 Vol.4, No.9B, 23-28 (2013) Agricultural Sciences http://dx.doi.org/10.4236/as.2013.49B004 Copyright © 2013 SciRes. OPEN ACCESS Synthesis and flocculation characteristics of cationic modified corncob: A novel polymeric flocculant Yanhua Pang1*, Yongsheng Din g 2, Jie Chen3, Weimin Gong3 1Center of Food Inspection, Liaoning Entry-Exit Inspection and Quarantine Bureau, Dalian, China; *Corresponding Author: cindy_gl@eyou.com 2College of Ocean Environment and Engineering, Shanghai Maritime University, Shanghai, China 3College of Environ ment al Science and Engineerin g, D alian Maritime Univer sity, Dalian, China Received Ju l y 2013 AB STRACT A novel organic polymeric flocculant was syn- thesized by grafting cationic etherifying mono- mer (3-chloro-2-hydroxypropyl) trimethyl ammo- nium chloride (CHPTAC) onto the backbone of corncob powder (CP, Φ ≤ 30 µm). The synthetic reaction between CP and CHPTAC was initiated by hydroxyl radical made from Fenton reagent (H2O2-FeSO4). The alkalization process and syn- thetic reaction conditions were optimized by va- rying several parameters affecting grafting effi- ciency, such as NaOH dose, monomer concen- tration, reaction temperature and time and dis- tilled water dose. The synthesized cationic flo c- culant was characterized by elemental analysis, Fourier-transform infrared spectrometer and scanning electron microscope techniques. It was concluded that the backbone of CP have been grafted cationic etherifying monomer. The floc- culation characteristics in 0.25% (w/v) kaolin suspensions was compared with some of the comm ercial f loccul ants a vailab le in markets , and the results demonstrated the synthesized catio- nic flocculation has superiority as a novel floc- culant. Keywords: Flocculation; Corncob; Polymeric Flocculant; CHPTAC 1. INTRODUCTION Flocculation [1-3] is a process of bringing together smaller particles to form larger particles, and settling of colloidal particles from stable suspensions caused by the addition of chemicals known as flocculants in minute quantities. It is well known that the use of organic and inorganic flocculants can improve the efficiency of sol- id/liquid separation in a wide range of papermaking, wastewater treatment and mineral processing industries [4-7]. The inorganic flocculants, which are mainly based on hydrolyzable salts of aluminum, cannot be removed easily during treatment, and the residue in water can cause some health problems [8]. Natural and synthetic poly- meric flocculants, which are able to destabilize colloidal suspensions, have been found wide application as floc- culating agents in the past dec ade. Water-soluble syn thetic polymers [9-11] have a disadvantage of non-biodegra- dability and cost-effective. Thus, the demand for effec- tive, cheap and