 Modern Research in Catalysis, 2013, 2, 157-163 http://dx.doi.org/10.4236/mrc.2013.24021 Published Online October 2013 (http://www.scirp.org/journal/mrc) Kinetic and Mechanistic Study of Oxidation of Piperazines by Bromamine-T in Acidic Medium Chandrashekar1,2, B. M. Venkatesha1*, S. Ananda3, Netkal M. Made Gowda4* 1Department of Chemistry, Yuvaraja’s College, University of Mysore, Mysore, India 2Department of Chemistry, PES College of Engineering, Mandya, India 3Department of Studies in Chemistry, Manasagangothri, University of Mysore, Mysore, India 4Department of Chemistry, Western Illinois University, Macomb, USA Email: *venkichem123@yahoo.in, *gn-made@wiu.edu Received December 25, 2012; revised April 24, 2013; accepted September 14, 2013 Copyright © 2013 Chandrashekar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Oxidations of piperazine, 1-methylpiperazine and 1-ethylpiperazine by bromamine-T (BAT) in buffered acidic medium have been kinetically studied at 303 K. The reaction shows a first-order dependence of the rate each on [BAT]0 and [piperazine]0, and an inverse fractional-order dependence on [H+]. The additions of halide ions and the reduction prod- uct of BAT, p-toluenesulfonamide, have no effect on the reaction rate. The variation of ionic strength of the solvent medium has no influence on the rate. Activation parameters have been evaluated from the Arrhenius and Eyring plots. A common mechanism consistent with the kinetic data has been proposed for all piperazines. The protonation constants of substrates have been evaluated. The Hammett linear free-energy relationship has been observed for the reaction with ρ = −0.5 indicating that the electron-donating groups enhance the reaction rate by stabilizing the transition state. An isokinetic relationship observed shows β = 368 K indicating the dominance of enthalpy factors on the reaction rate. Keywords: Piperazines; Oxidation Kinetics; Mechanism; Bromamine-T; Buffer 1. Introduction Piperazine is a heterocyclic nitrogenous compound [1] that has chemical similarity with piperidine as it has two opposing nitrogen atoms in the ring. In animals, includ- ing man, piperazine and its salts are known to be highly effective as anthelmintics [2]. It is used in the treatment of gout and is an excellent solvent for uric acid [2,3]. Many uses of piperazine derivatives have been suggested [4]. Their more important use is as intermediates for tran- quilizing agents and antihistamines, insecticides, fungi- cides, bactericides, analgesics, antispasmodics, filaricides and anthelmintics. Some piperazines have been investi- gated for the treatment of cancer [5,6], radiation sickness [7], and anginapectoris [8]. The literature survey shows that the kinetic investigations of reactions of piperazines with iron (II) and cobalt (III) have been reported by Aravindakshan et al. [9]. Aromatic N-halosulfonamides are mild oxidants containing a strongly polarized N- halogen bond where the halogen is in its +1 oxidation state. The prominent member of this group, chloramine-T (CAT), is a well-known analytical reagent and the me- chanistic aspects of many of its reactions have been documented [10,11]. Its bromine analogue, bromamine-T (BAT), is a better oxidizing agent than CAT and chlor- amine-B. However, meager information exists in the lit- erature on BAT-piperazines reactions [12-15]. Hence, the oxidation kinetics of piperazines adds much to the knowledge of redox chemistry. These facts prompted us to undertake the study of kinetics of oxidation of pipera- zines by BAT in acidic buffer medium with a view to elucidate the reaction mechanism. 