N. HUTNIK ET AL.
4
these process parameters on the final crystal size distri-
bution of struvite can be regarded only as a moderate.
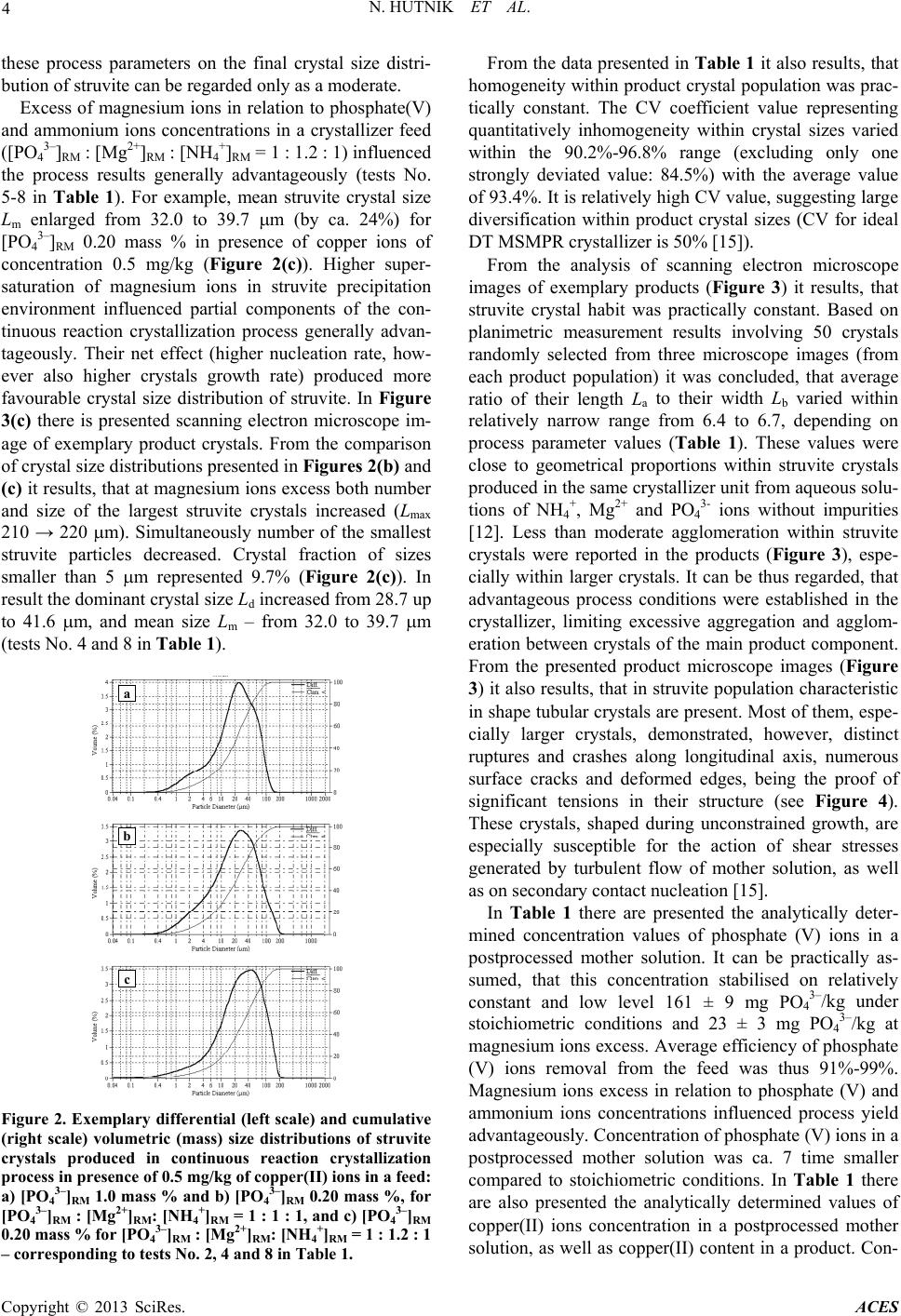
Excess of magnesium ions in relation to phosphate(V)
and ammonium ions concentrations in a crystallizer feed
([PO4
3–]RM : [Mg2+]RM : [NH4
+]RM = 1 : 1.2 : 1) influenced
the process results generally advantageously (tests No.