environmentally friendly natural floccu- lants for wast ewater treatment is increasing. With the characteristics of nontoxic and biodegradable, the so-called “green flocculants” such as starch [12,13], amylopectin [14], alginic acid [15], guar gum [16], and so for th, ha ve bee n paid mu ch att entio n. The chemical and physical properties of the natural polymers can be mod- ified by grafting a synthetic polymer onto the backbone of natural polymers. For highly negatively charged colloidal particles, cationic polymers are more used as flocculating agents to remove finely divided solids from aqueous suspension [17]. The natural corncob powder (CP), mainly composed of cellulose, lignose and pentosan, has abundant output in China. Composed of much hydroxyl radicals, CP may be grafting by cationic polymers and perform efficient flocculation characteristics. In view of several authors’ earlier findings, CP was chosen as the base pol ymer graft- ing by a quaternary ammonium compo und [18]. Howev- er, it has not been much s tudied as floccul ating agent for wastewater treatment. Highly charged cationic polymers such as polyamines and polyimines are costly. Therefore, it has been at- tempted to synthesize a cationic quaternary ammonium salt (3 -chloro-2-hydroxypropyl) trimethyl ammonium chlo- ride (CHPTAC). The present work aims to explore the feasibility of grafting flocculation (DXSL-I) of CP and CHPTAC, illustrate it s characteristics b y using elemental analysis, Fourier-transform infrared (FTIR) and scan-  Y. H. Pang et al. / Agricultural Sciences 4 (2013) 23-28 Copyright © 2013 SciRes. OPEN A CCESS ning electron microscope (SEM) and evaluate its floccu- lation performance. The reaction parameters affecting grafting efficiency, such as NaOH dose, monomer con- centration, reaction temperature and time and distilled water dose were also investigated. 2. EXPERIMENTAL 2.1. Materials Corncob powder (CP, (Ф ≤ 30 mm), obtained from Dalian Farm Product Processing Plant, was dried at 100˚C for 4 h before use. Triethylamine, Epoxy chloro- propane, all purchased from Shanghai Reagent Company, China, were of analytical reagent grade. Aluminum chlo- rate and Polyacrylamide are used as commercial floccu- lants for comparison test. All the reagents were used without further purification. Kaolin clay (Ф ≤ 2 mm) was purchased from Zhonglong Kaolin Ltd., Chi na. 2.2. Preparation of Cationic Monomer The reaction conditions were established according to our previous experience [18]. Epoxy chloropropane (20 ml) was mixed with excessive triethylamine in a sealed flask with constant stirring. The reaction was allowed to continue for 3 h at a reaction temperature of 0˚C - 5˚C, after which the sample was settled at ice bath for 2 h. The content of upper solution, in