2. Experimental The oxidant BAT was prepared and purified using the method of Nair and Indrasenan [16]. Its purity was checked by iodometric and spectroscopic data [16,17]. Aqueous solutions of BAT were prepared, standardized by the iodometric method, and preserved in amber-col- ored bottles until use, to prevent its photochemical dete- rioration. Piperazines (Spectrochem Co.) of acceptable grades of purity were used without further purification. Fresh aqueous solutions of piperazines were prepared whenever required. All other chemicals used were of ac- *Corresponding authors. C opyright © 2013 SciRes. MRC 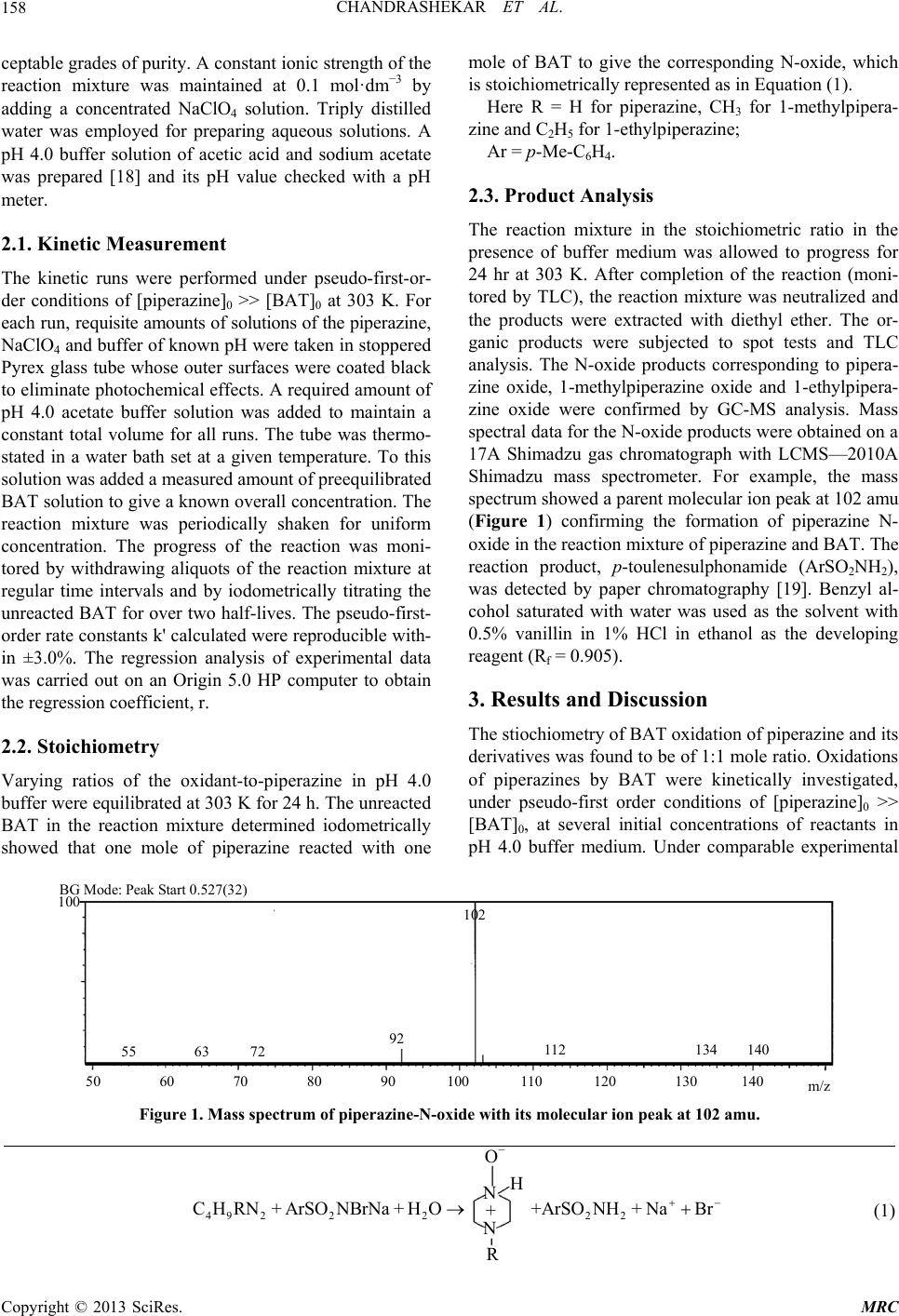 CHANDRASHEKAR ET AL. 158 ceptable grades of purity. A constant ionic strength of the reaction mixture was maintained at 0.1 mol·dm−3 by adding a concentrated NaClO4 solution. Triply distilled water was employed for preparing aqueous solutions. A pH 4.0 buffer solution of acetic acid and sodium acetate was prepared [18] and its pH value checked with a pH meter. 2.1. Kinetic Measurement The kinetic runs were performed under pseudo-first-or- der conditions of [piperazine]0 >> [BAT]0 at 303 K. For each run, requisite amounts of solutions of the piperazine, NaClO4 and buffer of known pH were taken in stoppered Pyrex glass tube whose outer surfaces were coated black to eliminate photochemical effects. A required amount of pH 4.0 acetate buffer solution was added to maintain a constant total volume for all runs. The tube was thermo- stated in a water bath set at a given temperature. To this solution was added a measured amount of preequilibrated BAT solution to give a known overall concentration. The reaction mixture was periodically shaken for uniform concentration. The progress of the reaction was moni- tored by withdrawing aliquots of the reaction mixture at regular time intervals and by iodometrically titrating the unreacted BAT for over two half-lives. The pseudo-first- order rate constants k' calculated were reproducible with- in ±3.0%. The regression analysis of experimental data was carried out on an Origin 5.0 HP computer to obtain the regression coefficient, r. 2.2. Stoichiometry Varying ratios of the oxidant-to-piperazine in pH 4.0 buffer were equilibrated at 303 K for 24 h. The unreacted BAT in the reaction mixture determined iodometrically showed that one mole of piperazine reacted with one mole of BAT to give the corresponding N-oxide, which is stoichiometrically represented as in Equation (1). Here R = H for piperazine, CH3 for 1-methylpipera- zine and C2H5 for 1-ethylpiperazine; Ar = p-Me-C6H4. 