genome is dsRNA co- mes from it being sensitive to RNase A digestion at low NaCl treatment, but was resistant to RNase A treatment at high NaCl concentration (Figure 6(a)). The PPUDV bacteriophage genome, on the other hand, was a ssDNA as confirmed by agarose gel. Boiled and un-boiled ge- nomes migrated to the same distance on the agarose gel (Figure 6(b)). 3.4. One Step Growth Curve of the Isolated Bacteriophages To reveal the nature of virus replication upon infection of host bacteria, one step growth curve assays were design- ed to allow estimating the titers of bacteriophage stocks through using a “Plaque Forming Assay” (PFU). This re- flects how much the original infected cells release viral progeny. Consequently, the titer calculation in PFU/ml were obtained through multiplying the number of plaques with dilution factor then dividing on the inoculum volu- me [24]. It relies first on constructing a table showing each titration with the resulting plaques. This helps in measuring the (PFU) as previously described as only the first titration is capable of forming plaques. The data from the plaque assays were analyzed. The number of PFU per bacterial cell was plotted on the Y axis against time required to form plaques on the X axis. Consequently, for each bacteriophage, the latent, burst period and burst size were as a result determined (Figure 7). For PPURV phage, the latent period was about 24 h, burst period was 47 h and the burst size was about 700 Figure 5. Identity and sizes of PPUDV and PPURV bac- teriophages. Both genomes were treated with DNase and RNase before they were loaded on 0.7% agarose gel elec- trophoresis. (a) the PPURV genome is an RNA phage. Lane1 contains 23 kb ladder and lane 2 contains undigested PPURV genome. Lane 3 contains PPURV genome treated with DNase and Lane 4 the PPURV genome treated with RNase. (b) the PPUDV genome is a DNA phage. Lane 1 contains a 23 kb ladder and lane 2 contains the undigested PPUDV genome. Lane 3 contains PPUDV genome treated with DNase and Lane 4 PPUDV genome treated with RNase. Figure 6. The PPURV is a dsRNA whereas PPUDV is a ssDNA phage. (a) Lane 1 contains a 23 kb ladder, lane 2 contains the PPURV genome treated with RNase A in the presence of 0.4 M NaCl and lane 3 contains the PPURV genome treated with RNase (a) at 0.1 M NaCl. (b) the PPUDV is a ssDNA phage. Lane 1 contains a 23 kb ladder, lane 2 contains the PPUDV genome incubated on ice and Lane 3 contains PPUDV genome after 6 min incubation in boiling water. All treatments and ladders were loaded on 0.7% agarose gels. Figure 7. One step growth curves for PPUDV and PPURV bacteriophages. The curves showed the latent, burst period and burst size for each phage through its replication after bacteria intrusion. It is based on counting the number of formed plaques that were developed, then measuring PFU and comparing it against time. Results are the measure of three time trials. For PPURV phage, the latent period was about 24 h, burst period was 47 h and the burst size of approximately 700 PFU per infected cell. Whereas, the latent period for PPUDV was about 36 h with burst period of about 70 h and a burst size of about 600 PFU/infected cell. PFU per infected cell. Whereas, the latent period for PPUDV was about 36 h with burst period of about 70 h and a burst size of about 600 PFU/cell. 3.5. SDS-PAGE Analysis of the Isolated PPUDV Bacteriophage The phage total proteins were analyzed on SDS-PAGE. Samples were electrophoresed on a 10% polyacrylamide gel in the presence of SDS. Stained gels showed three distinct protein bands for PPUDV phage particles (Fig- ure 8). The band sizes were estimated to be approxi- Open