Paper Menu >>
Journal Menu >>
 Open Journal of Urology, 2011, 1, 4-7 doi:10.4236/oju.2011.11002 Published Online February 2011 (http://www.SciRP.org/journal/oju) Copyright © 2011 SciRes. OJU A Two-Stage Buccal M u c osal Graft (BMG) for Managing Recurrent Proximal Penile Hypospadias in Pediatric & Adolescent Populations M. G. Elsheikh, A. Fayad Cairo University, Cairo, Egypt E-mail: mohamed.galalelsheikh@gmail.com , mohamed.galalelsheikh@gmail.com Received December 31, 2010; revised February 13, 2011; accepted February 20, 2011 Abstract Introductio n: The presence of a recur rent proxim al penile hypos padius represents a surgical challenge to the urologist due to the presence of e xcessive scarring an d fibrosis of the ti ssues. This problem is more pronounced in ci rcumcised patien ts, in whom there is no enough skin for one stage procedures. Buccal mucosal grafts represent a good surgical option. The aim of this study was to evaluate the results of two stages buccal mucosal urethroplasty in pediatric & adolescent patients, presenting with recurrent proximal penile hypospadias who are circumcised. Methods: Thirty seven pediatric & adoles- cent patients underwent two stages buccal mucosal urethroplasty for recurrent proximal penile hypospad ius. In all cases the buccal graft was place d dorsally followed by the second stage closure after 6 m onths. Results: The mean age was 17.7 (14-20) years. With a mean follow-up of 28.3 m onths, 33 patients (89.2%) ha d a final success ful outc om e. Of the 4 c ases that were co nsidered as fai lure, 3 patie nts (8.1%) deve loped uret hra-cutaneous fistula that required closu re after 3 m onths. The remaining patient developed meatal stenosis. Conclusion: Although buccal mucosal urethroplasty is a two staged procedure, i t is feasi ble opti on fo r pediatr ic & adol escent pat ients p resentin g with recurren t proxim al peni le hypos padius , who had n o skin available for p e n i l e fl a ps, with a success rate approaching 89.2%. Keywords: Buccal Graft Urethroplasty, Hypospadias 1. Introduction Hypospadias is a congenital abnormality occurring in 1 of 300 live births (1). Nowadays, most cases of hypospadias can be successfully repaired with a one-stage procedure in the first year of life with a success rate approaching 90% (2). The penile skin with the underlying dartos fascia provides together with the prepuce in uncircumcised children good tissue for primary repair together with managing any encountered complications. Unfortunately, the repeated attempts at surgical repair in recurrent failures after hypospadias repair wou ld result in densely scarred, immobile, hypovascular, or signifi- cantly shortened penis (3). The repair of these penile hypospadias represents a challenge for the urologists, the tissues being fibrosed, hypovascular and immobile. Furthermore, there is no enough healthy penile skin for r epair. 2. Patients and Methods This is a prospective study carried out between February 2006 and A pril 2009 on 37 pediatric & adolescent patients presenting with recurrent proximal penile hypospadias. The age of p atients range d between 14 and 20 years (mean 17.7). All patients had undergone a variety of primary hypospadias correctio ns in the past while 10 patients h ad two trials of previous repair. All patients were found to be circumcised at time of presentations. Thirty patients had penile chordee. The main complaint was proximal pen ile hypospadias, with ventral chordee in 30 pat ients. T he urethral plate was found to be fibrosed and scarred. All patients included in the study were found to be circumcised. Preoperatively, all patients underwent physical ex- amination to exclude BXO, urine analysis and culture. All our patients and their relatives were informed about the procedure and signed an appropriate consent. All of the patients were managed by 2 stages buccal mucosal urethroplasty. At the first stage, we