2.3. Product Analysis The reaction mixture in the stoichiometric ratio in the presence of buffer medium was allowed to progress for 24 hr at 303 K. After completion of the reaction (moni- tored by TLC), the reaction mixture was neutralized and the products were extracted with diethyl ether. The or- ganic products were subjected to spot tests and TLC analysis. The N-oxide products corresponding to pipera- zine oxide, 1-methylpiperazine oxide and 1-ethylpipera- zine oxide were confirmed by GC-MS analysis. Mass spectral data for the N-oxide products were obtained on a 17A Shimadzu gas chromatograph with LCMS—2010A Shimadzu mass spectrometer. For example, the mass spectrum showed a parent molecular ion peak at 102 amu (Figure 1) confirming the formation of piperazine N- oxide in the reaction mixture of piperazine and BAT. The reaction product, p-toulenesulphonamide (ArSO2NH2), was detected by paper chromatography [19]. Benzyl al- cohol saturated with water was used as the solvent with 0.5% vanillin in 1% HCl in ethanol as the developing reagent (Rf = 0.905). 3. Results and Discussion The stiochiometry of BAT oxidation of piperazine and its derivatives was found to be of 1:1 mole ratio. Oxidations of piperazines by BAT were kinetically investigated, under pseudo-first order conditions of [piperazine]0 >> [BAT]0, at several initial concentrations of reactants in pH 4.0 buffer medium. Under comparable experimental 50 60 70 80 90 100 110 120 130 140 m/z 55 63 92 112134 140 102 100 BG Mode: Peak Start 0.527(32) 72 Figure 1. Mass spectrum of piperazine-N-oxide with its molecular ion peak at 102 amu. + O − H 49 222 C H RN+ArSO NBrNa+H O22 +ArSONH+ NaBr (1) Copyright © 2013 SciRes. MRC 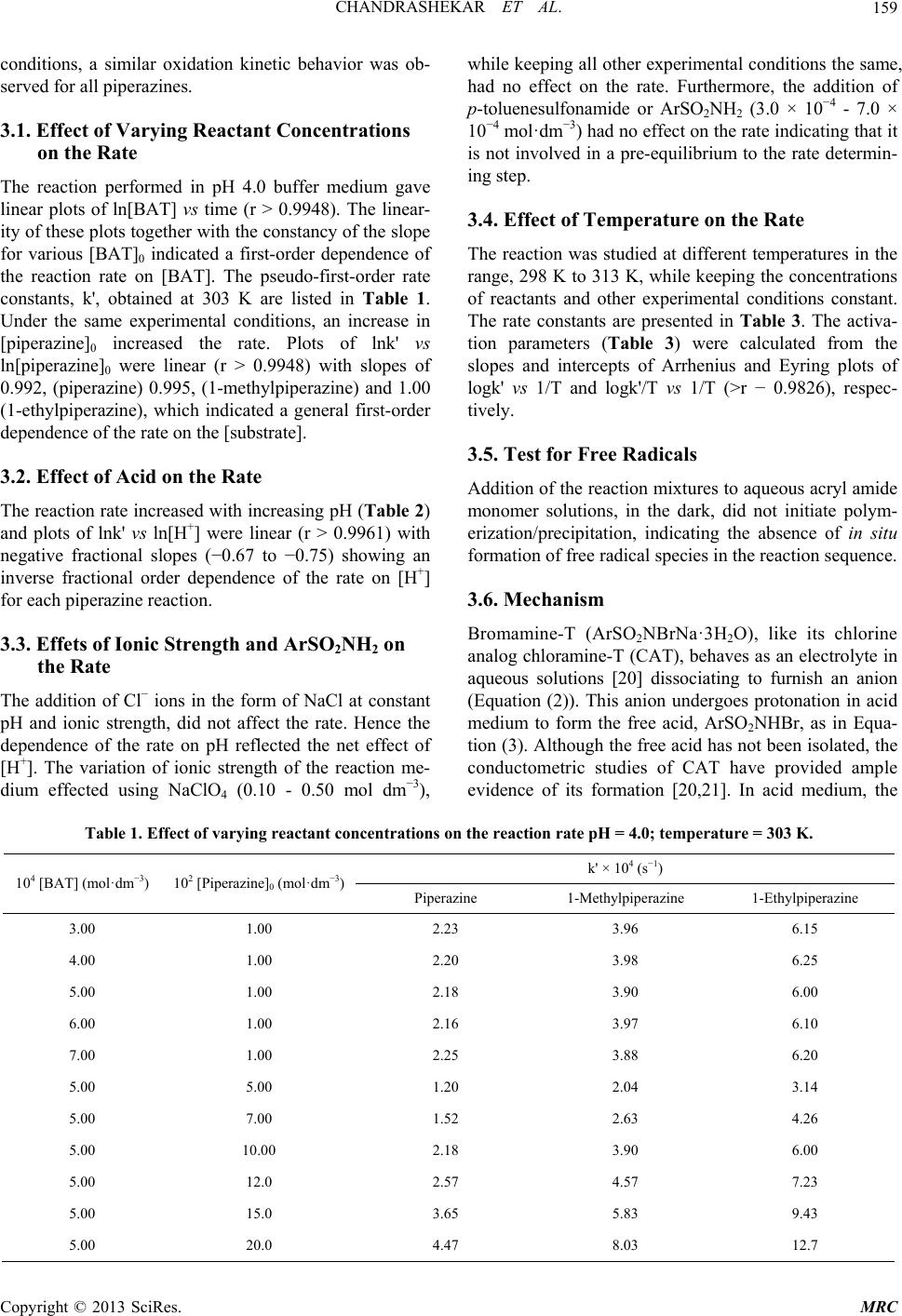 CHANDRASHEKAR ET AL. 159 conditions, a similar oxidation kinetic behavior was ob- served for all piperazines. 3.1. Effect of Varying Reactant Concentrations on the Rate The reaction performed in pH 4.0 buffer medium gave linear plots of ln[BAT] vs time (r > 0.9948). The linear- ity of these plots together with the constancy of the slope for various [BAT]0 indicated a first-order dependence of the reaction rate on [BAT]. The pseudo-first-order rate constants, k', obtained at 303 K are listed in Table 1. Under the same experimental conditions, an increase in [piperazine]0 increased the rate. Plots of lnk' vs ln[piperazine]0 were linear (r > 0.9948) with slopes of 0.992, (piperazine) 0.995, (1-methylpiperazine) and 1.00 (1-ethylpiperazine), which indicated a general first-order dependence of the rate on the [substrate]. 3.2. Effect of Acid on the Rate The reaction rate increased with increasing pH (Table 2) and plots of lnk' vs ln[H+] were linear (r > 0.9961) with negative fractional slopes (−0.67 to −0.75) showing an inverse fractional order dependence of the rate on [H+] for each piperazine reaction. 