which was the cationic monomer (3-chloro-2-hydroxypropyl) trimethyl ammo- nium chloride (CHPTAC), can be determined by using Ag+ solution. The quaternary ammonium monomer was decomposed easily under daylight or high temperature. Therefore, the use of instant prepared monomer was suggested. The reaction of quaternary ammonium group preparation was shown as followed: ClCH 2 CH O CH 2 CH 2 CH CH 2 O N + Cl N(C 2 H 5 ) 3 (C 2 H 5 ) 3 + Fenton reagent T< 5℃ (1) 2.3. Synthesis of Cationic Flocculant DXSL-I Corncob powder can be modified by incorporating a cationic monomer (3-chloro-2-hydroxypropyl) trimethyl ammonium chloride onto the backbone of the polysac- charide. The details of the synthesis and the reaction conditions are as follows. Corncob powder (2.0 g) was dissolved in distilled wa- ter (8.0 ml) at room temperature. As alkaline medium is essential to carry out the reaction, 0.002 g NaCl and 0.6 g NaOH were added to it. The reaction solution was al- kalized for 60 min at 40˚C - 50˚C, followed by adding Fenton reagent (H2O2 and Fe2+ were 0.8% - 1.2% and 0.005% of CP mass, respectively) into the solution to initiate the reactio n for 30 - 90 s. Re actio n was then c on- tinued for 3 h with excessive CHPTAC at 50˚C. The so- lution was thereafter cooled to room temperature, and the copolymer was precipitated by centrifugation. The resi- due was washed with distilled water several times until its pH reached 7 - 8 and there was not CHPTAC in the filtrate checked with Ag+ solution. After washing, it was dried in a vacuum oven. 2.4. Characterization of Cationic Flocculant DXSL-I 2.4.1. Elemental Analysis Elemental analysis of the cationic flocculant DXSL-I, CP and CHPTAC was undertaken with a LiquiTOC ele- mental analyzer (Elementar Analysensysteme GmbH, German). The estimation of only three elements, that is car bon, hydrogen and nitrogen, was undertaken. 2.4.2. FTIR Spectroscopy A 500 FTIR Spectrophotometer (Nicolet, USA) was used and the potassium bromide (KBr) pellet method was used for FTIR study. The FTIR spectrum of cationic flocculation DXSL-I, alkalized CP and CP were collected separately. 2.4.3. Scanning Electron Microscopy (SEM) The small granules left after the pulverized graft co- polymers and CP were sieved, and were subjected to SEM study. The samples were gold-coated, and a magni- fication of 2000 times was obtained. A Philips XL-30 Scanning Electro-Microscope (Holland) was used for SEM study. 2.5. Flocculation Test Flocculation capacity of DXSL-I and other commer- cial flocculants were evaluated using 0.25% (w/v) of kaoli n suspe nsio ns. After add ition of flocculants solutio n (80 - 100 ml), mixtures were mechanically stirred at a constant speed of 200 rpm for 2 min, followed by a slow stirring at 40 rpm for 10 min. Thereafter, the sample was left to settle for 5 min. At the end of the settling, 10 ml of the supernatant fluid was taken from the beaker surface, and its transmittance was measured at a wavelength of 610 nm by using JASCO V-500 Spectrometer (Japan). 