Paper Menu >>
Journal Menu >>
 Vol.3, No.2, 110-1 15 (2011) Health doi:10.4236/health.2011.32020 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Efficacy of a synbiotic chewable tablet in the prevention of antibiotic-associated diarrhea Charles Spielholz Nutraceutical Medical Research, 445 Hamilton Avenue, Suite 1102, White Plains, New York, 10601. USA. cspielholz@nutraceuticalmedicalresearch.com Received 4 January 2011; revised 24 January 2011; accepted 27 January 2011 ABSTRACT Infection by Clostridium difficile, a complication of treatment with antibiotics, causes antibiotic- associated diarrhea (AAD) and can lead to coli- tis and pseudomembranous colitis. Incidence of C. difficile infection is increasing among the elderly undergoing antibiotics therapy confined to health care facilities, conditions that are ex- pensive to treat, decrease the quality of life and are life threatening. Use of probiotics has been proposed as a method to decrease the inci- dence of AAD in health care facilities. To exam- ine the efficacy of using probiotics, 120 nursing home residents undergoing antibiotic therapy were provided w ith a synbiotic tablet containing two probiotics, Saccharomyces boulardii and Bacillus coagulans, and a prebiotic, fructooli- gosaccharide. Residents were evaluated retro- spectively for AAD and C. difficile infection. It was found that 95% of residents treated with antibiotics and taking the synbiotic tablet were free of AAD. More than 97% of the residents did not become infected with C. difficile. No adv erse effects were reported. Minor side effects, gas- trointestinal up set and nausea, were reported by less than 6% of the residents. The cause of the minor side effects was not know n. Only 2.5% of the residents stopp ed taking the synbiotic t ablet because of the gastrointestinal upset. These Results suggest that use of the synbiotic tablet prevents AAD and C. difficile infection in nurs- ing home residents undergoing antibiotic ther- apy. It is concluded that this synbiotic tablet provides an easy to administer and safe ap- proach to controlling AAD and C. difficile infec- tion in residents in nursing homes. Keywords: Synbiotic; Saccharomyces Boulardii; Bacillus Coagulans; Antibiotic-Associated Diarrhea; Clostridium Difficile 1. INTRODUCTION Antibiotics have significantly decreased mortality re- sulting from infectious disease and increased the success rates of many medical procedures such as surgery. However, use of an tib iotics also causes significant losses to the population of beneficial microbiota residing in the digestive tract. The digestive tract is the front line of defense against infection, representing a significant por- tion of the total immune system [1]. Loss of native pop- ulations of beneficial microbiota exposes the intestinal mucosa and allows non-beneficial and pathological spe- cies, including those that are antibiotic-resistant, to po- pulate the intestine [2 ,3]. This results in a variety of bio- logical changes in the digestive tract including changes in immune function, inflammatory response and normal metabolism [4]. Such changes increase susceptibility to antibiotic-associated diarrhea (AAD) and antibiotic- resistant infectious diseases [2]. Chronic use of broad-spectrum antibiotics in elderly residents in health care facilities, such as nursing homes, long-term care facilities, and hospitals, is leading to a serious increase in the incidence of AAD [5,6]. AAD occurs in 25% to 50% of residen ts taking antibiotic [7 ,8]. Clostridium difficile, a bacterial, spore-forming, anaero- bic species that infects the human digestive tract, is re- sistant to many commonly used