3.3. Effets of Ionic Strength and ArSO2NH2 on the Rate The addition of Cl− ions in the form of NaCl at constant pH and ionic strength, did not affect the rate. Hence the dependence of the rate on pH reflected the net effect of [H+]. The variation of ionic strength of the reaction me- dium effected using NaClO4 (0.10 - 0.50 mol dm−3), while keeping all other experimental conditions the same, had no effect on the rate. Furthermore, the addition of p-toluenesulfonamide or ArSO2NH2 (3.0 × 10−4 - 7.0 × 10−4 mol·dm−3) had no effect on the rate indicating that it is not involved in a pre-equilibrium to the rate determin- ing step. 3.4. Effect of Temperature on the Rate The reaction was studied at different temperatures in the range, 298 K to 313 K, while keeping the concentrations of reactants and other experimental conditions constant. The rate constants are presented in Table 3. The activa- tion parameters (Table 3) were calculated from the slopes and intercepts of Arrhenius and Eyring plots of logk' vs 1/T and logk'/T vs 1/T (>r − 0.9826), respec- tively. 3.5. Test for Free Radicals Addition of the reaction mixtures to aqueous acryl amide monomer solutions, in the dark, did not initiate polym- erization/precipitation, indicating the absence of in situ formation of free radical species in the reaction sequence. 3.6. Mechanism Bromamine-T (ArSO2NBrNa·3H2O), like its chlorine analog chloramine-T (CAT), behaves as an electrolyte in aqueous solutions [20] dissociating to furnish an anion (Equation (2)). This anion undergoes protonation in acid medium to form the free acid, ArSO2NHBr, as in Equa- tion (3). Although the free acid has not been isolated, the conductometric studies of CAT have provided ample evidence of its formation [20,21]. In acid medium, the Table 1. Effect of varying reactant concentrations on the reaction rate pH = 4.0; temperature = 303 K. k' × 104 (s−1) 104 [BAT] (mol·dm−3) 102 [Piperazine]0 (mol·dm−3) Piperazine 1-Methylpiperazine 1-Ethylpiperazine 3.00 1.00 2.23 3.96 6.15 4.00 1.00 2.20 3.98 6.25 5.00 1.00 2.18 3.90 6.00 6.00 1.00 2.16 3.97 6.10 7.00 1.00 2.25 3.88 6.20 5.00 5.00 1.20 2.04 3.14 5.00 7.00 1.52 2.63 4.26 5.00 10.00 2.18 3.90 6.00 5.00 12.0 2.57 4.57 7.23 5.00 15.0 3.65 5.83 9.43 5.00 20.0 4.47 8.03 12.7 Copyright © 2013 SciRes. MRC  CHANDRASHEKAR ET AL. 160 Table 2. Effect of varying pH on the reaction rate [pipera- zine]0 = 1.00 × 10−2 mol·dm−3; [BAT]0 = 5.00 × 10−4 mol·dm−3; temperature = 303 K. k' × 104 (s−1) pH 105 [H+] (mol·dm−3) Piperazine 1-Methylpiperazine 1-Ethylpiperazine 3.6 25.1 1.14 1.90 2.95 3.8 15.9 1.62 2.63 3.99 4.0 10.0 2.18 3.90 6.00 4.2 6.30 3.02 5.88 8.22 4.4 3.98 4.16 8.51 10.96 4.6 2.51 4.90 12.3 14.80 free acid undergoes a reaction to form dibromamine-T or DBT (ArSO2NBr2) and p-toluenesulfonamide (ArSO2NH2) (Equation (4)). ArSO2NHBr hydrolyzes to give HOBr as one of the products [Equation (5)]. In high acid concentrations, HOBr can get protonated to H2OBr+ as in Equation (6). 22 ArSONBrNaArSONBrNa 22 (2) 22 ArSO NBrHArSO NHBr (3) 22 2ArSO NHBrArSO NBr+ArSO NH (4) 22 22 ArSONHBrHOArSONH+ HOBr (5) 2 HOBr +HHOBr (6) In acidic solutions, the probable oxidizing species are the free acid (ArSO2NHBr), ArSO2NBr2 and HOBr. The involvement of ArSO2NBr2 in the mechanism leads to a second-order rate law, which is contrary to the experi- mental observations, as Equation (4) indicates. If a slow hydrolysis of ArSO2NHBr occurred as in Equation (5), leading to HOBr as the primary oxidizing species, a first- order retardation of the rate by the added ArSO2NH2 would be expected. This is contrary to the experimental