 Journal of Cancer Therapy, 2013, 4, 1366-1372 http://dx.doi.org/10.4236/jct.2013.48162 Published Online October 2013 (http://www.scirp.org/journal/jct) Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide Domenico Mastrangelo1, Lauretta Massai1, Giuseppe Fioritoni2, Antonio Iacone2, Paolo Di Bartolomeo3, Patrizia Accorsi3, Tiziana Bonfini3, Michela Muscettola1, Giovanni Grasso1 1Department of Medical, Surgical, and Neurological Sciences, University of Siena, Siena, Italy; 2Pescara Cell Factory Foundation, Pescara Main Hospital, Pescara, Italy; 3Department of Hematology, Pescara Main Hospital, Pescara, Italy. Email: mastrangelo@unisi.it Received September 23rd, 2013; revised October 17th, 2013; accepted October 23rd, 2013 Copyright © 2013 Domenico Mastrangelo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Arsenic Trioxide (ATO) is widely acknowledged as the treatment of choice for Acute Promyelocytic Leukemia (APL). It is a “two-sided” drug since it can induce differentiation or kill APL and other tumor cells according to the dosage. Part of the cytotoxic effects of ATO on APL cells is due to its pro-oxidant activity, a characteristic which ATO shares with a number of other compounds, including high doses of ascorbate (ASC). In a comparative investigation on the cy- totoxic effects of both ATO and ASC on HL60 (APL) cell lines, in vitro, we have been able to confirm the known cy- totoxic effects of ATO, but, more importantly, we have demonstrated that ASC is significantly more effective than ATO, in killing these cancer cells in vitro, when the concentrations are maintained within the millimolar (mM) range, i.e. the range of plasma concentrations at which ASC induces oxidative damage to tumor cells. Since these plasma lev- els can be reached only by the intravenous administration of high doses of ASC, we propose that intravenous high doses of ASC may represent a potentially revolutionary new approach in the management of APL. Keywords: HL60; Acute Promyelocytic Leukemia; High Doses of Ascorbate; Cell Count and Viability Assays 1. Introduction Acute Promyelocytic Leukemia (APL) is a rare, though extremely malignant subtype of acute myeloid leukemia (AML), which, only a few decades ago, Hillestad de- scribed as characterized by “a very rapid fatal course of only a few week’s duration” with a white blood cell (WBC) picture dominated by promyelocytes and a severe bleeding tendency [1,2]. The use of all-trans retinoic acid (ATRA) as a differ- entiating agent is well established, in the treatment of APL [3], but in the last two decades or more, Arsenic Trioxide (ATO), initially proposed by Chinese authors as the first line treatment of APL [4], has been largely ac- knowledged as the “most effective single agent” [5], “the most biologically active single drug” [6], and more re- cently, “a drug from a poison” to be used not only in the treatment of this leukemia, but also in other types of cancer [7], for its efficacy, cost effectiveness, and low toxicity [8,9]. Studies on HL60 (human promyelocytic leukemia) cell line, have shown that ATO can be viewed as a two- sided drug, inducing either differentiation or apoptosis on these cell lines [10], according to the dosage employed. These data have been confirmed by studies on retino- blastoma cell lines, showing that in low doses (≤1 μM) it induces differentiation, while in high doses (≥2 μM) it behaves as a pro-apoptotic drug and effectively inhibit tumor formation in vitro [11]. As reported by different Authors [12-14], oxidative stress is critical to the ATO-induced apoptotic process, with generation of ROS, including, among others, H2O2, which is considered a metabolite of the drug [15]. Inter- estingly, ATO shares this pro-oxidant activity with a number of other