antibiotics and is the cause of C. difficile-associated diarrhea (CDAD). CDAD represents 15% to 25% of all AAD occurring in health care facilities [8,9]. C. difficile is the cause of approxi- mately half of all cases of antibiotic-associated colitis and almost all cases of pseudomembranous colitis, a life threatening inflammation of the colon [9,11]. There are now over 250,000 C. difficile infections requiring hospi- talization in the United States each year [10] causing an estimated 15,000 to 30,000 deaths each year. Nationally, the cost of treating C. difficile infections is greater than $1 billion per year [4].  C. Spielholz / Health 3 (2011) 110-115 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 111111 Treatment of C. difficile infection requires discon- tinuation of antibiotic therapy, if appropriate, intrave- nous fluids and administration of either metronidazole or vancomycin [13-16]. Reinfection occurs in up to 30% of those successfully treated [18,19]. Furthermore, use of metronidazole or vancomycin contributes to the increase in antibiotic resistant species [17,19]. Therefore, sup- plemental and alternate methods using probiotics in the treatment and prevention of C. difficile infection have been proposed [20,21]. The idea behind administration of probiotics is to replace the normal population of beneficial microbes that are lost from the digestive tract during antibiotic treatment [21]. Probiotic microbes sti- mulate the immune system of the gut and suppress the growth of pathological species [2,3]. In this report, the results of a retrospective analysis of residents in nursing homes undergoing treatment with antibiotics and taking a chewable synbiotic tablet as an adjunctive preventative of AAD are presented. The syn- biotic tablet contained the nonpathogenic yeast Sac- charomyces boulardii, the bacteria Bacillus coagulans, and a prebiotic, fructooligosaccharide. The purpose of this retrospective study was to analyze and understand the feasibility of administering a synbiotic tablet to resi- dents in a nursing home environment, analyze the poten- tial efficacy with regard to AAD, C. difficile infection, and CDAD and to define any safety and tolerability is- sues. 2. METHODS Residents in 17 nursing homes receiving antibiotic treatment and taking the synbiotic tablet as part of their standard of care from September 2009 to November 2009 were evaluated retrospectively for the presence of AAD and C. difficile infection. The resident population analyzed in this study consisted of 120 people. There were 77 females representing 64.2% of the resident pop- ulation, and 43 males representing 35.8% of the study population. The residents ranged in age from 40 to 96 years with an average age of 80 10 years (Table 1). Twenty six of the residents, representing 20.8% of the study population, had a prior history of infection with C. difficile, and 82 of the residents, representing 68.3% of the population, d id not have a pr ior history of C. difficile infection. The C. difficile history of 15 of the 120 resi- dents, 12.5% of the study population, was not known (Table 1). The synbiotic tablet was in the form of a chewable tablet containing two probiotics and one prebiotic. The two probiotics were 7.5 b illion colon y forming un its (cfu ) of the yeast Saccharomyces boulardii and 1 billion cfu of the ba cteria Bacillus coagulans. The prebiotic present in the tablet was 500 mg of fructooligosaccharide. Resi- Table 1. Demographics of the study population. Study Population n = 120 Gender n (%) Female 77 (64.2%) Male 43 (35.8%) Age years A verage Age 80 10 Age Range 40-96 Prior C. difficile History n (%) No Prior History 