results. Hardy and Johnston [22], who have studied the pH-dependence of relative concentrations of the species present in acidified chloramine-T solutions of compara- ble molarities, have shown that ArSO2NHBr is the likely oxidizing species in acid medium. Furthermore, ultraviolet spectral measurements showed that the aqueous piperazine solutions have a sharp ab- sorption band at 235 nm, while the BAT solution exhibits a peak around 287 nm, both in pH 4.0 buffer solutions and water. A mixture of BAT and piperazine shows a max around 330 nm which indicates no direct reaction between BAB and piperazines and no deprotanation from BAB. However, piperazine in pH 4.0 buffer exhibits a max at 380 nm, which shows a longer shift indicating the formation of intermediate S' due to deprotanation of the substrate. Based on the preceding discussion, Scheme 1 below is proposed for the reaction. A detailed common mode of oxidation of piperazines by BAT in acidic buffer medium along with structures of intermediates is depicted in Scheme 2. 3.7. Rate Law Derivation From the slow step of Scheme 1, 2 RatekS' BAT (7) From step (1), 0 1 S' H SK (8) Also, 0 SSS t ' (9) Combination of Equations (8) and (9) leads to Equa- tion (10) 1 0 SS'H Piperazine K i) fast 2 k 22 complex S'ArSO NHBrXArSO NH ii) slow 2 222 XH OProducts ArSO NHHArSO NH iii) fast Here Ar = p-Me-C 6 H 4 Scheme 1. General mechanism for the oxidation of pipera- zines by BAT in pH 4 buffer. Table 3. Effect of varying temperature and activation parameters for the oxidation of piperazines by BAT in acidic buffer [piperazine]0 = 1.00 × 10−2 mol·dm−3; [BAT]0 = 5.00 × 10−4 mol·dm−3; pH = 4.0. k' 104 (s−1) H≠ S≠ G≠ Ea Substrate 298 303 308 313 (kJ mol−1) (JK−1 mol−1) (kJ mol−1) (kJ mol−1) Piperazine 1.47 2.18 3.38 7.41 79.0 −55.6 95.4 81.6 1-Methylpiperazine 2.61 3.90 6.45 10.7 71.0 −76.0 94.16 73.5 1-Ethylpiperazine 3.71 6.0 8.77 12.6 60.2 −106.6 92.7 62.7 Copyright © 2013 SciRes. MRC 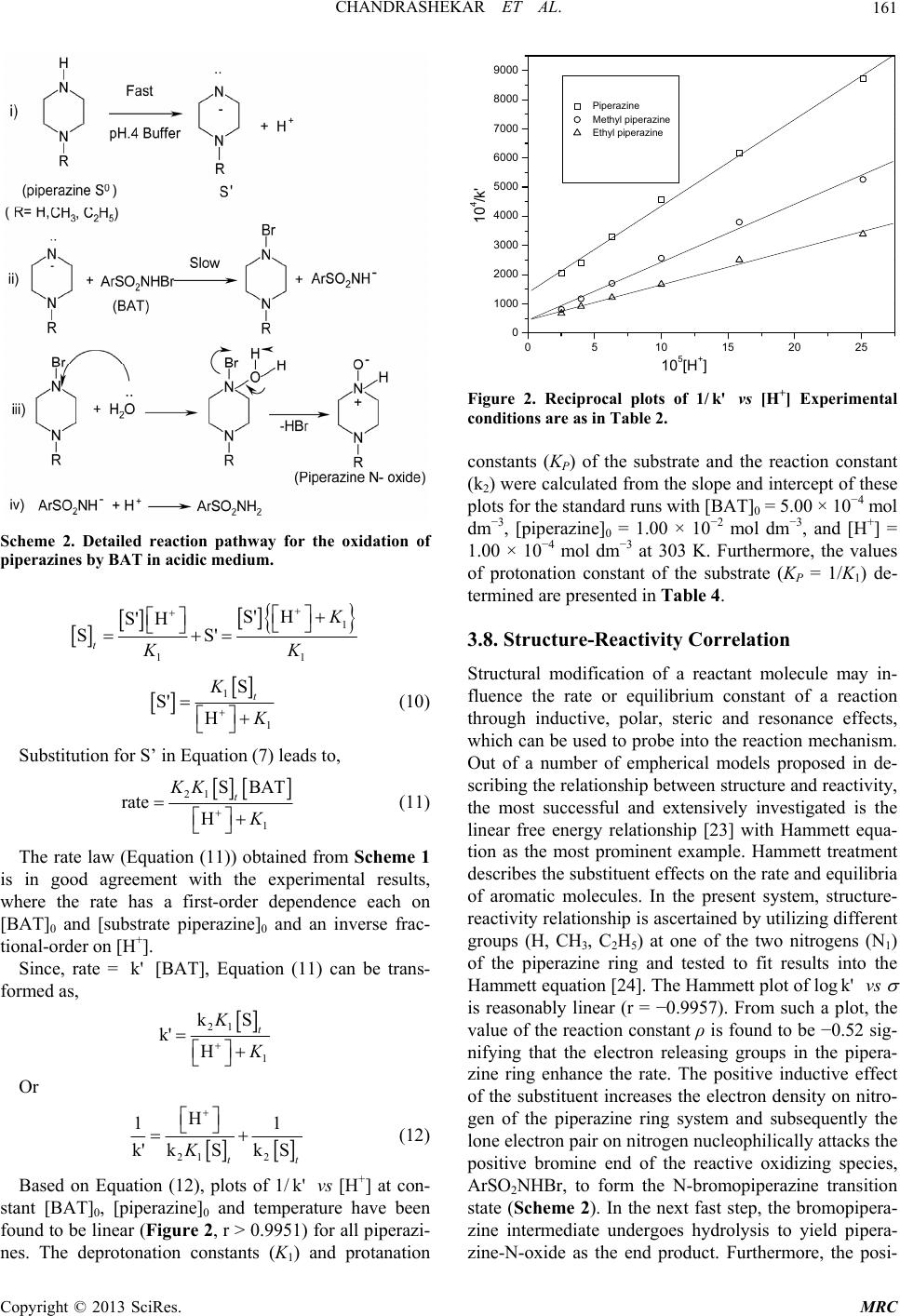 CHANDRASHEKAR ET AL. 161 Scheme 2. Detailed reaction pathway for the oxidation of piperazines by BAT in acidic medium. 1 11 S' H S' H SS' t KK 1 1 S S' H t K (10) Substitution for S’ in Equation (7) leads to, 21 1 SBAT rate H t KK K (11) The rate law (Equation (11)) obtained from Scheme 1 is in good agreement with the experimental results, where the rate has a first-order dependence each on [BAT]0 and [substrate piperazine]0 and an inverse frac- tional-order on [H+]. Since, rate = [BAT], Equation (11) can be trans- formed as, k' 21 1 kS k' H t K Or 21 2 H 1 k'kSk S tt K 1 (12) Based on Equation (12), plots of 1/k' vs [H+] at con- stant [BAT]0, [piperazine]0 and temperature have been found to be linear (Figure 2, r > 0.9951) for all piperazi- nes. The deprotonation constants (K1) and protanation 0510 15 20 25 0 1000 2000 3000 4000 5000 6000 7000 8000 9000 Piperazine Methyl piperazine Ethyl piperazi ne 104/k' 105[H+] Figure 2. Reciprocal plots of 1/ vs [H+] Experimental conditions are as in Table 2. k' constants (KP) of the substrate and the reaction constant (k2) were calculated from the slope and intercept of these plots for the standard runs with [BAT]0 = 5.00 × 10−4 mol dm−3, [piperazine]0 = 1.00 × 10−2 mol dm−3, and [H+] = 1.00 × 10−4 mol dm−3 at 303 K. Furthermore, the values of protonation constant of the substrate (KP = 1/K1) de- termined are presented in Table 4. 