compounds, including sodium ascorbate (ASC) administered in high doses by intravenous injec- tion [16-20]. Given the above mentioned similarities underlying the anticancer activity of ATO and ASC, and the high cyto- toxic potential shown by ASC in different cancer cell lines in vitro [16-21], we have undertaken the present investigation in order to assess the cytotoxic effect of ASC on HL60 cells in vitro in comparison with ATO, which is the drug of choice in the treatment of APL. Copyright © 2013 SciRes. JCT  Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide 1367 2. Materials and Methods HL60 (Human Promyelocytic Leukemia) cell lines were kindly supplied by the Department of Molecular and Developmental Medicine of the University of Siena. All reagents, including culture media, Sodium Ascorbate (ASC), Trypan Blue, Hoechst 33342, and Propidium Iodide (PI), were purchased from Sigma-Aldrich. Arse- nic trioxide (ATO chemical formula: As2O3) vials (Tri- senox) were kindly supplied by the Department of Hae- matology of the Pescara main hospital (Pescara, Italy). Automated cell count and viability was performed by using the “Muse”™ (Merck-Millipore) automated cell analyzer. A Zeiss Axioplan2 microscope was used for fluorescence microscopy and a Shandon cytocentrifuge for microscopic/morphologic analysis of cell suspen- sions. Cell counting, before and after exposure to increasing doses of either ATO and ASC was performed with both the manual (Trypan Blue Exclusion Test) and automated (“Muse”™) methods. The automated method, using the “Muse”™, was carried out according to the instructions supplied by the manufacturer which encompass an in house method of nuclear staining for the assessment of cell viability. The Trypan Blue Exclusion Test was per- formed according to the standard procedures [22,23]. At the end of each count and viability test HL60 cell suspen- sions were stained with Hoechst/PI as described else- where [24] and aliquots of about 1 × 105 cells were de- posited onto alcohol-washed glass microscope slides by using a cytocentrifuge, at 1000 r.p.m. for 5 minutes, and then observed under fluorescence microscopy. HL60 human promyelocytic leukemia cells were grown in RPMI supplemented with antibiotics, glutamine, and 20% FBS, at 37˚C in 5% CO2/95% air. During the phase of exponential growth, the cells were harvested and counted with the “Muse”™ automated cell counter/ analyzer and then diluted to a concentration of about (2 - 5) × 105/ml. Four dilutions of 2, 4, 6, and 8 μg/ml of ATO were used, starting from a stock solution of ATO commercial- ly available at a concentration of 1mg/ml (Trisnox™), by simply adding 2, 4, 6, and 8 μl, respectively, of Tris- enox™, to 1 ml of culture medium containing (2 - 5) × 105 HL60 cells. These concentrations were chosen ac- cording to the data reported by Yediou and coll. [10]. A 1 M solution of ASC was prepared fresh each time as described elsewhere [21], and aliquots of 1, 3, 5, and 7 μl of this stock solution were added to four different wells containing 1ml each of culture medium in which (2 - 5) × 105 HL60 cells were suspended, to a final concen- tration of 1, 3, 5, and 7 mM, respectively. ATO can be stored at room temperature for up to 36 months. Aliquots of 1 ml of culture medium containing (2 - 5) × 105 HL60 cells were loaded into each of 12 well plate, and added with the four different concentrations of ATO (2, 4, 6, and 8 μg/ml); the same procedure was used for ASC (1, 3, 5, and 7 mM). The cells were exposed for a total of 18 - 24 hours. One control (no treatment) sample was also included. At the end of the incubation period the cells were collected in vials, and mixed with the Muse™ Count & Viability Reagent, according to the procedure supplied by the