82 (68.3%) Known Prior History 25 (20.8%) Prior History Not Known 15 (12.5%) dents were started on the synbiotic tablet shortly after beginning treatment with an antibiotic. The synbiotic tablet was given twice a day. Administration of the syn- biotic tablet continued for two weeks after antibiotic treatment was completed at which time residents were evaluated for the presence of AAD and C. difficile infec- tion. Demographic data included the sex and age of each resident and prior medical history with regard to C. diffi- cile infection. In additio n, all adverse events, sid e effects, resident compliance, and ease of administration with regard to taking the synbiotic tablet were noted. All data were obtained from resident records through a nurse employed by each nursing home using a questionnaire. Residents were not known by name but were assigned a code to protect their specific identity and privacy. Statistical analysis of all numerical results was con- ducted by calculating the average and standard devia- tion. 3. Results A total of 128 nursing home residents being adminis- tered antibiotics were given a chewable synbiotic tablet twice a day from September, 2009 to November, 2009. Of the 128 residents offered the synbiotic tablet, 120 were evaluated for the purpose of this study and consti- tuted the study population. Eight residents were not eva- luated because the data received from the nursing home was incomplete. Most residents started the synbiotic regimen within 3 to 4 days after beginning antibiotic treatment. The aver- age delay between initial treatment with antibiotics and the start of ingestion of the synbiotic tablet was 2 4 days. Slightly more than one-half of the 120 residents, 64, began taking the synbiotic tablet immediately after beginning antibiotic treatment (that is, beginning at 0 days). 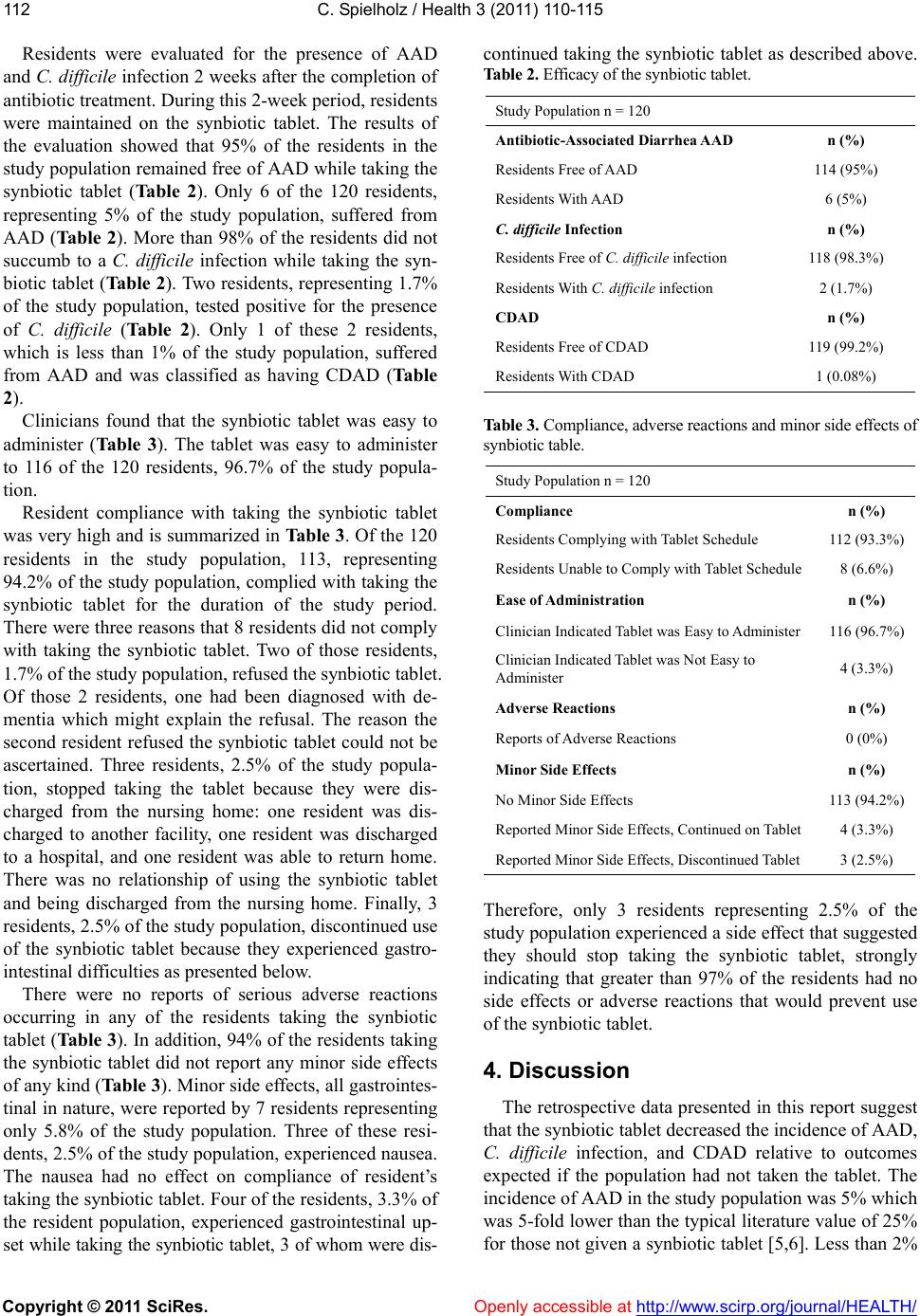 C. Spielholz / Health 3 (2011) 110-115 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 112 Residents were evaluated for the presence of AAD and C. difficile infection 2 weeks after the completio n of antibiotic treatment. During this 2-week period, residents were maintained on the synbiotic tablet. The results of the evaluation showed that 95% of the residents in the study population remained free of AAD while taking the synbiotic tablet (Ta ble 2). Only 6 of the 120 residents, representing 5% of the study population, suffered from AAD (Table 2). More than 98% of the residents did not succumb to a C. difficile infection while taking the syn- biotic tablet (Table 2). Two residents, representing 1.7% of the study population, tested positive for the presence of C. difficile (Table 2). Only 1 of these 2 residents, which is less than 1% of the study population, suffered from AAD and was classified as having CDAD (Table 2). Clinicians found that the synbiotic tablet was easy to administer (Table 3). The tablet was easy to administer to 116 of the 120 residents, 96.7% of the study popula- tion. Resident compliance with taking the synbiotic tablet was very high and is summarized in Table 3. Of th e 120 residents in the study population, 113, representing 94.2% of the study population, complied with taking the synbiotic tablet for the duration of the study period. There were three reasons that 8 residents did not comply with taking the synbiotic tablet. Two of those residents, 1.7% of the study population, refused the synbiotic tablet. Of those 2 residents, one had been diagnosed with de- mentia which might explain the refusal. The reason the second resident refused the synbiotic tablet could not be ascertained. Three residents, 2.5% of the study popula- tion, stopped