3.8. Structure-Reactivity Correlation Structural modification of a reactant molecule may in- fluence the rate or equilibrium constant of a reaction through inductive, polar, steric and resonance effects, which can be used to probe into the reaction mechanism. Out of a number of empherical models proposed in de- scribing the relationship between structure and reactivity, the most successful and extensively investigated is the linear free energy relationship [23] with Hammett equa- tion as the most prominent example. Hammett treatment describes the substituent effects on the rate and equilibria of aromatic molecules. In the present system, structure- reactivity relationship is ascertained by utilizing different groups (H, CH3, C2H5) at one of the two nitrogens (N1) of the piperazine ring and tested to fit results into the Hammett equation [24]. The Hammett plot of log vs is reasonably linear (r = −0.9957). From such a plot, the value of the reaction constant ρ is found to be −0.52 sig- nifying that the electron releasing groups in the pipera- zine ring enhance the rate. The positive inductive effect of the substituent increases the electron density on nitro- gen of the piperazine ring system and subsequently the lone electron pair on nitrogen nucleophilically attacks the positive bromine end of the reactive oxidizing species, ArSO2NHBr, to form the N-bromopiperazine transition state (Scheme 2). In the next fast step, the bromopipera- zine intermediate undergoes hydrolysis to yield pipera- zine-N-oxide as the end product. Furthermore, the posi- k' Copyright © 2013 SciRes. MRC  CHANDRASHEKAR ET AL. 162 Table 4. Protonation and deprotonation constants for the oxidation of piperazines by BAT in acidic medium at 303 K. Substrate 10−5 K1 (mol·dm−3) 104 KP (dm3·mol−1) k2 (dm3·mol−1s−1) Piperazine 4.65 2.14 0.072 1-Methylpiperazine 2.21 4.51 0.226 1-Ethylpiperazine 3.64 2.74 0.226 tive inductive effect of the substituent in the piperazine ring system increases in the order: H < CH3 < C2H5, which justifies the observed reactivity trend of piperazine < 1-methylpiperazine < 1-ethylpiperazine. 3.9. Isokinetic Relationship The largest activation energy for the slowest reaction (Table 3) indicates that the reaction is enthalpy con- trolled, within the reaction series. The variation in the rate may be caused by changes in either the enthalpy or entropy of activation or both. In this study, enthalpy and entropy of activation are correlated by H≠ = H≠ 0 + S≠, which is called the isokinetic relationship where β is the isokinetic temperature. When the experimental temperature T < β, the reaction rate is controlled mainly by the enthalpy change. In the present case, the piperazi- nes oxidations are linearly related by plotting H≠ vs S≠ (Figure 3, r = 0.9995). From the slope, the value of isokinetic temperature (β) is computed to be 368 K. The determined β value of 368 K being higher than the ex- perimental temperature of 303 K, suggests that