Access FNS  Isolation and Characterization of Bacteriophages from Laban Jameed 62 Figure 8. SDS-PAGE gel image of PPUDV phage proteins. Lane 1 shows three bands and lane 2, empty well with no phage sample. Lane 3 contains high molecular weight pro- ein ladder (Sigma/S8320). As shown, at least three major bands (27, 40 and 45 kDa) were detected. mately 27 KDa, 40 KDa and 45 KDa. 4. Discussion 4.1. Bacteriophages PPUDV and PPURV Genome Characterizations Dairy products are essential components in food indus- tries and individual nutrition in Palestine. The quality of dairy products is quite critical and any contamination is considered detrimental and results in major economic losses. Contamination that is caused by bacteriophages generally decreases the quality of food products and is considered undesirable by dairy industries. We reported the isolation of two bacteriophages from a traditional dairy product called laban jameed: a dsRNA PPURV and ssDNA PPUDV hosting on Bacillus amylo- liquefaciens and Lactobacillus helveticus, respectively. The majority of identified Bacillus amyloliquefaciens and Lactobacillus helveticus bacteriophage genomes be- long to dsDNA [25-28]. However, little is known about dsRNA and ssDNA bacteriophages that host on Bacillus amyloliquefaciens and Lactobacillus helveticus strains. A dsDNA Lactobacillus helveticus with genome size 36,566 bp from Grana Padano cheese product was isolated [28]. Also, Sechaud et al. [26] characterized 35 cheese whey Lactobacillus helveticus dsDNA bacteriophages, but without providing information on their genome size. Most lactic acid bacteriophages are believed to be dsDNA [5,27]. In addition, the isolation of 235 baceriophages affecting L. helveticus, L. delbrueckii subsp bulgaricus and L. delbrueckii subsp lactis strains, all with dsDNA and belonged to Siphoviridae, Myoviridae and Podoviri- dae were also described [27]. There are no reports of dsRNA bacteriophages that host on Bacillus amyloliquefaciens, although a dsDNA PBA12 Bacillus amyloliquefaciens bacteriophage was isolated from soil [25]. While Qiao et al. (2010) [29] described a dsRNA bacteriophage from radish leaves with genome size of 12684 bp hosting on Pseudomonas sy- ringae bacteria. But so far, this study represents the first isolation of dsRNA phage that hosts on Bacillus amylo- liquefaciens and ssDNA phage that hosts on Lactobacillus helveticus from dairy jameed food product. The bacte- riophage genomes were isolated under reducing condi- tions to minimize degradation. The -mercaptoethanol was added to inhibit the activity of DNase and RNase degrading enzymes by weakening their disulfide bonds [12]. To liberate phage genomes from DNA and RNA binding proteins, phage proteins were denatured and pre- cipitated through the addition of NaOH and SDS and the phage genomes were precipitated from the supernatant through the addition of sodium acetate in the presence of -mercaptoethanol as reported by other studies [14,30]. The PPUDV phage was found to be a DNA genome as no bands appeared on the 0.7% agarose gel when treated with DNase. This is in contrast to PPURV phage which was insensitive to DNase. Unlike PPUDV, it was responsive to RNase digestion as observed on the agarose gel (Figures 5(a) and (b)). Both PPUDV and PPURV genomes ap- peared to have the same sizes, approximately 20 kb ± 1 kb as observed on agarose gel using a 23 kb ladder. The nature of the bacteriophage genomes was further studied. The RNA genome was determined to be a dsRNA (Figure 6(a)), whereas the DNA genome was a ssDNA genome (Figure 6(b)). There are other assays, such as the Hyperchromicity test, ds/ssRNase specific