taking the tablet because they were dis- charged from the nursing home: one resident was dis- charged to another facility, one resident was discharged to a hospital, and one resident was able to return home. There was no relationship of using the synbiotic tablet and being discharged from the nursing home. Finally, 3 residents, 2.5% of the study population, discontinued use of the synbiotic tablet because they experienced gastro- intestinal difficulties as presented below. There were no reports of serious adverse reactions occurring in any of the residents taking the synbiotic tablet (Table 3). In ad dition, 94% of the residents taking the synbiotic tablet did not report any minor side effects of any kind (Table 3). Minor side effects, all gastrointes- tinal in nature, were reported by 7 residents representing only 5.8% of the study population. Three of these resi- dents, 2.5% o f the stud y population, experien ced naus ea. The nausea had no effect on compliance of resident’s taking the synbiotic tablet. Four of the residents, 3.3% of the resident population, experienced gastrointestinal up- set while taking the synbiotic tablet, 3 of whom were dis- continued taking the synbiotic tablet as described above. Table 2. Ef ficacy of the s ynbiotic tablet. Study Population n = 120 Antibiotic-Associated Diarrhea AAD n (%) Residents Free of AAD 114 (95%) Residents With AAD 6 (5%) C. difficile Infection n (%) Residents Free of C. difficile infection 118 (98.3%) Residents With C. difficile infection 2 (1.7%) CDAD n (%) Residents Free of CDAD 119 (99.2%) Residents With CDAD 1 (0.08%) Table 3. Compliance, adverse reactions and minor side effects of synbiotic table. Study Population n = 120 Compliance n (%) Residents Complying with Tablet Schedule 112 (93.3%) Residents Unable to Comply with Tablet Schedule 8 (6.6%) Ease of Administration n (%) Clinician Indicated Tablet was Easy to Admi nister 116 (96.7%) Clinician Indicated Tablet was Not Easy to Administer 4 (3.3%) Adverse Reactions n (%) Reports of Adverse Reactions 0 (0%) Minor Side Effects n (%) No Minor Side Effects 113 (94.2%) Reported Minor Side Effects, Continued on Tablet 4 (3.3%) Reported Minor Side Effects, Discontinued Tablet 3 (2.5%) Therefore, only 3 residents representing 2.5% of the study population experienced a side effect that suggested they should stop taking the synbiotic tablet, strongly indicating that greater than 97% of the residents had no side effects or adverse reactions that would prevent use of the synbiotic tablet. 4. Discussion The retrospective data presen ted in this report suggest that the synbiotic tablet de creased the incidence of AAD, C. difficile infection, and CDAD relative to outcomes expected if the population had not taken the tablet. The incidence of AAD in the study population was 5% which was 5-fold lower than the typical literature value of 25% for those not given a synbiotic tablet [5,6]. Less than 2%  C. Spielholz / Health 3 (2011) 110-115 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 113113 of the study population was infected with C. difficile, which is 4- to 16-fold lower than the expected 8% to 33% incidence of infection observed in health care fa- cilities [22]. Less than 1% of the study population was afflicted with CDAD, which is as much 15-fold lower than the predicted rates of CDAD for residents in health care facilities. The low incidence of C. difficile infection observed in the study population is also notable because it is at least 60% lower than the incidence expected from recurring infections. Of the residents in the study population, ap- proximately 21% had a prior history of C. difficile