the reac- tion is enthalpy controlled. The existence of isokinetic relationship is very valuable to the mechanistic chemist as this can be used as a supportive evidence for the me- chanism along with other data. The large negative value of S≠ indicates a more ordered associative transition state with less degree of freedom. The near constant G≠ values show an identical common mechanistic pathway in the oxidation of all the piperazines studied. Further- -110-100-90 -80 -70 -60 -50 60 65 70 75 80 H ≠ (kJ mol −1 ) S (JK −1 mol −1 ) Figure 3. Isokinetic plot of H≠ vs S≠. more, the independent nature of the rate towards the ad- dition of p-toulenesulfonamide, halide ion and neutral salts supports the proposed mechanism and the rate law derived. 4. Conclusion Kinetics of oxidation of three piperazines using Broma- mine-T as the oxidant was carried out in acid medium. A mechanism has been proposed and the rate law has been derived. The Hammett correlation of the substituent ef- fect shows a linear free energy relationship with = −0.52 indicating that electron-donating centers enhance the rate of reaction. An isokinetic study indicates that enthalpy rather than entropy factors controls the reaction rate. 5. Acknowledgements Chandrashekar thanks the management of PES College of Engineering, Mandya, Karnataka for granting permis- sion to undertake this study and for encouragement. REFERENCES [1] W. R. Maynard Jr., “Gravimetric and Infra-Red Spectro- photometric Determination of Piperazine,” Journal of the Association of Official Analytical Chemists, Vol. 42, 1961, pp. 610-612. [2] G. L. Crenshaw and R. J. Shaver, “Down to Earth,” Dow Chemical Company, Michigan, 1956. [3] L. S. Goodman and A. Gilman, “The Pharmacological Basis of Therapeutics,” 2nd Edition, Macillan Company, New York, 1956. [4] R. M. Ioset, “Personal Communication,” Dow Chemical Company, Midland, Michigan, 1961. [5] T. J Mc Nair, F. A. Wibin, E. T. Hoppe, J. L. Schmidt and F. A. de Peyster, “Antitumor Action of Several New Piperazine Derivatives Compared to Certain Standard Anticancer Agents,” Journal of Surgical Research, Vol. 3, No. 3, 1963, pp. 130-136. http://dx.doi.org/10.1016/S0022-4804(63)80014-1 [6] V. A. Mikhalev, M. I. Dorokhova and N. E. Smolina, “Prospidine and Some Other Derivatives of Steropoly Piperazine,” Meditsinskaia promyshlennost’ SSSR, Vol. 17, No. 1, 1963, pp. 17-20. [7] W. O. Foye and D. H. Kay, “Antiradiation Compounds III. N-2-Mercaptoethylpiperazines” Journal of Pharma- Copyright © 2013 SciRes. MRC  CHANDRASHEKAR ET AL. 163 ceutical Sciences, Vol. 51, No. 11, 1962, pp. 1098-1101. http://dx.doi.org/10.1002/jps.2600511120 [8] A. Jouve, A. Grass and R. Benyamine, “Hemodynamic Studies during Angina Pectoris,” Vie Med., Vol. 44, 1963, pp. 115-120. [9] K. K Aravindakshan and K. Muraleedharan, “Kinetics of Non-Isothermal Decomposition of Polymeric Complexes of N,N’-Bis(dithiocarboxy)piperazine with Iron(III) and Cobalt(III)” Thermochimica Acta, Vol. 159, 1990, pp. 101-107. http://dx.doi.org/10.1016/0040-6031(90)80098-J [10] M. M. Campbell and G Johnson, “Chloramine T and Re- lated N-Halogeno-N-Metallo Reagents,” Chemical Re- views, Vol. 78, No. 1, 