enzyme deg- radation and RNase III specific for breaking down dsRNA that were reportedly used to differentiate between ds/ss genomes, but these methods were reported to possess low efficiency in working on small amounts of RNA [13,30]. In this study, the RNase A assay was preferred and used. The RNase A digests both ssRNA and dsRNA under low NaCl concentration (0.1 M), while it digests only ssRNA under high NaCl concentration (0.3 M) [13-17,30,31]. A previous study reported the resistance of dsRNA of vi- ruses infected plant and fungi to RNase A treatment under 0.3 M NaCl even after 24 h incubation [13]. Following treatment with high salt concentration, clear bands were still visible, but disappeared under low (0.1 M) salt treat- ment. In addition, ssRNA extracted from other viruses was also examined. It completely disappeared under 0.1 Open Access FNS  Isolation and Characterization of Bacteriophages from Laban Jameed 63 M and 0.3 M NaCl treatments [13]. The PPUDV bacte- riophage genome, on the other hand, was a ssDNA. The ssDNAs are usually smaller in size than dsDNAs, thus having more fragile structures and are expected therefore to run much faster on agarose gels. The heated PPUDV genome migrated similarly to the un-boiled PPUDV ge- nome on the gel, thus confirming its ssDNA identity. The single strandedness entity was further confirmed by the resistance of genomes to restriction enzyme treatment that cut dsDNA, no bands were detected in both reactions incubated at 8 h or 16 h (data not shown). The ssDNA phage genomes are mostly related to either the Microviridae, circular ssDNA genomes with nonen- veloped and isometric shapes, or Inoviridae families, cir- cular ssDNA genomes with nonenveloped and filamen- tous shapes as appeared by morphological studies using the electron microscope [32,33]. Phages that belong to the Cystoviridae family are dsRNA and possess segmented genomes with enveloped capsids and spherical shapes for the phage genomes. However, up to 2009, 88% of com- pletely sequenced genomes in GenBank phage databases are related to dsDNA, mainly distributed among the Caudoviridae orders. The PPUDV and PPURV bacte- riophages are therefore possibly belong to the Microviri- dae, or Inoviridae (ssDNA nucleic acid) or Cystoviridae (dsRNA) family, respectively. To identify the phases of bacteriophage infection, one step growth curves were constructed. These curves were performed using the double layer plaque assay, a com- monly used and highly accurate, fast and easy to handle method [21]. It comprises an agar layer at the plate surface, overlaid with another soft layer (0.7%) of agar containing a phage-bacterial suspension. The phage-bacterial sus- pension is first incubated at 37˚C to ensure phage ad- sorption to bacteria, then the soft agar is added in a steril- ity conditions to avoid any other microorganism con- taminations. The latent period, burst period and burst size are then determined. The latent period illustrates the time period from the adsorption of phage to the host bacteria, until the onset of cell lysis and burst. Host cells rapidly lyse and release infective phage at burst period, occurring after the latent period. The average number estimated for phage progeny to be formed per infected bacterial host cell symbolizes the burst size (Figure 7). Results are recorded as the mean of three trials; measures of PFUs were approximately conserved. This means the relationship between phage titer and phage infection on the related host cells are the same in the three trials. Both PPURV and PPUDV bacte- riophages have long latent periods. This indicates that further conditions should be manipulated