performed a urethroscopy under general anesthesia to assess the ure- thra, then while the patient in the lithotomy position, all the scarred urethral plate was completely removed through a two para-urethral incision that extends to the tip 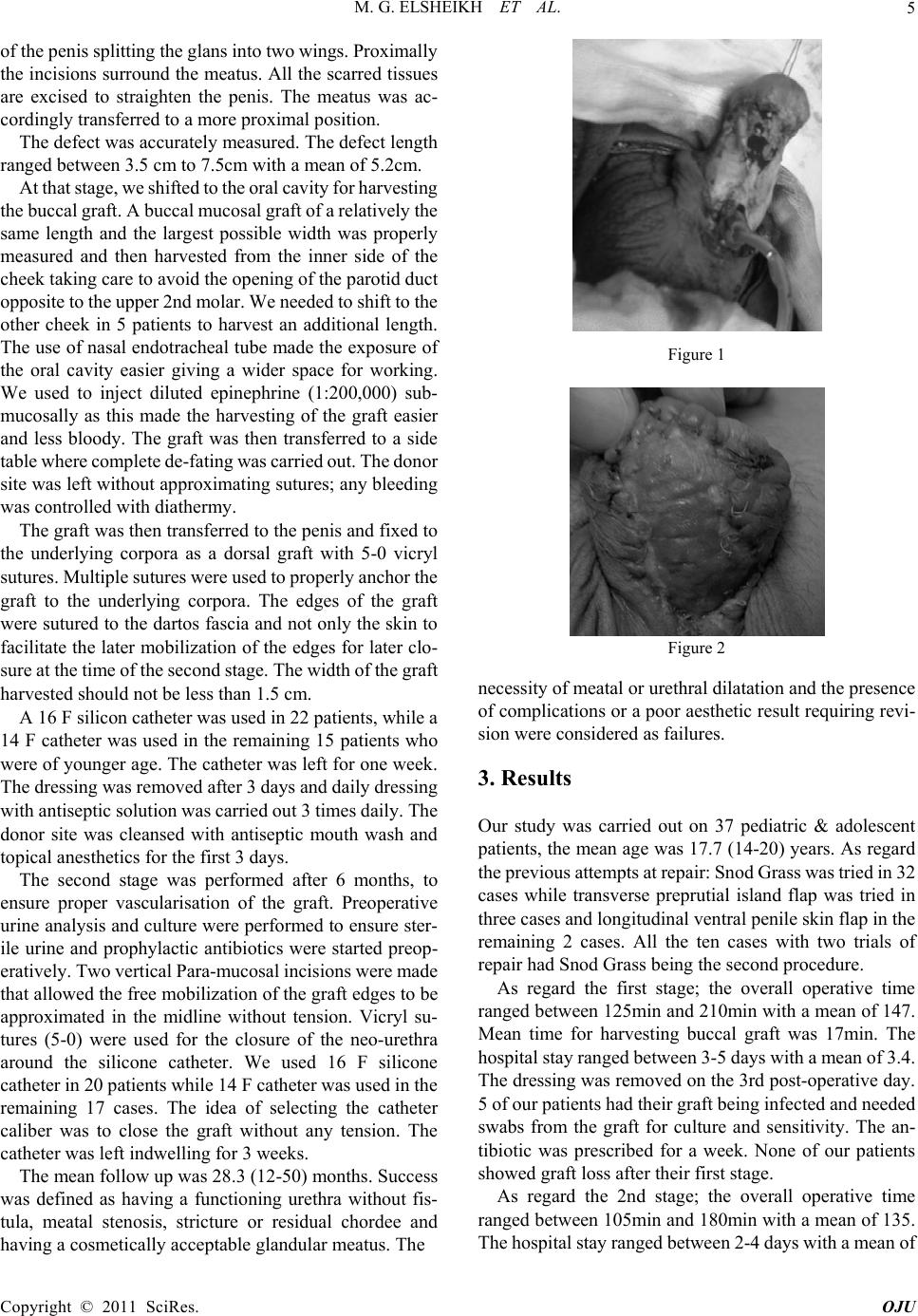 M. G. ELSHEIKH ET AL.5 of the penis splitting the glans into two wings. Proximally the incisions surround the meatus. All the scarred tissues are excised to straighten the penis. The meatus was ac- cordingly transferred to a more proximal po s ition. The defect was accurately measured. The defect length ranged between 3.5 cm to 7.5cm with a mean of 5.2cm. At that stage, we shifted to the oral cavity for harvesting the buccal graf t. A buccal mucosal graft of a relatively the same length and the largest possible width was properly measured and then harvested from the inner side of the cheek taking care to avoid the opening of the parotid duct opposite to the upper 2nd molar. We needed to shift to the other cheek in 5 patients to harvest an additional length. The use of nasal endotracheal tube made the exposure of the oral cavity easier giving a wider space for working. We used to inject diluted epinephrine (1:200,000) sub- mucosally as this made the harvesting of the graft easier and less bloody. The graft was then transferred to a side table where complete de-fating was carried out. T he donor site was left without approximating sutures; any bleeding was controll ed with diathermy. The graft was then transferred to the penis and fixed to the underlying corpora as a dorsal graft with 5-0 vicryl sutures. Multiple sutures were used to properly anchor the graft to the underlying corpora. The edges of the graft were sutured to the dartos fascia and not only th e skin to facilitate the later mobilization of the edges for later clo- sure at th e tim e of the second s tage. The widt h of t he gr aft harvested should no t be less than 1.5 cm. A 16 F silicon catheter was used in 22 patients, while a 14 F catheter was used in the remaining 15 patients who were of younger ag e. The catheter was left for one week. The dressi ng w as rem ov ed a ft er 3 days a nd d ai ly dressi ng with antiseptic solution was carried out 3 times daily. The donor site was cleansed with antiseptic mouth wash and topical anesthetics for the first 3 days. The second stage was performed after 6 months, to ensure proper vascularisation of the graft. Preoperative urine analysis and culture were performed to ensure ster- ile urine and prophylactic antibiotics were started preop- eratively. Two vertical Para-m ucosal incisions were made that allowed the free mobilization of the graft edges to be approximated in the midline without tension. Vicryl su- tures (5-0) were used for the closure of the neo-urethra around the silicone catheter. We used 16 F silicone catheter in 20 patients while 14 F catheter was used in the remaining 17 cases. The idea of selecting the catheter caliber was to close the graft without any tension. The catheter was left indwelling for 3 weeks. The mean follow up was 28.3 (12-50) months. Success was defined as having a functioning urethra without fis- tula, meatal stenosis, stricture or residual chordee and having a cosmetically acceptable glandular meatus. The Figure 1 Figure 2 necessity of meatal or urethral dilatation and the presence of complications or a poor aesthetic result requiring revi- sion were considered as failures. 