infec- tion. Based on recurrence rate of 25% [7,17,22], more than 5% of the study population would have been ex- pected to present a C. difficile infection; however only 1.7% of the study population presented a C. difficile in- fection, indicating that the synbiotic tablet suppressed C. difficile infection by 66%. The data presented in this report agree well with pr ior studies using probiotic or synbiotic supplements. In this report, the synbiotic tablet prevented AAD, C. difficile infection, and CDAD by an estimated 75% or more rela- tive to expected values. Reported clinical trials have shown that probiotic supplements containing S. boulardii or B. coagulans decreased levels of AAD by 50% to 85% [23,25] and CDAD by 45% to 85% [26-28]. Me- ta-analysis of multiple clinical trials has shown that pro- biotics decrease ADD and CDAD by 40% to 60% [29,30]. The mechanisms by which the components of the synbiotic tablet used in this study, S. boulardii, B. co- agulans, and fructooligosaccharide, function to reduce AAD, C. difficile infection, and CDAD have begun to be elucidated. Each of the components of the synbiotic tab- let appear to inhibit AAD, C. difficile infection, and CDAD through different mechanisms. Studies have shown that S. boulardii, can up-regulate IgA expression against C. difficile toxin A [31,32]. S. boulardii may a ls o inhibit C. difficile toxin directly [33-35]. Furthermore, S. boulardii, has been shown to inhibit inflammatory sig- naling pathways which may result in decreased damage to the digestive tract by pathological species [36,37]. In combination with fructooligosaccharide, B. coagu- lans has been shown to have properties that inhibit spe- cies that cause AAD [38]. Fructooligosaccharide has been shown to increase the growth of beneficial bacteria such as bifidobacteria [39]. Bifidobacteria cause an in- crease in short-chain fatty acid concentration and a de- crease in the pH in the colon which results in condition s that are not conducive to the growth of certain patho- genic organisms. This may help restore colonization resistance in the digestive tract [40,41]. Use of the syn- biotic tablet in this study containing components that function through different mechanisms increases the probability of success. No serious adverse reactions were reported while any of the residents were taking the synbiotic tablet. Minor side effects involved nausea or gastrointestinal upset. It is not clear that the synbiotic tablet was the actual cause of the nausea or gastrointestinal upset. The synbiotic tablet was easy to administer with clinicians finding that nearly 97% of the residents had no difficulty ingesting the tablet. Resident compliance with tak ing the synbiotic tablet was very high, with over 93% of residents using the tablet as directed, indicating that the synbiotic tablet was well tolerated by the study population. There are limitations regarding the interpretation of the results of this retrospectiv e study. The first is that th e evaluations for AAD and the presence of C. difficile were performed 2 weeks after the completion of antibi- otic treatment. Although 80% of AAD cases occur 4 to 5 days after commencing antibiotic treatment, AAD can occur up to 2 months after the initial treatment with an- tibiotic. Therefore it is po ssible that some cases of AAD and C. difficile infection were missed. Second, this stud y did not examine a dose response. It is possible that an increased dose of the synbiotic may further inhibit C. difficile infections [5,42]. Finally, this study focused solely on adult residents in nursing homes and not pa- tients in hospitals. However, it is believed that the results of