3. RESULTS AND DISCUSSION 3.1. Synthesis Mechanism Corncob, mainly consisted of polysaccharide, was a natural polymer. There were many -OH groups on its microparticle’s surface [19]. Cationic modification was based on these radicals. The synthetic reactions were etherifying processes as shown in Eq s.2 and 3. Matrix OH OH n CH2CH CH2N+ O Cl OH CH2CH CH2N+ OH Matrix O OH n Cl (C2H5)3(C2H5)3 + 1 -1 - (2)  Y. H. Pang et al. / Agricultural Sciences 4 (2013) 23-28 Copyright © 2013 SciRes. OPEN ACCESS CH2CH CH2N+ O Cl (C2H5)3 CH2CH CH2N+ OH Matrix O Cl (C2H5)3 n Matrix OH n +(n) (3) Reactions were theoretically divided into several steps, including the free radical chain reaction and ion reaction [20,21]. The formation of free OH groups could be ex- pressed as followed equations: (4) Matrix OH Matrix O OH OH 2 ++ (5) The synthetic reaction was actually an electr o -nuc- leophilic process as shown below: Matrix O OH n CH 2 CH CH 2 N + O Cl OH CH 2 CH CH 2 N + OH Matrix O OH n Cl (C 2 H 5 ) 3 (C 2 H 5 ) 3 - + 1 - 1 - δ + δ (6) Some possible unwished negative reactions may hap- pen as indicated from Eq s.7 to 10, where included free radical transfers and exterminations, as well as the spli- tup of etherified monomer by hydrolyzation. (7) (8) (9) CH 2 CH CH 2 N + OH OH Cl Matrix OCH 2 CH CH 2 N + OH Cl OH OH 2 (R) 3 (R) 3 + (10) 3.2. Synthesis Conditions and Influence Factors 3.2.1. NaOH Dosage, A lkalizing Temperature and Time In order to evaluate the effect of NaOH dose on the grafting of CHPTAC onto CP, a set of experiments were carried out, in which the dose was changed from 0.10 g to 6.00 g, while all the other parameters were kept un- changed. The results showed an increase in the grafting percentage with the increase in NaOH dose. However, beyond a value of 0.6 g, a significant decrease in grafting percentage was observed. According to synthesis reac- tion (Eq.2 ) , the low dose OH- was he lpful to chan ge OH group into ionic oxygen, of which the nucleophilic ca- pacity was strengthened consumedly. However, overdose of NaOH could make the epoxy ring broken up and ac- celerate the quaternary ammonium radical decomposed, resulting in grafting efficiency reduced. The similar re- sults were found after a series of experiments about alka- lization conditions. There is also an increase in the graft- ing percentage with the alkalizing temperature and time, but a reduction occurred with the alkalizing temperature raised and time prolonged. The density of free radicals was l ess, r esulti ng in re duct ion of grafti ng