3. Results Our study was carried out on 37 pediatric & adolescent patients, the mean age was 17.7 (14-20) years. As regard the previous attempts at repair: Snod Grass was tried in 32 cases while transverse preprutial island flap was tried in three cases and longitudinal ventral penile skin flap in the remaining 2 cases. All the ten cases with two trials of repair had Snod Grass being the second procedure. As regard the first stage; the overall operative time ranged between 125min and 210min with a mean of 147. Mean time for harvesting buccal graft was 17min. The hospital stay ra nge d betwee n 3-5 d ays wi th a mean of 3.4. The dressing was removed on the 3rd post-operative day. 5 of our p ati ents ha d t hei r gra f t bein g i nfecte d a nd ne ede d swabs from the graft for culture and sensitivity. The an- tibiotic was prescribed for a week. None of our patients showed graft loss after their first stage. As regard the 2nd stage; the overall operative time ranged between 105min and 180min with a mean of 135. The hospital stay ranged bet ween 2-4 days w ith a mean of Copyright © 2011 SciRes. OJU  M. G. ELSHEIKH ET AL. 6 3.16. Of the 37 cases included in the study, 33 (89.2%) were classified as successes and 4 cases (10.8%) as failures. The failures were due to urethro-cutaneous fistula in 3 cases (8.1%) and meatal stenosis in one case (2.7%). The fistula developed immediately after removal of urethral catheter after the second stage. The patient was considered as failure and underwent closure of the fistula successfully 3 months later. Meatal stenosis was the other cause of failure; the patient was 18 years old, and pre- sented with gradual decrease in the urinary flow after removal of the uret hral catheter after successful 2nd stage. He needed meatal d ilatation for 2 weeks ther eafter. None of the patients who were considered as success needed any further intervention during the follow-up. Further- more, none of our patients experienced any degree of penile chordee. Also, no one complained from any oral problems during the follow-up. 4. Discussion The management of complications in pediatric and ado- lescent age groups, in whom multiple attempts of hypo- spadias repair had failed, is a surgical challenge and still represents a complex problem for reconstructive urolo- gists. This is attribute d to the fact that repeated attem pts at repair would result in tissues that are fibrosed, hypovas- cular and immobile. The deficiency of enough unscarred penile skin, being used for previous attempt at repair, adds another difficulty. Historically, various tissues have been used to repair the damaged urethra including genital (penile and scrotal) skin, extra-genital skin, bladder mucosa and buccal mu- cosa. These tissues have been used as either pedicled flaps with their own blood supply or as free tissue grafts. The most common graft materials in use today are buccal mucosa, preputial skin (when available) and penile and preputial skin flaps with their own blood supply. The use of buc cal mucosa (B M) in urethral surgery was first describ ed by Humby in 1941, (4) however, it was not reported again until the late 1980s. The main reported advantages of the buccal mucosa that favors its use in substitution urethroplasty is it’s na- ture being wet epithelium, it is readily harvested, it has been shown to be resistant to recurrence of strictures (especially in the presence of balanitis xerotica obliterans, BXO). The presence of a dense submucosa with a dense capillary network facilitates early imbibition and early inosculation (5). It was not until 1992 when Burger et al. reported the first use of BM for repair of complications following childhood hypospadias surgery (6). In 1995, Bracka conducted his study on managing complications after hypospadias repair and he concluded that a two-stage repair by splitting the glans and lining it with penile skin or BM grafts was extremely adaptable and successful (7). In studying the outcomes of buccal mucosa (BM) penile urethroplasty, most of the studies reported a high success rate (8). In 1998 Mundy et al reported a success rate of 100% on 8 cases, as 2 stages buccal mucosal urethroplasty due to penile stricture caused by BXO (9). Three years later, he published his results on 39 cases underwent 2 stages procedures and followed –up for at least 2 years and re- ported a success of 83% (5). Dubey et al reported a series of 43 patients undergoing dorsal-onlay BM urethroplasty for penile strictures. 