1978, pp. 65-79. http://dx.doi.org/10.1021/cr60311a005 [11] K. K. Banerji, B. Jayaram and D. S. Mahadevappa, “Mechanistic Aspects of Oxidations by N-Metallo-N- Haloarylsulfonamides,” Journal of Scientific & Industrial Research, Vol. 46, 1987, pp. 65-76. [12] F. Ruff and A. F. Kucsman, “Oxidation of Dialkylsul- hides with BAT in Alkaline Buffer Solutions,” Journal of the Chemical Society, Perkin Transactions II, 1990, p. 1075. [13] D. S. Mahadevappa and Puttaswamy, “Kinetics of Oxida- tion of Aliphatic Ketones with Bromamine-T in Acid Me- dium,” Bulletin of the Chemical Society of Japan, Vol. 61, No. 2, 1988, pp. 543-547. http://dx.doi.org/10.1246/bcsj.61.543 [14] K. S. Rangappa, H. Ramachandra, D. S. Mahadevappa and N. M. Made Gowda, “Osmium (VIII) Catalyzed Ki- netics and Mechanism of Indoles Oxidation with Aryl-N- Haloamines in Alkaline Medium,” International Journal of Chemical Kinetics, Vol. 28, No. 4, 1996, pp. 265-274. http://dx.doi.org/10.1002/(SICI)1097-4601(1996)28:4<26 5::AID-KIN4>3.0.CO;2-T [15] Puttaswamy and R. Ramachndrappa, “Ruthenium(III)- Catalyzed Oxidation of Substituted Ethanols by Sodium N-Bromo-p-Toluenesulfonamide in Hydrochloric Acid Medium,” Transition Metal Chemistry, Vol. 24, No. 3, 1999, pp. 326-332. http://dx.doi.org/10.1023/A:1006931803005 [16] C. G. R Nair and P Indrasenan, “New Redox Titrants in Non-Aqueous or Partially Aqueous Media—VI1: Poten- tiometric Determinations Using Dibromamine-T and Some Further Applications of Dichloramine-T,” Talanta, Vol. 23, No. 3, 1976, pp. 239-241. http://dx.doi.org/10.1016/0039-9140(76)80178-6 [17] M. S. Ahmed and D. S. Mahadevappa, “Bromamine-B as a New Oxidimetric Titrant,” Talanta, Vol. 27, No. 8, 1980, pp. 669-670. http://dx.doi.org/10.1016/0039-9140(80)80207-4 [18] R. M. C. Dawson, D. C. Elliott, W. Elliott and K. M. Jones, “Data for Biochemical Research,” 3rd Edition, Oxford Science Publications, 1986. [19] D. S. Mahadeveppa and N. M. Made Gowda, “Estimation of Glutathione with Chloramine-T and Dichloramine-T,” Talanta, Vol. 22, No. 9, 1975, pp. 771-773. http://dx.doi.org/10.1016/0039-9140(75)80226-8 [20] E. Bishop and V. J. Jennings, “Titrimetric analysis with chloramine-T—I: The status of chloramine-T as a ti- trimetric reagent,” Talanta, Vol. 1, No. 3, 1958, pp. 197- 212. http://dx.doi.org/10.1016/0039-9140(58)80034-X [21] D. S. Mahadevappa and Rangaswamy, “Physico Chemi- cal Properties of Chloramine-T. II. Conductometric study of the Interaction of Chloramine-T with Chromium(III), Aluminium(III) and Iron(III) Solutions,” Revue Roumaine de Chimie, Vol. 22, 1977, pp. 1233-1241. [22] F. F Hardy and J. P. J Johnston, “The Interactions of N- Bromo-N-Sodiobenzenesulphonamide (Bromamine-B) with p-Nitrophenoxide Ion,” Journal of the Chemical Society, Perkin Transactions II, No. 6, 1973, pp. 742-746. [23] R. D Gilliom, “Introduction to Physical Organic Chemis- try”, Addison Wesley, London, 1970, p. 144. [24] L. P. Hammett, “The Effect of Structure upon the Reac- tions of Organic Compounds. Benzene Derivatives,” Jour- nal of the American Chemical Society, Vol. 59, No. 1, 1937, pp. 96-103. http://dx.doi.org/10.1021/ja01280a022 Copyright © 2013 SciRes. MRC
|