efficie ncy with the alkalizing temperature and time increased. After the opti mization experiments, it was obtained the NaOH do- sage is 0.6 g, alkalizing temperature is 40˚C - 50˚C and alkalizatio n time is 60 min. 3.2.2. Monomer Concentration As sho wn in Fi gu re 1 , the gr afting p ercenta ge sig nifi- cantly increased with incr easing mono mer concentration. The presence of a hi gh concentration of CHPTAC in the medium provided a greater availability of CHPTAC mo- lecules to react with the CP macroradicals, leading to a higher synthesis percentage. However, overdose of mo- nome r co sts hi gh l y and unbeneficial. 3.2.3. Orthogonal Experi m en t In addition to monomer concentration, distilled water medium, synthesis temperature and time were also the infl uence factor s gove rnin g the gra fting e fficie ncy. There - fore, a series of experiments were carried out by ortho- gonal method to optimize the reaction conditions affect- ing flocculation efficiency. Taking CP (2.0 g), we ex- amined the influence factors including the reaction tem- perature (30˚C - 60˚C), reaction time (2 - 5 h) and dis- tilled water (4 - 16 ml), respectively. T he final optimized condition was obtained as follo ws: synthesis temperature 50˚C, synthesis time 3.0 h, and distilled water 8.0 ml. The orthogonal experimental parameters were shown in Table 1. 0.02 0.04 0.06 0.08 0.10 0 20 40 60 80 100 Grafting percentage/% M onom er Concentration /M Figure 1. Effect of monomer concentration on grafting percen- tage (C P dos a ge : 2.0 g). Table 1. The orthogonal experimen tal parameters. No. Reaction temperature (˚C) React io n tim e (h) Water (ml) 1 30 2 4 2 40 3 8 3 50 4 12 4 60 5 16 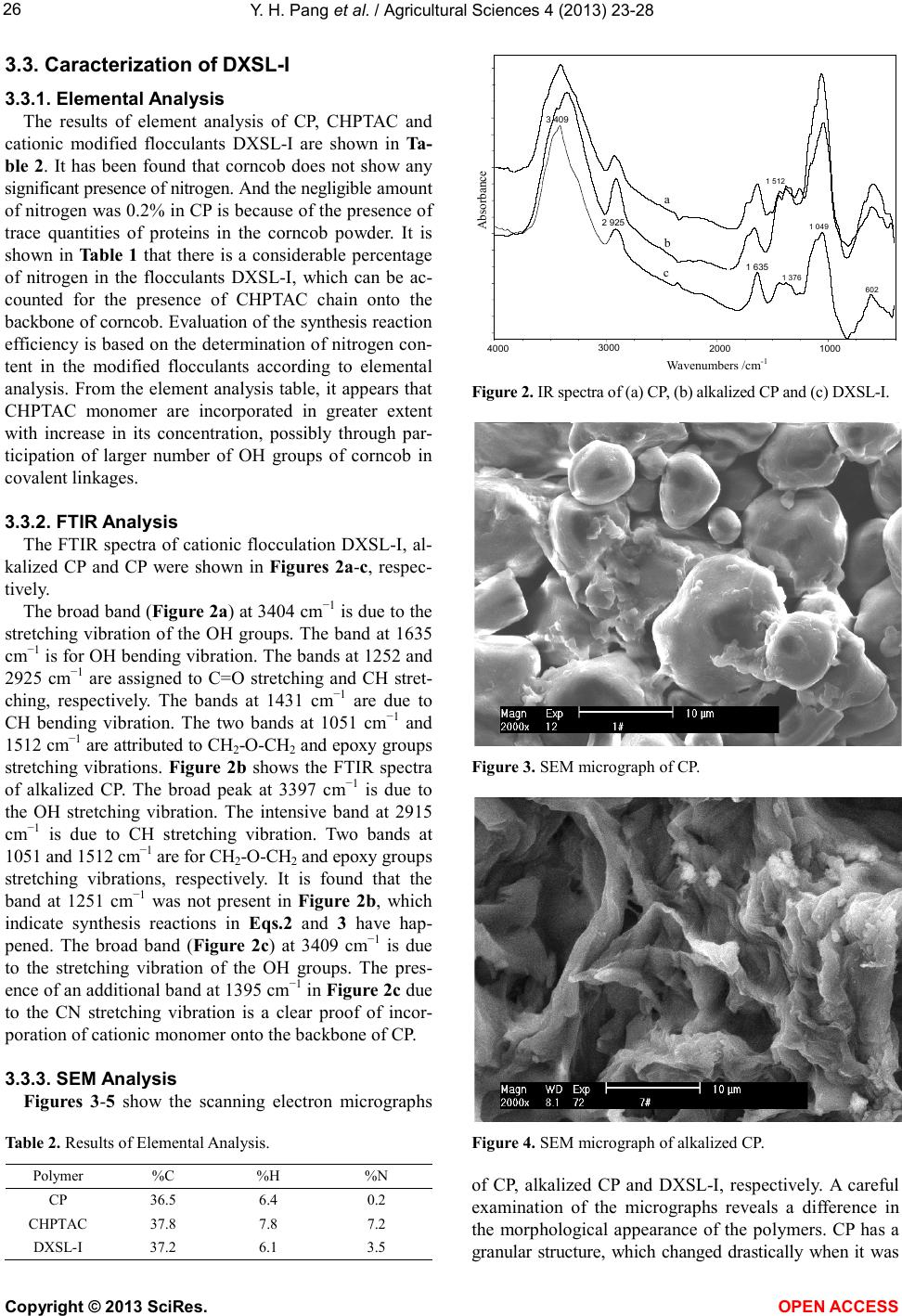 Y. H. Pang et al. / Agricultural Sciences 4 (2013) 23-28 Copyright © 2013 SciRes. OPEN A CCESS 3.3. Caracterization of DXSL-I 3.3.1. Elemental Analysis The results of element analysis of CP, CHPTAC and cationic modified flocculants DXSL-I are shown in Ta- ble 2. It has been found that corncob does not show any significant presence of nitrogen. And the negligible amount of nitrogen was 0.2% in CP is because of the presence of trace quantities of proteins in the corncob powder. It is shown in Table 1 that there is a considerable percentage of nitrogen in the flocculants DXSL-I, which can be ac- counted for the presence of CHPTAC chain onto the backbone of corncob. Evaluation of the synthesis reaction efficie ncy is ba sed on t he dete rminatio n of nit rogen co n- tent in the modified flocculants according to elemental analysis. From the element analysis table, it appears that CHPTAC monomer are incorporated in greater extent with increase in its concentration, possibly through par- ticipation of larger number of OH groups of corncob in cova lent li nkages . 