manufacturer, for automated counting and viability analysis. Namely, 10 μl of each sample were added to 190 μl of the Muse™ Count & Viability Reagent, which differentially stains viable and non-vi- able cells based on their permeability to the two DNA binding dyes present in the reagent. A specific Software Module then performs calculations automatically and displays data in two dot plots as shown in Figure 1. Each experiment was repeated at least twice, and a to- tal of six experiments was carried out, with the auto- mated (“Muse”™) cell counting and viability tests, with and without (control sample) increasing doses of ATO and ASC, as reported. The results have been cumula- tively analyzed by calculating the mean, standard error, standard deviation, and 95% confidence intervals (95% CI) of the percentages of living cells for each experiment. The statistics was performed by calculating the two- tailed “p” value of the difference between the mean per- centage of viable cells in each of the four doses of ASC (1, 3, 5, and 7 mM) as compared to ATO (2, 4, 6, and 8 μg/ml). Aliquots of cells were also stained with Hoechst/PI, according to the standard protocols, deposited onto glass microscope slides with a cytocentrifuge, and finally ob- served under fluorescence microscopy [25]. For statisti- cal analysis, the mean values and related 95% confidence intervals were calculated for each set of repeated meas- urement by using the SPSS statistical package, version 10. 3. Results The results of this experiment are reported in Table 1 and summarized in the diagram of Figure 2. As shown in the graph, the mean percentages of live HL60 cells (the “y” axis) proportionally decrease by increasing the con- centrations of both ASC and ATO (the “x” axis). How- ever, at any given concentration, ASC always kills more HL60 cells than ATO. The two tailed “p” value of the difference in the percentage of live cells between ASC and ATO suggests that ASC 1, and 3 mM is more effec- tive than ATO 2, and 4 μg/ml, respectively, in reducing the survival of HL60 cells in culture even though the difference does not appear statistically significant. How- ever, at 5 mM, ASC is significantly more effective (p = 0.0038) than ATO (6 μg/ml) and the difference becomes highly statistically significant (p = 0.0001) in favor of Copyright © 2013 SciRes. JCT 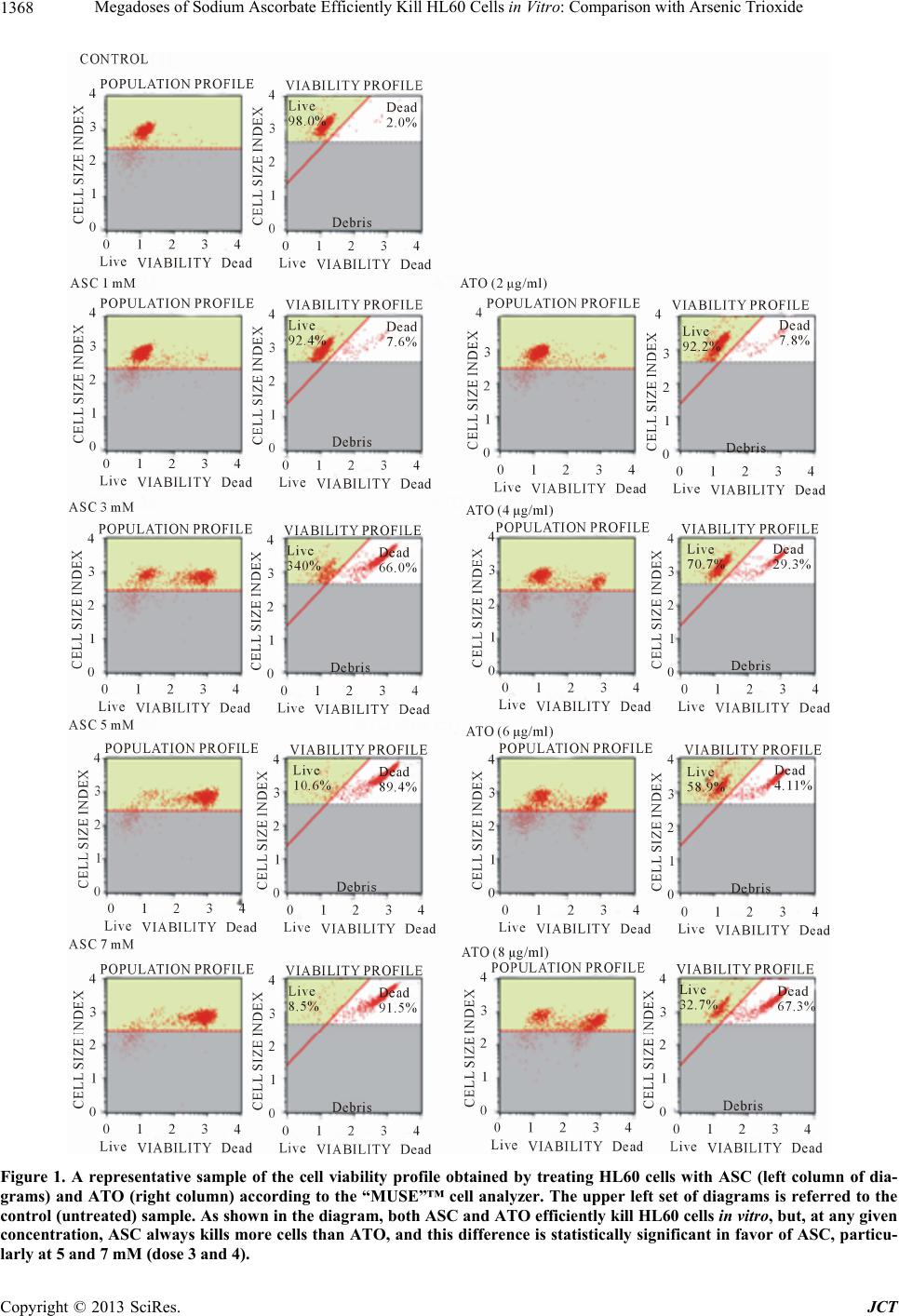 Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide Copyright © 2013 SciRes. JCT 1368 Figure 1. A representative sample of the cell viability profile obtained by treating HL60 cells with ASC (left column of dia- grams) and ATO (right column) according to the “MUSE”™ cell analyzer. The upper left set of diagrams is referred to the control (untreate d) sample. As shown in the diagram, both ASC and ATO effic iently kill HL60 cells in vitro, but, at any given concentration, ASC always kills more c ells than ATO, and this difference is statistically significant in favor of ASC, particu- larly at 5 and 7 mM (dose 3 and 4).  Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide 1369 dose 1 dose 2 dose 3dose 4 0 50 100 control ASC ATO Figure 2. Comparison of the mean percentages of viable HL60 cells (“y” axis) after treatment with ASC (blue bars) and ATO (red bars). The green bar indicates the untreated (control) cells. The statistical analysis of these data reveals that at “dose 3” (ASC 5 mM and ATO 6 µg/ml) and “dose 4” (ASC 7 mM and ATO 8 µg/ml) ASC is significantly more efficient than ATO in killing HL60 cells in vitro. Table 1. Mean percentages of viable cells in the untreated (control) and treated (ASC and ATO) HL60 cells, at dif- ferent dosages, in six consecutive experiments. EXP.N. Control (% vital) DOSE ASC (% vital) ATO (% vital) 1 96.5 1 91.9 93.2 2 