as to detect optimum bacteriophage activity after infecting their host bacteria. Thus, such conditions could be the culture media itself, as skim milk broth and agar that were used. Other studies preferred the MRS broth media being used for the detection of dairy bacteriophages [34,35]. Three major protein bands appeared for PPUDV bac- teriophage with estimated molecular weights of ap- proximately, 27, 40 and 45 kDa (Figure 8). These results indicate that aggregations of several proteins accumulate at each band and/or that some proteins are repetitive copies encoded from the same gene. This is very common in viruses, for example, the capsid surface proteins are usually repetitive of small number of genes [36]. For PPURV bacteriophage, it was difficult to obtain good resolution of the total PPURV proteins (data not shown). This is probably due to the fact that RNA binding proteins are susceptible to degradation. 4.2. Bacterial Hosts of PPUDV and PPURV The molecular characterization was used to identify the two host bacteria that were susceptible to bacteriophage lysis. Two phylogenetic markers that are universally dis- tributed in bacteria and commonly used in bacterial strain identifications, the 16S rRNA and recA genes were tar- geted [37-39]. The16S rRNA is used as a useful marker in bacterial characterization as it has conserved sequence and function among bacteria, though it shows several limitations due to its inability to differentiate between closely related species that share 99% or higher sequence identity, like in some LAB strains [38]. Such inability was clearly observed in the case of the L. plantarum and L. pentosus [38]. Therefore, to further confirm the identity of the host bacteria in this study, the recA gene was also used as a marker commonly used in lactic acid bacterial iden- tifications. In this regard, the recA gene has a fundamental advantage over the gene encoding the 16S rRNA, its abil- ity to differentiate closely related species. Sequence analysis and GenBank blast of the two marker genes used for the identification of BC4 and BC1 revealed their identities as Bacillus amyloliquefaciens for BC4 and Lactobacillu s helveticu s for the BC1 (Figures 3 and 4). The 16S rRNA was less informative in determin- ing the relatedness of the BC4 bacteria. Using the highest maximum identity score, it was initially difficult to de- termine whether BC4 was a close relative to Bacillus vallismortis, or Bacillus subtilis, or Bacillus amylolique- faciens. The use of the recA easily confirmed, however, the identity of the BC4 host to be more closely related to Bacillus amyloliquefaciens (Figure 3). The B. amyloliq- uefaciensis is a gram positive bacteria, discovered and normally found in soil. It is considered a useful industrial microorganism, representing, for example, an ample sour- ce for producing amylase enzyme commercially used in starch hydrolysis [40,41]. It is also a source of protease enzyme that catalyzes the breakdown of proteins and is used in detergent industries [40]. There is no previous study to report the natural presence of the B. amylolique- Open Access FNS  Isolation and Characterization of Bacteriophages from Laban Jameed 64 faciens in dairy products and that it plays a role in milk fermentation similar to LAB. However, it was shown to be a useful source for a milk-clotting enzyme in some in- dustrial dairy processing fermentations [42] and in the fermentation of other food products, such as soybean- fermented food [43]. Otherwise, the explanation for its existence is to be a result of jameed contamination with soil. During the process of jameed drying, it is usually kept uncovered on