3.3.2. FTIR Analysis The FTIR spectra of cationic flocculation DXSL-I, al- kalized CP and CP were shown in F i g ure s 2a-c, respec- tively. The broad band (Figure 2a) at 3404 cm−1 is due to the stretching vibration of the OH groups. The band at 1635 cm−1 is for OH b end ing vib rat io n. T he b ands a t 125 2 and 2925 cm−1 are assigned to C=O stretching and CH stret- ching, respectively. The bands at 1431 cm−1 are due to CH bending vibration. The two bands at 1051 cm−1 and 1512 cm−1 are attribu ted to CH2-O-CH2 and epoxy groups stretching vibrations. Figure 2b shows the FTIR spectra of alkalized CP. The broad peak at 3397 cm−1 is due to the OH stretching vibration. The intensive band at 2915 cm−1 is due to CH stretching vibration. Two bands at 1051 and 1512 cm−1 are for CH2-O-CH2 and epoxy groups stretching vibrations, respectively. It is found that the band at 1251 cm−1 was not present in Figure 2b, which indicate synthesis reactions in Eqs.2 and 3 have hap- pened. The broad band (Figure 2c) at 3409 cm−1 is due to the stretching vibration of the OH groups. The pres- ence of an additional band at 1395 cm−1 in Fig u re 2c due to the CN stretching vibration is a clear proof of incor- poration of cationic monomer onto the backbone of CP. 3.3.3. SEM Analysis Fig u re s 3-5 show the scanning electron micrographs Table 2. Results of Elemental Analysis. Po l ym er %C %H %N CP 36.5 6.4 0.2 CH PT AC 37.8 7.8 7.2 DXSL-I 37.2 6.1 3.5 Figur e 2. IR spectra of (a) CP, (b) alkalized CP and (c) DXSL-I. Figure 3. SEM micrograph of CP. Figure 4. SEM micro graph of alkalized CP. of CP, alkalized CP and DXSL-I, respectively. A careful examination of the micrographs reveals a difference in the morphological appearance of the polymers. CP has a granular structure, which changed drastically when it was 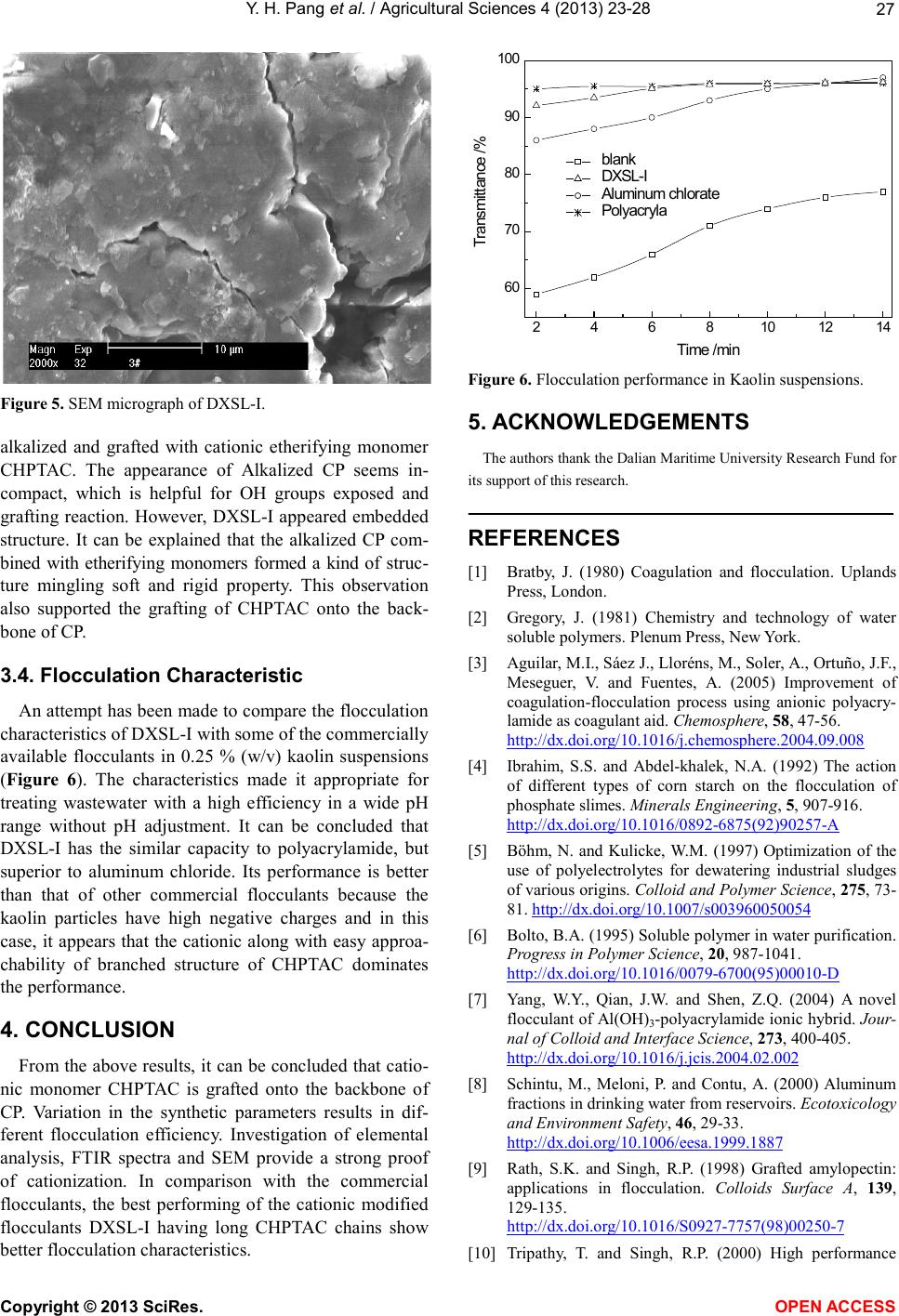 Y. H. Pang et al. / Agricultural Sciences 4 (2013) 23-28 Copyright © 2013 SciRes. OPEN ACCESS Figure 5. SEM micrograph of DXSL-I. alkalized and grafted with cationic etherifying monomer CHPTAC. The appearance of Alkalized CP seems in- compact, which is helpful for OH groups exposed and grafting reaction. However, DXSL-I appeared embedded structure. It can be explained that the alkalized CP com- bined with etherifying monomers formed a kind of struc- ture mingling soft and rigid property. This observation also supported the grafting of CHPTAC onto the back- bone of CP. 3.4. Flocculation Characteristic An attempt has been made to compare the flocculation characteristics of DXSL-I with some of the commercially available flocculants in 0.25 % (w/v) kaolin suspensions (Figure 6). The characteristics made it appropriate for treating wastewater with a high efficiency in a wide pH range without pH adjustment. It can be concluded that DXSL-I has the similar capacity to polyacrylamide, but superior to aluminum chloride. Its performance is better than that of other commercial flocculants because the kaolin particles have high negative charges and in this case, it appears that the cationic along with easy approa- chability of branched structure of CHPTAC dominates the performance. 