61.5 74.8 3 19.9 59.7 4 15.4 44.1 2 98 1 92.4 92.2 2 34 70.7 3 10.6 58.9 4 8.5 32.7 3 97.4 1 89.3 73.2 2 48.3 28.5 3 30.6 21.7 4 2.2 21.9 4 97.2 1 82 93.1 2 13 81.8 3 4.3 62.5 4 2.2 45.4 5 97.2 1 84.3 95.2 2 59.3 86.5 3 34.4 69.8 4 8.6 39.2 6 91.2 1 70.3 94.3 2 15.5 86.8 3 11.7 65.8 4 5.8 50.1 ASC when ASC 7 mM is compared to ATO 8 μg/ml. The LC50 for ATO is above 6 µg/ml, as reported by Yediou and coll.10, while for ASC it is in between 3 and 5 mM, as reported by different Authors [16-21]. The Trypan Blue analysis, even if not informative re- garding the apoptotic condition of treated HL60 cells, confirmed the data obtained with “Muse”™. Regarding the Hoechst/PI staining, as shown in Figure 3 the cellular changes induced by ATO seems to be sub- stantially different from those obtained with the exposure to millimolar (mM) doses of ASC. Namely, while ATO, at the maximum dosage tested herein, clearly shows signs of apoptotic cell damage, encompassing, nuclear fragmentation and chromatin condensation, ASC treated HL60 cells are clearly necrotic, as shown by the red color (a) (b) (c) (d) (e) Figure 3. Hoechst/PI fluorescent microscopy staining of untreated (controls) and treated (ASC and ATO) HL60 cells. 400× magnification. Untreated (control) cells (a) ap- pear uniformly blue with nuclei show ing a loose chr omatin, and no signs of necrosis or pyknosis. This feature is com- patible with a well preserved viability, as also confirmed by the “MUSE”™. The same cells, treated with 3 and 5 mM ASC ((b) and (c) respectively), appear uniformly stained in red (PI) thus indicating an advanced necrotic condition. On the contrary, HL60 cells treated with ATO at 4 and 6 µg/ml ((d) and (e) respectively), are almost uniformly stained in blue, but the condensed chromatin and nuclear fragmen- tation are indicative of early/late stage apoptotic condition. Copyright © 2013 SciRes. JCT  Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide 1370 of the nuclei which were stained by PI, but not by Heo- chst 33342. These results confirm our previous observa- tion of the effects of mM doses of ASC on Y79 retino- blastoma cells in vitro [21]. 4. Discussion Historically, four periods have been identified, in the treatment of APL, which have marked a progressive im- provement in the cure of this disease, namely: 1) chemo- therapy (CT) alone (with antracyclines and Ara-C), from 1957 to 1985; 2) all-trans retinoic acid (ATRA), intro- duced in 1985; 3) Arsenic Trioxide (As2O3-ATO) intro- duced in the mid 1990s; 4) Combination of ATRA and ATO, in 1998. With all these achievements, APL has passed from a 5-year disease-free survival (5yDFS) of about 35% - 45%, to a 6 year disease-free survival (6yDFS) of 86% (±10%) [26]. The role of ROS, H2O2, redox potential, and oxidative stress in the pro-apoptotic, anticancer activity of ATO, further illustrated in detail by de Thé & coll. [27], Selva- raj & coll. [28], Sanchez & coll. [29], among others, sug- gests that drugs with oxidizing potential may represent the new frontier in cancer treatment [30], especially if we consider that cancer cells have more commonly exhaust- ed their antioxidant machinery [31] and therefore are more vulnerable to oxidizing compounds. ASC (Vitamin C) was first isolated in 1928 by the Hungarian biochemist Dr. Szent-Gyorgyi who received the Nobel Prize for this discovery in 1937 [32]. The history of ASC as an anticancer molecule is very controversial [33]. McCormik, nearly 60 years ago, sug- gested that ASC protects against cancer by increasing collagen synthesis [34,35] while Ewan Cameron hypo- thesized that ASC could have anti-cancer effects by in- hibiting hyaluronidase and thereby preventing cancer spread [36]. Although successful clinical trials had been reported, by