the ground in sunny areas for a few days to reduce the moisture content and so it is possible that this strain is just a contaminant from the nearby soil. Milk microbial contaminants belong mostly to the genus Bacillus, including the B. amyloliquefaciens and the B. cereus, which produce a toxin that can cause diarrhea and another that causes vomiting [44]. Bacillus cereus spores are heat-resistant and may survive pasteurization. There have even been very rare cases linked to dried milk and dried infant formulas [45]. The source of contamination in dairy products could be multifarious, like soil, air and water [44,46]. The dairy industries implement several strategies to reduce contaminations, such as pasteurization under high temperature, but even so, contamination still could occur due to some microbial heat resistant spores. Dairy processing that relies on traditional methods is subject to a higher possibility of contaminations. Tradi- tional laban jameed production is dependent on personnel in milk transport and processing under mostly unsterile environment and storage conditions. The BC1 host was clearly identified to be a close rela- tive to the Lactobacillus helveticus strains (Figure 4). This strain is one of the common lactic acid producing gram positive bacteria [6,47]. It helps in maintaining good food flavor and acidic conditions that inhibit the spoilage of milk products. Furthermore, the relationships of PPUDV and BC1 and PPURV and BC4 host bacteria with other related species were determined by the phylogenetic trees, which were constructed using the highest identities as appeared by the two markers, recA and 16S rRNA se- quence blasts. The trees included the most common Lac- tobacillus bacteria that occupy the highest percentage of the microbial population; L. pentosus, L. casei, L. fer- mentum, L. plantarumand L. paraplantarum, while those with lower identities were masked and excluded. 5. Conclusions Several studies demonstrated the isolation of dairy prod- uct bacteriophages, but so far no phages have been iso- lated from laban jameed. Most of the isolated dairy bac- teriophages were characterized as dsDNA. To our know- ledge, this study reports the first isolation of ssDNA and dsRNA bacteriophages from laban jameed dairy product. Moreover, as there is a significant increase in antibi- otic resistance among bacteria, there is a notion to use phage therapy as an alternative for antibiotics. The iso- lated bacteriophages from jameed milk may have the potential for future uses as alternatives for antibiotics if they prove to lyse pathogenic bacteria, particularly those that exist in close proximity to milk, such as those caus- ing mastitis disease. It is believed that this study may provide further information on the complex interactions between phages and their hosts, and promote studies on phage therapy. 6. Acknowledgements The authors would like to thank Dr. Robin Abu Ghazaleh, Dr. Rami Arafeh, and Dr. Sameer Barghouthi for care- fully reading the manuscript and Dr. Yaqoub Ashhab for advice, Mrs. Amal Abu Rayan for carrying out the se- quencing, and Mrs. Asma Tamimi and Mr. Hasan Alta- rada for technical support. REFERENCES [1] A. Alomari, M. J. Quasem and A. S. Mazahreh, “Micro- biological Analysis of Solar and Freeze-Dried Jameed Produced from Cow and Sheep Milk with the Addition of Carrageenan Mix to the Jameed Paste,” Pakistan Journal of Nutrition, Vol. 7, No. 6, 2008, pp. 726-729. http://dx.doi.org/10.3923/pjn.2008.726.729 [2] A. S. Mazahreh, F. A. Al-Shawabkeh and M. J. Quasem, “Evaluation of the Chemical and Sensory Attributes of Solar and Freeze-Dried Jameed Produced from Cow and Sheep