4. CONCLUSI O N From the above results, it can be concluded that catio- nic monomer CHPTAC is grafted onto the backbone of CP. Variation in the synthetic parameters results in dif- ferent flocculation efficiency. Investigation of elemental analysis, FTIR spectra and SEM provide a strong proof of cationization. In comparison with the commercial flocculants, the best performing of the cationic modified flocculants DXSL-I having long CHPTAC chains show better flocculatio n characteristics. 2 4 6 810 12 14 60 70 80 90 100 bl ank DXSL -I Aluminum chlorate Polyac ryla Transm ittance /% Time /min Figure 6. Flocculation performance in Kaolin suspensions. 5. ACKNOWLEDG E ME NTS The authors thank the Dalian Maritime University Research Fund for its support of this research. REFERENCE S [1] Bratby, J. (1980) Coagulation and flocculation. Uplands Press, London. [2] Gregory, J. (1981) Chemistry and technology of water sol uble pol ymers. Plenum Press, New York. [3] Aguilar, M.I., Sáez J., Lloréns, M., Soler, A., Ortuño, J.F. , Meseguer, V. and Fuentes, A. (2005) Improvement of coagulat i on-flocculation process using anionic polyacry- lamide as coagulant ai d. Chemosphere, 58, 47-56. [4] Ibrahim, S.S. and Abdel-khalek, N.A. (1992) The action of different types of corn starch on the flocculation of phosphate slimes. Minerals Engineering, 5, 907-916. http://dx.doi.org/10.1016/j.chemosphere.2004.09.008 [5] Böhm, N. and Kulicke, W.M. (1997) Optimization of the use of polyelectrolytes for dewatering industrial sludges of various origins. Colloid and Polymer Science, 275, 73- 81. http://dx.doi.org/10.1016/0892-6875(92)90257-A [6] Bolto, B.A. (1995) Soluble polymer in water purification. Progress in Polymer Science, 20, 987 -1041. http://dx.doi.org/10.1007/s003960050054 [7] Yang, W.Y., Qian, J.W. and Shen, Z.Q. (2004) A novel flocculant of Al(OH)3-polyacrylamide ionic hybrid. Jour- nal of Colloid and Interface Science, 273, 400-405. http://dx.doi.org/10.1016/0079-6700(95)00010-D [8] Schintu, M., Meloni, P. and Contu, A. (2000) Aluminum fractions in drinking water from reservoirs. Ecotoxicology and Environment Safety, 46, 29-33. http://dx.doi.org/10.1016/j.jcis.2004.02.002 [9] Rath, S.K. and Singh, R.P. (1998) Grafted amylopectin: applications in flocculation. Colloids Surface A, 139, 129-135. http://dx.doi.org/10.1006/eesa.1999.1887 [10] Tripathy, T. and Singh, R.P. (2000) High performance