Cameron and the twofold Nobel laureate Linus Pauling, on terminal cancer patients, Charles Mo- ertel, at Mayo Clinic, reported negative results, and his trials were credited as the definitive proof of the ineffi- cacy of ASC in treating cancer [36,37]. In Moertel’s trial, however, ASC was given orally, while Cameron and Pauling had used both the intravenous and oral route of administration simultaneously. Report survey data on intravenous ASC show that ASC is used in doses up to 200 g per infusion, for a variety of pathological condi- tions, with very few adverse effects [38], and that, when taken orally, plasma ASC concentrations never raise be- yond the level of 100 μmol/l, due to the limited bioavail- ability of the molecule and renal excretion [39]. On the contrary, when administered by intravenous infusion, ASC reaches plasmatic concentrations in the order of millimolar range, which would never be obtained by the administration of oral doses [18-20,40,41], and behaves as a very powerful oxidant, suitable for the therapeutic use in cancer [42]. The anticancer effects of ASC have been widely dem- onstrated in a number of different tumor cell lines [17- 20], and phase I clinical trials have been already per- formed, in different cancers, showing that, even in very high doses, it is well tolerated [43] and has the potential to selectively kill cancer cells due to its capacity of pro- ducing intracellular H2O2, with oxidation of different cellular components, and cell death [17-20,31,40]. Although the use of ASC in the treatment of APL has been already proposed by both Yedjiou & coll. [10] and Bachleitner-Hofmann and coll. [44], in combination with ATO, ASC has never entered in the routine treatment of APL; this can be explained by the substantial difference in the dosage proposed by both Yedjiou and Bachleitner, when compared to our protocol. In fact, while it has been observed that adding ASC in the treatment of APL may slightly increase the cytotoxic potential of ATO, the dose of ASC recommended by both Authors, never over- comes 150 µM, which is within the range of plasma concentrations that can be reached by oral administration of ASC. More importantly, at plasma concentrations in the range of micromoles (µM), ASC behaves as an “anti- oxidant”, and, as such, it has also been reported to protect HL60 cells from arsenic toxicity [45] and antagonize the toxic effects of chemotherapeutic agents [46]. On the contrary, at concentrations in the order of magnitude of the millimoles (mM), used in the present investigation, it clearly shows pro-oxidant effects, on tumor cells, which lead to an increased production of H2O2 and other ROS and consequent oxidative damage of different cell struc- tures, as reported by several Authors [17-20,31,40]. The data reported herein substantially confirm the ef- fectiveness of ATO in the treatment of APL, but add new relevant information, by showing that ASC, when used in mM concentration, as those that can be reached by intra- venous injection of pharmacologic doses, is significantly more effective than ATO in killing HL60 cells in vitro. The advantages of ASC, as compared to ATO, in the treatment of APL are clear: 1) In phase I clinical trials on metastatic pancreatic cancer [43], ASC has been administered at doses varying from 50 to 100 grams per infusion, three times a week for eight weeks, with plasma levels up to 30 mM and no major side effects and; 2) Due to its peculiar “metabolic” effect, ASC has been widely reported to selectively kill cancer cells, be- ing substantially harmless for normal cells. Given all the above, the Authors strongly believe that high (“pharmacologic”) doses of ASC may represent a second revolution in the treatment of APL, and suggest to start a phase