Milk with the Addition of Carrageenan Mix to the Jameed Paste,” American Journal of Agricultural and Biological Sciences, Vol. 3, No. 3, 2008, pp. 627-632. http://dx.doi.org/10.3844/ajabssp.2008.627.632 [3] A. Al-Saed, R. Al-Groum and M. Al-Dabbas, “Imple- mentation of Hazard Analysis Critical Control Point in Jameed Production,” Food Science and Technology In- ternational, Vol. 18, No. 3, 2012, pp. 229-239. http://dx.doi.org/10.1177/1082013211427783 [4] A. Bosch, M. Golowczyc, A. Abraham, G. Garrote, G. De Antoni and O. Yantorno, “Rapid Determination of Lacto- bacilli Isolated from Kefir Grains by FT-IR Spectros- copy,” International Journal of Food Microbiology, Vol. 111, No. 3, 2006, pp. 280-287. http://dx.doi.org/10.1016/j.ijfoodmicro.2006.05.010 [5] G. De Antoni, M. Zago, O. Vasek, G, Giraffe, D. Carmi- nati, B. M. Marco, J. Reinheime and V. Suarez, “Lacto- bacillus plantarum Bacteriophages Isolated from Kefir Grains: Phenotypic and Molecular Characterization,” Journal of Dairy Research, Vol. 77, No. 1, 2010, pp. 7-12. http://dx.doi.org/10.1017/S0022029909990203 [6] B. M. Marcó, S. Moineau and A. Quiberoni, “Bacterio- phages and Dairy Fermentations,” Landes Bioscience, Vol. 2, No. 3, 2012, pp. 149-158. [7] B. Del Rio, G. A. Binetti, C. M. Martín, M. Fernández, H. A. Magadán and A. M. Alvarez, “Multiplex PCR for the Detection and Identification of Dairy Bacteriophages in Milk,” Food Microbiology, Vol. 24, No. 1, 2007, pp. 75-81. http://dx.doi.org/10.1016/j.fm.2006.03.001 Open Access FNS  Isolation and Characterization of Bacteriophages from Laban Jameed 65 [8] S. Mills, P. R. Ross, H. Neve and A. Coffey, “Bacterio- phage and Anti-Phage Mechanisms in Lactic Acid Bacte- ria,” Microbiological and Functional Aspects, 2011, pp. 165S-186S. [9] N. W. I. Haq, N. M. Akhtar, S. Andleeb and I. Qadri, “Bacteriophages and Their Implications on Future Bio- technology: A Review,” Virology Journal, Vol. 9, No. 9, 2012, pp. 1-8. http://dx.doi.org/10.1186/1743-422X-9-9 [10] S. J. Labrie, J. E. Samson and S. Moineau, “Bacterio- phage resistance mechanisms,” Nature Reviews Microbi- ology, Vol. 8, 2010, pp. 317-327. http://dx.doi.org/10.1038/nrmicro2315 [11] E. J. Garneau and S. Moineau, “Bacteriophages of Lactic Acid Bacteria and Their Impact on Milk Fermentations,” Microbial Cell Factories, Vol. 10, Supl. 1, 2011, pp. 1-10. http://dx.doi.org/10.1186/1475-2859-10-S1-S20 [12] I. Manasrah and S. Barghouthi, “Multiplex PCR Detec- tion of Bacterial and Viral Meningitis in Cerebrospinal Fluid,” The 7th Palestinian Conference of Laboratory Medicine, Bethlehem, 15-17 March 2012. [13] J. T. Morris and A. J. Dodds, “Isolation and Analysis of Double-Stranded RNA from Virus-Infected Plant and Fungal Tissue,” Phytopathology, Vol. 69, No. 8, 1979, p. 855. [14] G. W. P. Chu and G. E. Westaway, “Replication Strategy of Kunjin Virus: Evidence for Recycling Role of Replica- tive Form RNA as Template in a Semi Conservative and Asymmetric Replication,” Virology Journal, Vol. 140, No. 1, 1985, pp. 68-79. http://dx.doi.org/10.1016/0042-6822(85)90446-5 [15] E. G. Westaway, A. A Khromykh and J. M. Mackenzie, “Nascent Flavivirus RNA Colocalized in Situ with Dou- ble-Stranded RNA in Stable Replication Complexes,” Virology Journal, Vol. 258, No. 1, 1999, pp. 108-117. http://dx.doi.org/10.1006/viro.1999.9683 [16] P. Targett-Adams, S. Boulant and J. McLauchlan, “Visu- alization of Double-Stranded RNA in Cells Supporting Hepatitis C Virus RNA Replication,” Journal of Virology, Vol. 82, No. 5, 2008, pp. 2182-2195. http://dx.doi.org/10.1128/JVI.01565-07 [17] A. Ablasser, F. Bauernfeind, G. Hartmann, E. Latz, K. A. Fitzgerald and V. Hornung, “RIG-I-Dependent