28 of their patients h ad a one -st a ge procedure, while 15 of their patients required two-stages. The mean follow-up was shorter for two-stage reconstruction (24.2 months) com- pared to one-st age proce dures (34 m onths). They reporte d success rate of 86.7% for two-stage procedures, with the majority of recurrences being managed successfully with DVIU (10). The same authors reported the outcomes of the 2 stage procedure on 14 cases with strictures due to BXO, after a mean follow-up of 32.5 months, they re- ported a success rate of 78.6% (11). Our success rate was 89.2%, a result which is compa- rable to that publi shed by Mundy et al (5) and Dubey et al in his first series (10) but much better than that reported by the same author in his second series (11).This might be attributed to the fact that most published series on two stages buccal urethroplasty was done for BXO, this is not the case in our study as no ne o f o ur pat i e nt s had BXO. In our study, none of our patients developed contracture of the initial graft, and none of the succeeded cases required more than the two previously planned surgeries. That’s why we do not agree with Shukla et al. in 2004 (12) , who concluded his study that a 2-stage repair is actually a mis- nomer and that about 70% of cases required at least one additional procedure (a mean of extra 1.6 pro cedures). 5. Conclusion Although, there is no single technique that can fit for all situations, the use of 2 stage buccal mucosal uret hroplasty appears to be a successful and feasible option for man- agement of patients with recurrent proximal penile hy- pospadias in the absence of sufficient penile skin with a success rate approaching 89.2%. Although most of our patients have been follow ed up for more th an 2 year s yet, longer follow-u p is still required. 6. References [1] L. S. Baskin, “Hypospadias and Urethral Development”, Copyright © 2011 SciRes. OJU  M. G. ELSHEIKH ET AL. Copyright © 2011 SciRes. OJU 7 Journal of Urology, Vol. 163, 2000, pp. 951-956. [2] G. Manzoni, A. Braccka, E. Palminteri and G. Marrocco, “Hypospadias Surgery: When, What and by Whom?” BJU International, Vol. 94, 2004, pp. 1188-1195. [3] J. F. Stec ker, C. E. Horton, C. J. Devine and J. B . McCraw, “Hypospadias Cripples,” Urologic Clinics North Amem- rica, Vol. 8, 1981, pp. 539-544. [4] G. Humby, “A One-Stage Operation for Hypospadias,” British of Journal Surgery, Vol. 29, 1941, p. 84. [5] D. E. Andrich and A. R. Mundy, “Substitution Urethro- plasty with Buccal Mucosal-Free Grafts,” Journal of Urology, Vol. 65, 2001, p. 1131. [6] R. A. Burger, S. C. Muller, H. El-Damanhoury, A. Tschakaloff, H. Riedmiller and R. Hohenfellner, “The Buccal Mucosal Graft for Urethral Reconstruction: A Preliminary Report,” Journal of Urology, Vol. 147, 1992, pp. 662-664. [7] A. Bracka, “A Versatile Two-Stage Hypospadias Repair,” British Journal of Plastic Surgery, Vol. 48, 1995, pp. 345-352. [8] J. M. Patterson and C. R. Chapple, “Surgical Techniques in Substitut ion Urethroplas ty Using Buccal M ucosa for the Treatment of Anterior Urethral Strictures,” European Urology, Vol. 53 (6), 2008, pp. 1162-1171. [9] S. N. Venn and A. R. Mundy, “Urethroplasty for Balanitis Xerotica Obliterans,” British Journal of Urology, Vol. 81, 1998, p. 735. [10] D. Dubey, A. Kumar, A. Mandhani, et al., “Buccal Mu- cosal Urethroplasty: A Versatile Technique for All Ure- thral Segme nts,” BJU Interna tional, Vol. 95 , 2005 , p. 625 . [11] D. Dubey, A. Sehgal, A. Srivastava, et al., “Buccal Mu- cosal Urethroplasty for Balanitis Xerotica Obliterans Re- lated Urethral Strictures: The Outcome of 1 and 2-Stage Techniques,” Journal of Urology, Vol. 179, 2005, p. 463. [12] A. R. Shukla, R. P. Pate l an d D . A. Canni ng, “Th e 2-Stag e Hypospadias Repair. Is It a Misnomer?” Journal of Urol- ogy, Vol. 172, 2004, pp. 1714-1716. |