I-IV clinical investigation on ASC in the treatment of selected cases of APL, in order to verify its effectiveness in the treatment of this leukemia. Copyright © 2013 SciRes. JCT  Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide 1371 5. Acknowledgements The authors thank Dr. Felice Arcuri, of the Department of Molecular and Developmental Medicine of the Uni- versity of Siena (Italy), for contributing to this work by supplying HL60 cell lines. REFERENCES [1] L. K. Hillestad, “Acute Promyelocytic Leukemia,” Acta Medica S c andinavica, Vol. 159, No. 3, 1957, pp. 189-194. http://dx.doi.org/10.1111/j.0954-6820.1957.tb00124.x [2] R. P. Warrell Jr., H. de Thé, Z. Y. Wang and L. Degos, “Acute Promyelocytic Leukemia,” New England Journal of Medicine, Vol. 329. 1993, pp. 177-189. http://dx.doi.org/10.1056/NEJM199307153290307 [3] D. Nowak, D. Stewart and H. P. Koeffler, “Differentia- tion Therapy of Leukemia: 3 Decades of Development,” Blood, Vol. 113, No. 16, 2009, pp. 3655-3665. http://dx.doi.org/10.1182/blood-2009-01-198911 [4] K. H. Antman, “Introduction: The History of Arsenic Trioxide in Cancer Therapy,” The Oncologist, Vol. 6, Suppl. 2, 2001, pp. 1-2. http://dx.doi.org/10.1634/theoncologist.6-suppl_2-1 [5] B. L. Powell, “Arsenic Trioxide in Acute Promyelocytic Leukemia: Potion Not Poison,” Expert Reviews in Anti- cancer Therapy, Vol. 11, No. 9, 2011, pp. 1317-1319. http://dx.doi.org/10.1586/era.11.128 [6] M. A. Sanz, D. Grimwade, M. S. Tallman, et al., “Man- agement of Acute Promyelocytic Leukemia: Recommen- dations from an Expert Panel on Behalf of the European Leukemia Net,” Blood, Vol. 113, No. 9, 2009, pp. 1875- 1891. http://dx.doi.org/10.1182/blood-2008-04-150250 [7] Y. Rao, R. H. Li and D. Q. Zhang, “A Drug from Poison: How the Therapeutic Effect of Arsenic Trioxide on Acute Promyelocytic Leukemia Was Discovered,” Science China Life Science, Vol. 56, No. 6, 2013, pp. 495-502. http://dx.doi.org/10.1007/s11427-013-4487-z [8] J. Q. Mi, J. M. Li, S. X. Shen, S. J. Chen and Z. Chen, “How to Manage Acute Promyelocytic Leukemia,” Leu- kemia, Vol. 26, No. 8, 2012, pp. 1743-1751. http://dx.doi.org/10.1038/leu.2012.57 [9] S. J. Chen, G. B. Zhou, S. W. Zhang, J. H. Mao, H. de Thé and Z. Chen, “From an Old Remedy to a Magic Bul- let: Molecular Mechanisms Underlying the Therapeutic Effects of Arsenic in Fighting Leukemia,” Blood, Vol. 117, No. 24, 2011, pp. 6425-6437. http://dx.doi.org/10.1182/blood-2010-11-283598 [10] C. Yedjou, P. Tchounwou, J. Jenkins and R. McMurray, “Basic Mechanisms of Arsenic Trioxide (ATO)-Induced Apoptosis in Human Leukemia (HL60) Cells,” Journal of Hematology and Oncology, Vol. 3, No. 28, 2010, pp. 1-9. [11] J. H. Kim, J. H. Kim, Y. S. Yu, D. H. Kim, C. J. Kim and K. W. Kim, “Antitumor Activity of Arsenic Trioxide on Retinoblastoma: Cell Differentiation and Apoptosis De- pending on Arsenic Trioxide Concentration,” Investiga- tive Ophthalmology and Visual Sciences, Vol. 50, No. 4, 2009, pp. 1819-1823. http://dx.doi.org/10.1167/iovs.08-2623 [12] A. Emadi and S. D. Gore, “Arsenic Trioxide: An Old Drug Rediscovered,” Blood, Vol. 24, No. 4, 2010, pp. 191-199. [13] S. Alarifi, D. Ali, S. Alkahtani, M. A. Siddiqui and B. A. Ali, “Arsenic Trioxide-Mediated Oxidative Stress and Genotoxicity in Human Hepatocellular Carcinoma Cells,” OncoTargets and Therapy, Vol. 6, 2013, pp. 65-84. [14] R. C. Sun, P. G. Board and A. C. Blackburn, “Targeting Metabolism with Arsenic Trioxide and Dichloroacetate in Breast Cancer Cells, Oxidative Damage to Cancer Cells,” Molecular Cancer, Vol. 10, No. 142, 2011, pp. 1-15. [15] J. Zhou, J. Ye, X. Zhao, A. Li and J. Zhou, “JWA Is Re- quired for Arsenic Trioxide Induced Apoptosis in HeLa and MCF-7 Cells via Reactive Oxygen Species and Mi- tochondria Linked Signal