Sensing of Poly (dA:dT) through the Induction of an RNA Poly- merase III-Transcribed RNA Intermediate,” Nature Im- munology, Vol. 10, No. 10, 2009, pp. 1065-1073. http://dx.doi.org/10.1038/ni.1779 [18] J. Sambrook and W. D. Russel, “Molecular Cloning La- boratory Manual,” Cold Spring Harbor Laboratory Press, Vol. 3, 2001. [19] A. M. Al-Manasra and F. Al-Razem, “Cloning and Ex- pression of a New Bacteriophage (SHPh) DNA Ligase Isolated from Sewage,” Journal of Genetic Engineering and Biotechnology, Vol. 10, No. 2, 2012, pp. 177-184. http://dx.doi.org/10.1016/j.jgeb.2012.05.005 [20] Z. Lu, F. Breidt Jr., P. H. Fleming, E. Altermann and R. T. Klaenhammer, “Isolation and Characterization of a Lac- tobacillus plantarum Bacteriophage, AJL-1, from a Cu- cumber Fermentation,” International Journal of Food Microbiology, Vol. 84, No. 2, 2003, pp. 225-235. http://dx.doi.org/10.1016/S0168-1605(03)00111-9 [21] L. Moce-Llivina, F. Lucena and J. Jofre, “Double-Layer Plaque Assay for Quantification of Enteroviruses,” Ap- plied and Environmental Microbiology, Vol. 70, No. 5, 2004, pp. 2801-2805. http://dx.doi.org/10.1128/AEM.70.5.2801-2805.2004 [22] E. G. Felis and F. Dellaglio, “Taxonomy of Lactobacilli and Bifidobacteria,” Current Issues Intestinal Microbiol- ogy, Vol. 8, No. 2, 2005, pp. 44-61. [23] C. Canchaya J. M. Claesson, F. G. Fitzgerald, D. Sin- deren and W. P. O’Toole, “Diversity of the Genus Lac- tobacillus Revealed by Comparative Genomics of Five Species,” Microbiology, Vol. 152, No. 11, 2006, pp. 3185-3196. http://dx.doi.org/10.1099/mic.0.29140-0 [24] A. M. W. Mullan, “Plaque Formation,” 2002. http://www.dairyscience.info/enumeration-of-lactococcal- bacteriophages/plaque-formation.html [25] R. J. Erickson and F. E. Young, “Characterization of a Virulent Bacteriophage for Bacillus subtilis (var. Amylo- liquefaciens),” Applied Microbiology, Vol. 27, No. 3, 1974, pp. 600-602. [26] L. Sechaud, M. Rousseau, B. Fayard, M. L. Callegari, P. Queen and J. P. Accolas, “Comparative Study of 35 Bac- teriophages of Lactobacillus helveticus: Morphology and Host Range,” Applied and Environmental Microbiology, Vol. 58, No. 3, 1991, pp. 10-11. [27] M. Villion and S. Moniteau, “Bacteriophages of Lactoba- cillus,” Frontiers in Bioscience, Vol. 14, No. 5, 2009, pp. 1661-1683. http://dx.doi.org/10.2741/3332 [28] M. Zago, E. Scaltriti, L. Rossetti, L. Guffanti, A. Armi- ento, E. M . Fornasari, S. Grolli, D. Carminati, E. Brini, P. Pavan, A. Felsani, A. D’Urzo, S. Moles, B. J. Claude, R. Grandori, R. Ramoni and G. Giraffa, “Genome Charac- terization of the Dairy Lactobacillus helveticus Bacterio- phage ΦAQ113,” Applied Environmental Microbiology, Vol. 79, No. 15, 2013, pp. 1-29. http://dx.doi.org/10.1128/AEM.00620-13 [29] X. Qiao, Y. Sun, J. Qiao, F. Di Sanzo and L. Mindich, “Characterization of Φ2954, a Newly Isolated Bacterio- phage Containing Three dsRNA Genomic Segments,” BMC Microbiology, Vol. 10, No. 55, 2010, pp.1-7. http://dx.doi.org/10.1186/1471-2180-10-55 [30] G. V. Edy, M. Szekely, T. Loviny and C. Dreyer, “Action of Nucleases on Double-Stranded RNA,” European Jour- nal of Biochemistry, Vol. 61, No. 2, 1976, pp. 563-572. http://dx.doi.org/10.1111/j.1432-1033.1976.tb10051.x [31] R. M. Dayer, O. Ghayour and S. M. Dayer, “Mechanism of the Bell-Shaped Profile of RibonucleaseA Activity: Molecular Dynamic Approach,” Protein Journal, Vol. 31, No. 7, 2012, pp. 573-579. http://dx.doi.org/10.1007/s10930-012-9435-4 [32] H.