5-8 in Table 1). For example, mean struvite crystal size
Lm enlarged from 32.0 to 39.7 m (by ca. 24%) for
[PO4
3–]RM 0.20 mass % in presence of copper ions of
concentration 0.5 mg/kg (Figure 2(c)). Higher super-
saturation of magnesium ions in struvite precipitation
environment influenced partial components of the con-
tinuous reaction crystallization process generally advan-
tageously. Their net effect (higher nucleation rate, how-
ever also higher crystals growth rate) produced more
favourable crystal size distribution of struvite. In Figure
3(c) there is presented scanning electron microscope im-
age of exemplary product crystals. From the comparison
of crystal size distributions presented in Figures 2(b) and
(c) it results, that at magnesium ions excess both number
and size of the largest struvite crystals increased (Lmax
210 → 220 m). Simultaneously number of the smallest
struvite particles decreased. Crystal fraction of sizes
smaller than 5 m represented 9.7% (Figure 2(c)). In
result the dominant crystal size Ld increased from 28.7 up
to 41.6 m, and mean size Lm – from 32.0 to 39.7 m
(tests No. 4 and 8 in Table 1).
a
b
c
Figure 2. Exemplary differential (left scale) and cumulative
(right scale) volumetric (mass) size distributions of struvite
crystals produced in continuous reaction crystallization
process in presence of 0.5 mg/kg of copper(II) ions in a feed:
a) [PO43–]RM 1.0 mass % and b) [PO43–]RM 0.20 mass %, for
[PO43–]RM : [Mg2+]RM: [NH4+]RM = 1 : 1 : 1, and c) [PO43–]RM
0.20 mass % for [PO43–]RM : [Mg2+]RM: [NH4+]RM = 1 : 1.2 : 1
– corresponding to te sts No. 2, 4 and 8 in Table 1.
From the data presented in Table 1 it also results, that
homogeneity within product crystal population was prac-
tically constant. The CV coefficient value representing
quantitatively inhomogeneity within crystal sizes varied
within the 90.2%-96.8% range (excluding only one
strongly deviated value: 84.5%) with the average value
of 93.4%. It is relatively high CV value, suggesting large
diversification within product crystal sizes (CV for ideal
DT MSMPR crystallizer is 50% [15]).
From the analysis of scanning electron microscope
images of exemplary products (Figure 3) it results, that
struvite crystal habit was practically constant. Based on
planimetric measurement results involving 50 crystals
randomly selected from three microscope images (from
each product population) it was concluded, that average
ratio of their length La to their width Lb varied within
relatively narrow range from 6.4 to 6.7, depending on
process parameter values (Table 1). These values were
close to geometrical proportions within struvite crystals
produced in the same crystallizer unit from aqueous solu-
tions of NH4
+, Mg2+ and PO4
3- ions without impurities
[12]. Less than moderate agglomeration within struvite
crystals were reported in the products (Figure 3), espe-
cially within larger crystals. It can be thus regarded, that
advantageous process conditions were established in the
crystallizer, limiting excessive aggregation and agglom-
eration between crystals of the main product component.
From the presented product microscope images (Figure
3) it also results, that in struvite population characteristic
in shape tubular crystals are present. Most of them, espe-
cially larger crystals, demonstrated, however, distinct
ruptures and crashes along longitudinal axis, numerous
surface cracks and deformed edges, being the proof of
significant tensions in their structure (see Figure 4).
These crystals, shaped during unconstrained growth, are
especially susceptible for the action of shear stresses
generated by turbulent flow of mother solution, as well
as on secondary contact nucleation [15].
In Table 1 there are presented the analytically deter-
mined concentration values of phosphate (V) ions in a
postprocessed mother solution. It can be practically as-
sumed, that this concentration stabilised on relatively
constant and low level 161 ± 9 mg PO4
3–/kg under
stoichiometric conditions and 23 ± 3 mg PO4
3–/kg at
magnesium ions excess. Average efficiency of phosphate
(V) ions removal from the feed was thus 91%-99%.
Magnesium ions excess in relation to phosphate (V) and
ammonium ions concentrations influenced process yield
advantageously. Concentration of phosphate (V) ions in a
postprocessed mother solution was ca. 7 time smaller
compared to stoichiometric conditions. In Table 1 there
are also presented the analytically determined values of
copper(II) ions concentration in a postprocessed mother
solution, as well as copper(II) content in a product. Con-
Copyright © 2013 SciRes. ACES