Pathway,” Toxicology and Ap- plied Pharmacology, Vol. 230, No. 1, 2008, pp. 33-40. http://dx.doi.org/10.1016/j.taap.2008.01.041 [16] S. Ohno, Y. Ohno, N. Suzuki, G. I. Soma and M. Inoue, “High-Dose Vitamin C (Ascorbic Acid) Therapy in the Treatment of Patients with Advanced Cancer,” Antican- cer Research, Vol. 29, 2009, pp. 809-816. [17] Q. Chen, M. Graham Espey, M. C. Krishna, et al., “Phar- macologic Ascorbic Acid Concentrations Selectively Kill Cancer Cells: Action as a Pro-Drug to Deliver Hydrogen Peroxide to Tissues,” Proceedings of the National Acad- emy of Sciences, Vol. 102, No. 38, 2005, pp. 13604- 13609. http://dx.doi.org/10.1073/pnas.0506390102 [18] Q. Chen, M. G. Espey, A. Y. Sun, et al., “Pharmacologic Doses of Ascorbate Act as a Prooxidant and Decrease Growth of Aggressive Tumor Xenografts in Mice,” Pro- ceedings of the National Academy of Sciences, Vol. 105, No. 32, 2008, pp. 11105-11109. http://dx.doi.org/10.1073/pnas.0804226105 [19] P. Chen, J. Yu, B. Chalmers, et al., “Pharmacological Ascorbate Induces Cytotoxicity in Prostate Cancer Cells through ATP Depletion and Induction of Autophagy,” Anticancer Drugs, Vol. 23, No. 4, 2012, pp. 437-444. http://dx.doi.org/10.1097/CAD.0b013e32834fd01f [20] P. Chen, J Stone, G Sullivan, J. A. Drisko and Q. Chen, “Anti-Cancer Effect of Pharmacologic Ascorbate and Its Interaction with Supplementary Parenteral Glutathione in Preclinical Cancer Models,” Free Radicals in Biology and Medicine, Vol. 51, No. 3, 2011, pp. 681-687. http://dx.doi.org/10.1016/j.freeradbiomed.2011.05.031 [21] D. Mastrangelo, L. Massai, L. Micheli, M. Muscettola, G. Cevenini and G. Grasso, “High Doses of Ascorbate Kill Y79 Retinoblastoma Cells in Vitro,” Journal of Clinical and Experimental Ophthalmolology, Vol. 4, No. 1, 2013, pp. 1-8. [22] W. Strober, “Trypan Blue Exclusion Test of Cell Viabil- ity,” Current Protocols in Immunology, 2001, Appendix 3: Appendix 3B. [23] M. J. Stoddart, “Cell Viability Assays: Introduction,” Me- thods in Molecular Biology, Vol. 740, 2011, pp. 1-6. http://dx.doi.org/10.1007/978-1-61779-108-6_1 [24] D. Lu, X. C. Bai, L. Gui, et al., “Hydrogen Peroxide in the Burkitt’s Lymphoma Cell Line Raji Provides Protec- Copyright © 2013 SciRes. JCT  Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide Copyright © 2013 SciRes. JCT 1372 tion against Arsenic Trioxide-Induced Apoptosis via the Phosphoinositide-3 Kinase Signalling Pathway,” British Journal of Haematology, Vol. 125, No. 4, 2004, pp. 512- 520. http://dx.doi.org/10.1111/j.1365-2141.2004.04940.x [25] Y. J. Lee and E. Shacter, “Oxidative Stress Inhibits Apop- tosis in Human Lymphoma Cells,” Journal of Biological Chemistry, Vol. 274, No. 28, 1999, pp. 19792-19798. http://dx.doi.org/10.1074/jbc.274.28.19792 [26] Z. Y. Wang and Z. Chen, “Acute Promyelocytic Leuke- mia: From Highly Fatal to Highly Curable,” Blood, Vol. 11, No. 5, 2008, pp. 2505-2515. http://dx.doi.org/10.1182/blood-2007-07-102798 [27] H. de Thé, M. Le Bras and V. Lallemand-Breitenbach, “Acute Promyelocytic Leukemia, Arsenic, and PML Bod- ies,” Journal of Cell Biology, Vol. 198, No. 1, 2012, pp. 11-21. http://dx.doi.org/10.1083/jcb.201112044 [28] V. Selvaraj, M. Yeager-Armstead and E. Murray, “Pro- tective and Antioxidant Role of Selenium on Arsenic Tri- oxide-Induced Oxidative Stress and Genotoxicity in the Fish Hepatoma Cell Line PLHC-1,” Environmental Toxi- cology and Chemistry, Vol. 31, No. 12, 2012, pp. 2861- 2869. http://dx.doi.org/10.1002/etc.2022 [29] Y. Sanchez, D. Amràn, E. de Blas and P. Aller, “Arsenic Trioxide as an Antitumor Agent: Mechanisms of Action and Strategies of Sensitization,” Journal of Applied Bio- medicine, Vol. 8, 2010, pp. 199-208. [30] B. Halliwell, “Oxidative Stress and Cancer: Have We Moved Forward?” Review Article, Biochemical