-W. Ackermann, “Bacteriophage Taxonomy,” Micro- biology Australia, 2011, pp. 90-94. [33] www.ictvonline.org/virusTaxonomy.asp [34] T. S. Abedon, “Selection for Bacteriophage Latent Period Length by Bacterial Density: A Theoretical Examina- tion,” Microbial Ecology, Vol. 18, No. 2, 1989, pp. 79-88. http://dx.doi.org/10.1007/BF02030117 Open Access FNS  Isolation and Characterization of Bacteriophages from Laban Jameed Open Access FNS 66 [35] J. A. Cann, “Principles of Molecular Virology,” 4th Edi- tion, Academic Press, 2005, p. 352. [36] S.C. Harrison, “Viruses,” Current Opinion in Structural Biology, Vol. 2, No. 2, 1992, pp.293-299. http://dx.doi.org/10.1016/0959-440X(92)90160-9 [37] M. Collins, U. Rodrigues, C. Ash, M. Aguirre, E. A. J. Farrow, M. A. Murcia, A. B. Phillips, M. A. Williams and S. Wallbanks, “Phylogenetic Analysis of the Genus Lac- tobacillus and Related Lactic Acid Bacteria as Deter- mined by Reverse Transcriptase Sequencing of 16S rRNA,” Federation of European Microbiological Socie- ties, Vol. 77, No. 1, 1991, pp. 5-12. http://dx.doi.org/10.1111/j.1574-6968.1991.tb04313.x [38] S. Torriani, E. G. Felis and F. Deliaglio, “Differentiation of Lactobacillus plantarum, L. pentosus and L. paraplan- tarum by recA Gene Sequence Analysis and Multiplex PCR Assay with recA Gene-Derived Primers,” Applied and Environmental Microbiology, Vol. 67, No. 8, 2001, pp. 3450-3454. http://dx.doi.org/10.1128/AEM.67.8.3450-3454.2001 [39] M. M. Patil, A. Pal, A. Anand and V. K. Ramana, “Iso- lation and Characterization of Lactic Acid Bacteria from Curd and Cucumber,” Indian Journal of Biotechnology, Vol. 9, No. 2, 2010, pp. 166-172. [40] G. F. Priest, M. Goodfello, A. L. Shute and W. C. R. Ber- kely, “Bacillus amyloliquefaciens sp. nov., nom. rev,” In- ternational Journal of Systematic Bacteriology, Vol. 37, No. 1, 1987, pp. 69-71. http://dx.doi.org/10.1099/00207713-37-1-69 [41] D. Gangadharan, S. Sivaramakrishnan, K. M. Nampoo- thiri and A. Pandey, “Solid Culturing of Bacillus amylo- liquefaciens for Alpha Amylase Production,” Food Technology and Biotechnology, Vol. 44, No. 2, 2006, pp. 269-274. [42] Z. Ding, W. Wang, B. Wang, A. Ouyang, Si. Xiao, Y. Wang, S. Liu, M. Ding, L. Zhang and G. Shi, “Production and Characterization of Milk-Clotting Enzyme from Ba- cillus amyloliquefaciens JNU002 by Submerged Fermen- tation,”European Food Research & Technology, Vol. 234, No. 3, 2012, pp. 415-421. http://dx.doi.org/10.1007/s00217-011-1650-2 [43] M.-H. Joo, S.-H. Hur, Y.-S. Han and J.-Y. Kim, “Isola- tion, Identification and Characterization of Bacillus Strains from the Traditional Korean Soybean-fermented Food Chungkookjang,” Journal of Applied Biological Chemistry, Vol. 50, No. 4, 2007, pp. 202-210. [44] J. M. González, F. Gorgoroso, M. S. Reginensi, A. J. Olivera and J. Bermúdez Polyphasic, “Identification of Closely Related Bacillus Subtilis and Bacillus amylo- liquefaciens Isolated from Dairy Farms and Milk Pow- der,” Journal of Microbiology, Biotechnology and Food Sciences, Vol. 2, No. 5, 2012, pp. 2326-2331. [45] K. J. Boor, “Fluid Dairy Product Quality and Safety: Looking to the Future,” Journal of Dairy Science, Vol. 84, No. 1, 2001, pp. 1-11. http://dx.doi.org/10.3168/jds.S0022-0302(01)74445-1 [46] A. Coorevits, V. De Jonghe, J. V. androemme, R. Reek- mans, J. Heyrman, W. Messens, P. De Vos and M. Heyn- drickx, “Comparative Analysis of the Diversity of Aero- bic Spore-Forming Bacteria in Raw Milk from Organic and Conventional Dairy Farms,” Systematic and Applied Microbiology, Vol. 31, No. 2, 2008, pp. 126-140. http://dx.doi.org/10.1016/j.syapm.2008.03.002 [47] L. Slattery, J. O’Callaghan, F. G. Fitzgerald, T. Beresford and P. R. Ross, “Invited Review: Lactobacillus helveti- cus-A Thermophilic Dairy Starter Related to Gut Bacte- ria,” Journal of Dairy Science, Vol. 93, No. 10, 2010, pp. 4435-4454. http://dx.doi.org/10.3168/jds.2010-3327
|