this report will be applicable to settings other than nursing homes. The data presented in this report suggest that the syn- biotic tablet can prevent AAD and C. difficile infection in residents in a nursing home who receive antibiotics. Development of an approach using synbiotics to replace beneficial microbes lost in the digestive tract during an- tibiotic treatment could significantly reduce the inci- dence of antibiotic-resistant infections and the preva- lence of AAD in health care facilities. Use of synbiotics could also decrease reliance on antibiotics and thus re- duce the rise in antibiotic-resistant pathogens. Further- more, synbiotics could reduce the treatment costs asso- ciated with residents suffering from antibiotic-resistant infections and AAD. For example, it has been shown that decreases in CDAD observed with ad ministration of probiotics are associated with decreases in colitis [26]. The synbiotic tablet used in this study can be incorpo- rated into the health care protocols of health care facili- ties as an easy to administer, safe, acceptable, and easy to tolerate approach to preventing AAD and C. difficile infections. REFERENCES [1] Furness, J.B., Kunze, W.A. and Clerc, N. (1999) Nutrient tasting and signaling mechanisms in the gut. II. The in-  C. Spielholz / Health 3 (2011) 110-115 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 114 testine as a sensory organ: neural, endocrine, and im- mune responses. American Journal of Physiology, 277, 922-928. [2] Borriello, S.P. and Barclay, F.E. (1986) An in-vitro model of colonisation resistance to Clostridium difficile infec- tion. Journal of Medical Microbiology, 21, 299-309. doi:10.1099/00222615-21-4-299 [3] Borriello, S.P. (1990) The influence of the normal flora on Clostridium difficile colonisation of the gut. Annals of Medicine, 22, 61-7. doi:10.3109/07853899009147244 [4] Isakow, W., Morrow, L.E. and Kollef, M.H. (2007) Pro- biotics for preventing and treating nosocomial infections: review of current evidence and recommendations. Chest, 132, 286-294. doi:10.1378/chest.06-2156 [5] Garibaldi, R.A. (1999) Residential care and the elderly: the burden of infection. Journal of Hospital Infection, 43, supplement, S9-18. doi:10.1016/S0195-6701(99)90061-0 [6] Makris, A.T. and Gelone, S. (2007) Clostridium difficile in the long-term care setting. Journal of the American Medical Directors Association, 8, 290-299. doi:10.1016/j.jamda.2007.01.098 [7] Gao, X.W., Mubasher, M., Fang, C.Y., Reifer, C. and Miller, L.E. (2010) Dose-response efficacy of a proprie- tary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibi- otic-associated diarrhea and Clostridium diffi- cile-associated diarrhea prophylaxis in adult patients. The American Journal of Gastroenterology, 105, 1636-1641. doi:10.1038/ajg.2010.11 [8] Katz, J.A. (2006) Probiotics for the prevention of antibi- otic-associated diarrhea and Clostridium difficile diarrhea. Journal of Clinical Gastroenterology, 40, 249-255. doi:10.1097/00004836-200603000-00017 [9] Pochapin, M. (2000) The effect of probiotics on Clos- tridium difficile diarrhea. American Journal of Gastroen- terology, 95, S11-S13. doi:10.1016/S0002-9270(99)00809-6 [10] Bartlett, J.G., Chang, T.W. Gurwith, M., Gorbach, S.L. and Onderdonk, A.B. (1978) Antibiotic-associated pseu- domembranous colitis due to toxin-producing clostridia. The New England Journal of Medicine, 298, 531-534. doi:10.1056/NEJM197803092981003 [11] Bartlett, J.G., Willey, S.H., Chang, T.W. and Lowe, B. (1979) Cephalosporin-associated pseudomembranous co- litis due to Clostridium difficile. The Journal of the American Medical, 242, 2683-2685. doi:10.1001/jama.242.24.2683 [12] Ricciardi, R., Rothenberger, D.A., Madoff R.D., and Baxter, N.N. (2007) Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Archives of Surgery, 142, 624-631. doi:10.1001/archsurg.142.7.624 [13] Fekety, R. (1997) Guidelines for the diagnosis and man- agement of Clostridium difficile-associated diarrhea and colitis, American College of Gastroenterology, Practice Parameters Committee. American Journal of Gastroen- terology, 92, 739-750. [14] Aslam, S., Hamill, R.J. and Musher, D.M. (2005) Treat- ment of Clostridium difficile-associated disease: old therapies and new strategies. The Lancet Infectious Dis- eases, 5, 549-557. doi:10.1016/S1473-3099(05)70215-2 [15] Gerding, D.N., Muto, C.A. and Owens Jr., R.C. (2008) Treatment of Clostridium difficile infection. Clinical In- fectious Diseases, 46, supplement, 32-S42. doi:10.1086/521860 [16] Halsey, J. (2008) Current and future treatment modalities for Clostridium difficile-associated disease. American Journal of Health-System Pharmacy, 65, 705-715. doi:10.2146/ajhp070077 [17] Musher, D.M., Aslam, S., Logan, N., Nallacheru, S., Bhaila, I., Borchert, F. and Hamill, R.J. (2005) Relatively poor outcome after treatment of Clostridium difficile co- litis with metronidazole. Clinical Infectious Diseases, 40, 1586-1590. doi:10.1086/430311 [18] Gerding, D.N., Johnson, S., Peterson, L.R., Mulligan, M.E. and Silva Jr., J. (1995) Clostridium diffi- cile-associated diarrhea and colitis. Infection Control and Hospital Epidemiology, 16, 459-477. doi:10.1086/648363 [19] Pepin, J., Valiquette, L., Alary, M.E., Villemure, P., Pel- letier, A., Forget, K., Pepin, K. and Chouinard, D. (2004) Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Canadian Medical Association Journal, 171, 466-472. [20] Plummer, S., Weaver, M.A., Harris, J.C., Dee, P. and Hunter, J. (2004) Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. diffi- cile diarrhea. International Microbiology, 7, 59-62. [21] Hedge, D.D., Strain, J.D., Heins, J.R. and Farver, D.K. (2008) New advances in the treatment of Clostridium dif- ficile infection (CDI). Journal of Therapeutics and Clin- ical Risk Management, 4, 949-964. [22] Barbut, F. and Petit, J.C. (2001) Epidemiology of Clos- tridium difficile-associated infections. Clinical Microbi- ology and Infection, 7, 405-410. doi:10.1046/j.1198-743x.2001.00289.x [23] Surawicz, C.M., Elmer, G.W., Speelman, P., McFarland, L.V., Chinn, J. and van Belle, G. (1989) Prevention of an- tibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology, 96, 981-988. [24] McFarland, L.V., Surawicz, C.M., Greenberg, R.N., El- m er, G. W. , M o y er, K . A . M e l c her, S.A., Bowen, K.E. and Cox, J.L. (1995) Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. American Journal of Gastroenterology, 90, 439-448. [25] Can, M., Besirbellioglu, B.A., Avci, I.Y., Beker, C.M. and Pasha, A. (2006) Prophylactic Saccharomyces bou- lardii in the prevention of antibiotic-associated diarrhea: a prospective study. Medical Science Monitor, 2, 19-22. [26] Surawicz, C.M., Mc Farland, L.V. , Elmer, G. and Chinn, J. (1989) Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Ameri- can Journal of Gastroenterology, 84. 