Journal, Vol. 411, 2007, pp. 1-11. [31] J. Verrax, H. Taper and P. Buc Calderon, “Targeting Can- cer Cells by an Oxidant-Based Therapy,” Current Mole- cular Pharmacolology, Vol. 1, 2008, pp. 80-92. [32] A. Szent-Gyorgyi, “Observations on the Function of Per- oxidase Systems and the Chemistry of the Adrenal Cortex: Description of a New Carbohydrate Derivative,” Bioche- mical Journal, Vol. 22, 1928, pp. 1387-1409. [33] S. J. Padayatty and M. Levine, “Reevaluation of Ascorbate in Cancer Treatment: Emerging Evidence, Open Minds and Serendipity,” Journal of the American College of Nu- trition, Vol. 19, No. 4, 2000, pp. 423-425. http://dx.doi.org/10.1080/07315724.2000.10718941 [34] W. J. McCormick, “Cancer: The Preconditioning Factor in Pathogenesis; A New Etiologic Approach,” Archives of Pediatrics, Vol. 71, 1954, pp. 313-322. [35] W. J. McCormick, “Cancer: A Collagen Disease, Secon- dary to a Nutritional Deficiency,” Archives of Pediatrics, Vol. 76, 1959, pp. 166-171. [36] E. Cameron and D. Rotman, “Ascorbic Acid, Cell Prolif- eration, and Cancer,” The Lancet, Vol. 299, No. 7749, 1972, p. 542. http://dx.doi.org/10.1016/S0140-6736(72)90215-2 [37] C. G. Moertel, T. R. Fleming, E. T. Creagan, et al., “High- Dose Vitamin C versus Placebo in the Treatment of Pa- tients with Advanced Cancer Who Have Had No Prior Chemotherapy: A Randomized Double-Blind Compari- son,” New England Journal of Medicine, Vol. 312, No. 3, 1985, 137-141. http://dx.doi.org/10.1056/NEJM198501173120301 [38] S. J. Padayatty, A. Y. Sun, Q. Chen, M. G. Espey, J. Drisko and M. Levine, “Vitamin C: Intravenous Use by Complementary and Alternative Medicine Practitioners and Adverse Effects,” PLoS ONE, Vol. 5, No. 7, 2010, Article ID: e11414. http://dx.doi.org/10.1371/journal.pone.0011414 [39] M. Levine, C. Conry-Cantilena, Y. Wang, et al., “Vita- min C Pharmacokinetics in Healthy Volunteers: Evidence for a Recommended Dietary Allowance,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 93, No. 8, 1996, pp. 3704-3709. http://dx.doi.org/10.1073/pnas.93.8.3704 [40] J. Verrax and P. B. Calderon, “Pharmacologic Concentra- tions of Ascorbate Are Achieved by Parenteral Admini- stration and Exhibit Antitumoral Effects,” Free Radicals in Biology and Medicine, Vol. 47, No. 1, 2009, pp. 32-40. http://dx.doi.org/10.1016/j.freeradbiomed.2009.02.016 [41] S. J. Padayatty, H. Sun, Y. Wang, et al., “Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use,” Annals of Internal Medicine, Vol. 140, No. 7, 2004, pp. 533-537. http://dx.doi.org/10.7326/0003-4819-140-7-200404060-0 0010 [42] B. Frei and S. Lawson, “Vitamin C and Cancer Revis- ited,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 105, No. 32, 2008, pp. 11037-11038. http://dx.doi.org/10.1073/pnas.0806433105 [43] D. A. Monti, E. Mitchell, A. J. Bazzan, et al., “Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metas- tatic Pancreatic Cancer,” PLoS ONE, Vol. 7, No. 1 2012, Article ID: e29794. http://dx.doi.org/10.1371/journal.pone.0029794 [44] T. Bachleitner-Hofmann, B. Gisslinger, E. Grumbeck and H. Gisslinger, “Arsenic Trioxide and Ascorbic Acid: Syn- ergy with Potential Implications for the Treatment of Acute Myeloid Leukaemia?” British Journal of Haema- tology, Vol. 112, No. 3, 2001, pp. 783-786. http://dx.doi.org/10.1046/j.1365-2141.2001.02608.x [45] N. Karasavvas, J. M. Carcamo, G. Stratis and D. W. Golde, “Vitamin C Protects HL60 and U266 Cells from Arsenic Toxicity,” Blood, Vol. 105, No. 10, 2005, pp. 4004-4012. http://dx.doi.org/10.1182/blood-2003-03-0772 [46] M. L. Heaney, J. R. Gardner, N. Karasavvas, et al., “Vi- tamin C Antagonizes the Cytotoxic Effects of Antineo- plastic Drugs,” Cancer Research, Vol. 66, No. 19, 2008, pp. 8031-8038. http://dx.doi.org/10.1158/0008-5472.CAN-08-1490
|