http://dx.doi.org/10.1016/S0927-7757(98)00250-7  Y. H. Pang et al. / Agricultural Sciences 4 (2013) 23-28 Copyright © 2013 SciRes. OPEN A CCESS flocculating agent based on partially hydrolysed sodium alginate-g-p o l ya c r yl amide. European Polymer Journal, 36, 1471 -1476. [11] Mishra, A., Srinivasan, R., Bajpai, M. and Dubey, R. (2004) Use of po lya c r yla mi d e -grafted plantago psyllium mucilage as a flo cculant for t reatment of textile waste wa- ter. Colloid and Polymer Science, 282, 722-727. http://dx.doi.org/10.1016/S0014-3057(99)00201-3 [12] Burr, R.C., Fanta, G.F., Doane, W.M. and Russel, C.R. (1975) Starch graft co po lymer s for wat er tr eat men t. Starch, 27, 155-159. http://dx.doi.org/10.1007/s00396-003-1003-1 [13] Khalil, M .I. and Aly, A. A. (2 002 ) Prepar ation an d evalua- tion of some anionic starch derivatives as flocculants. Starch-Starke, 54, 132-139. http://dx.doi.org/10.1002/star.19750270504 [14] Larsson, A. and Wall, S. (1998) Flocculation of cationic amylopect in starch and colloidal silicic acid. The effect of various kinds of salt. Colloids Surface A, 139, 259-270. http://dx.doi.org/10.1002/1521-379X(200204)54:3/4<132 ::AID-STAR132>3.0.CO;2-E [15] Choong, J., Young, J.Y. and Wolfgang, H.H. (2005) En- vironmental effects and desorption characteristics on heavy metal removal using carboxylated alginic acid. Bioresource Technology, 96, 15-19. http://dx.doi.org/10.1016/S0927-7757(98)00326-4 [16] Nayak, B.R. and Singh, R.P. (2001) Development of graft copolymer flocculating agents based on hydroxypropyl guar gum and acrylamide. Journal of Applied Polymer Scien ce, 81, 1776-1785. http://dx.doi.org/10.1016/j.biortech.2004.03.001 [17] Ellis, H.A., Utah, S.I., Ogunrinde, A. and Ogedengbe, M.O. (1982) Preparation of some cationic starches as flocculants for water. Water Research, 16, 1433-1435. http://dx.doi.org/10.1002/app.1610 [18] Sheng, X.Q., Ding, Y.S. and Gong, W.M. (2002) Prepara- tion and evaluation of cationic natural polymer flocculant. Journal of Dalian Maritime University, 28, 72-75. (in Chin es e) http://dx.doi.org/10.1016/0043-1354(82)90231-7 [19] Lv, X.X., Li, H.Y. and Huang, K.Y. (2004) Effect of dif- ferent pretreatment methods on constituents of corn-cob. Journal of Chemical Indu stry of Forest Products, 2, 11- 13. (in Chinese) [20] Ren, J.L., Sun, R.C., Liu, C.F., Chao, Z.Y. and Luo, W. (2006) Two-step prep aration and thermal character ization of cationic 2-hydroxypropyltrimethylammonium chloride hemicell ul ose po lymers fro m sug arcan e bagass e. Po l ym er Degradation and Stability, 91, 2579-2587. [21] Ju, B.Z., Zhang, S.F. and Yang, J.Z. (2001) Progress in preparation of cationic starch using the dry process. Fine Chemicals, 18, 46-49. (in Ch in ese) http://dx.doi.org/10.1016/j.polymdegradstab.2006.05.008
|