1285-1287. [27] McFarland, L.V., Surawicz, C.M., Greenberg, R.N., Fe- kety, R.G., Elmer, W., Moyer, K.A., Melcher, S.A. Bo- wen, K.E., Cox, J.L., Noorani, Z., Harrington, G., Rubin, M. and Greenwald, D. (1994) A randomized pla- cebo-controlled trial of Saccharomyces boulardii in com- bination with standard antibiotics for Clostridium difficile disease. The Journal of the American Medical, 271, 1913-1918. doi:10.1001/jama.271.24.1913 [28] Surawicz, C.M., McFarland, L.V., Greenberg, R.N., Ru-  C. Spielholz / Health 3 (2011) 110-115 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 115115 bin, M., Fekety, R., Mulligan, M.E., Garcia, R.J., Brandmarker, S., Bowen, K., Borjal, D. and Elmer, G.W. (2000) The search for a better treatment for recur- rent Clostridium difficile disease: use of high-dose van- comycin combined with Saccharomyces boulardii. Clin- ical Infectious Diseases, 31, 1012-1017. doi:10.1086/318130 [29] McFarland, L.V. (2006) Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. American Journal of Gastroenterology, 101, 812-822. doi:10.1111/j.1572-0241.2006.00465.x [30] Szajewska, H. and Mrukowicz, J. (2005) Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea. Alimentary Pharmacology and Therapeutics, 22, 365-372. doi:10.1111/j.1365-2036.2005.02624.x [31] Buts, J.P., De Key ser, N. and De Raedemaeker, L. (1994) Saccharomyces boulardii enhances rat intestinal enzyme expression by endoluminal release of polyamines. Pedi- atric Research, 36, 522-527. doi:10.1203/00006450-199410000-00019 [32] Qamar, A., Aboudola, S., Warny, M., Mitchetti, P., Pot- houlakis, C., Lamont, J.T. and Kelly, C.P. (2001) Sac- charomyces boulardii stimulates intestinal immu- noglobulin A immune response to Clostridium difficile toxin A in mice. Infection and Immunity, 69, 2762-2765. doi:10.1128/IAI.69.4.2762-2765.2001 [33] Castagliuolo, I., LaMont, J.T., Nikulasson, S.T. and Pot- houlakis, C. (1996) Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat il- eum. Infection and Immunity, 64, 5225- 5232. [34] Castagliuolo, I., Riegler, M.F., Va lenick, L., LaMont, J.T. and Pothoulakis, C. (1993) Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infection and Immu- nity, 67, 302-307. [35] Pothoulakis, C., Kelly, C.P., Joshi, M.A., Gao, N., O'Keane, C.J., Castagliuolo, I. and LaMont, J.T. (1993) Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastro- enterology, 104, 1108-15. [36] Chen, X., Kokkotou, E.G., Mustafa, N., Bhaskar, K.R., Sougioltzis, S., O'Brein, M., Pothoulakis, C. and Kelly, C.P. (2006) Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. The Journal of Biological Chemistry, 281, 24449-24454. doi:10.1074/jbc.M605200200 [37] Sougioultzis, S., Simeonidis, S., Bhaskar, K.R., Chen, X., Anton, P.M., Keates, S., Pothoulakis, C. and Kelly, C.P. (2006) Saccharomyces boulardii produces a soluble an- ti-inflammatory factor that inhibits NF-kappaB-mediated IL-8 gene expression. Biochemical and Biophysical Re- search Communications, 343, 69-76. doi:10.1016/j.bbrc.2006.02.080 [38] La Rosa, M., Bottaro, G., Gulino, N., Gambuzza, F., Di- Forti, F., Ini, G. and Tornambe, E. (2003) Prevention of antibiotic-associated diarrhea with Lactobacillus sporo- gens and fructo-oligosaccharides in children. A multicen- tric double-blind vs placebo study. Minerva Pediatrica, 55, 447-452. [39] Gibson, G.R., Beatty, E.R., Wang, X. and Cummings, J.H. (1995) Selective stimulation of bifidobacteria in the hu- man colon by oligofructose and inulin. Gastroenterology, 108, 975-982. doi:10.1016/0016-5085(95)90192-2 [40] Losada, M.A. and Olleros, T. (2002) Towards a healthier diet for the colon: the influence of fructooligosaccharides and lactobacilli on intestinal health. Nutrition research, 22, 71-84. doi:10.1016/S0271-5317(01)00395-5 [41] Garleb, K., Snowden, M., Wolf, B. and Chow, J. (2002) Application of fructooligosaccharides to medical foods as fermentable dietary fiber. Bioscience and Microflora, 21, 43-54. [42] Doron, S.I., Hibberd, P.L. and Gorbach, S.L. (2008) Pro- biotics for prevention of antibiotic-associated diarrhea. Journal of Clinical Gastroenterology, 42, supplement 2, S58-S63. doi:10.1097/MCG.0b013e3181618ab7 |

