Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 15 0-155 doi:10.4236/jbnb.2011.22019 Published Online April 2011 (http://www.scirp.org/journal/jbnb) Copyright © 2011 SciRes. JBNB In Vivo Evaluation of Functionalized Biomimetic Hydroxyapatite for Local Delivery of Active Agents Johan Forsgren1, Ulrika Brohede2, Sonya Piskounova3, Albert Mihranyan1, Sune Larsson4, Maria Strømme1, Håkan Engqvist1 1Department of Engineering Sciences, Uppsala University, Uppsala, Sweden; 2Sandvik AB, Stockholm, Sweden; 3Department of Materials Chemistry, Uppsala University, Uppsala, Sweden; 4Department of Orthopaedics, Uppsala University Hospital, Uppsala, Sweden. E-mail: hakan.engqvist@angstrom.uu.se Received November 3rd, 2010; revised January 19th, 2011; accepted January 22nd, 2011. ABSTRACT This study was carried out to investiga te the biological respo nse in vivo to biomimetic hydroxyapatite implant coatings functionalized with bisphosphonates and bone morphogenetic proteins. The functionalization was carried out by a sim- ple soaking procedure in the operating room immediately prior to surgery. Cylindrical titanium samples with and without coatings were implanted in the distal femoral epiphysis of sheep and retrieved after 6 weeks. The histological analysis proved tha t all samples were integrated well in the tissu e with no signs of intolerance. Fewer osteoclasts were observed in the vicinity of bisphosphonate-functionalized samples and the bone was denser around these samples com- pared to the other samples. Samples functionalized with bone morphogenetic protein induced more bone/implant con- tact but showed a more inconsisten t outcome with reduced bone density around the samples. This study demonstrates a simple method to functionalize implant coatings, which provides surgeons with an option of patient-specific function- alization of implants. The ob served biologica l impact due to the delivery o f active molecules from the coatings su ggests that this strategy may also be employed to deliver antibiotics from similar coa tings. Keywords: Implant, Titanium, Hydroxyapatite, Bisphosphonate, Bone Morp hogenetic Protein-2 1.Introduction The success rate of hip and knee arthroplasties is high; more than 90% of the patients are predicted to live more than 10 years with their implant before revision surgery is needed [1,2]. But despite the high success rate, the number of revision surgeries is still far from satisfactory. Only in the U.S., 36,000 revision total hip arthroplasties and 32,700 revision total knee arthroplasties were per- formed in 2003, and the annual number is expected to increase steadily [3]. Revision surgeries due to implant failure are often complicated, expensive and painful for afflicted patients. In a stud y from 1995 it was shown that patients undergoing revision orthopedic surgery need to stay longer at the hospital and the time in the operating room is significantly higher compared with primary sur- gery [4]. In two retrospect studies of revision hip and knee arthroplasties, as much as 39% of the hip surgeries [1] and 63% of the knee surgeries [5] were performed within 5 years following primary surgery. Infection and aseptic loosening are two common indications for early implant failure of uncemented prostheses [1,2]. Surgical site infections account for 38% of all nosocomial infec- tions in the US [6] and the incidence rate can be as high as 10% at spinal surgery [7]. In a recent retrospect study of 9245 patients undergoing primary hip or knee arthro- plasty, 65% of the developed infections appeared within one year after primary surgery [8]. These findings show that perioperative infections associated with the surgical wound is a challenge that needs to be addressed if the number of revision surgeries is to be reduced. The interface between prosthetic implant and tissue is known to be a region of local immune depression with reduced resistance to microbes, often referred to as an immune-incompetent fibro-inflammatory zone [9]. In addition, implant migration due instability in the inter- face between bone and implant can damage the tissues  In Vivo Evaluation of Functionalized Biomimetic Hydroxyapatite for Local Delivery of Active Agents 151 and almost completely deplete the immune defenses [10]. Thus, these peri-prosthetic regions are highly sensitive for bacteria colonization and biofilm formation. To reduc e the risk for bacteria colonization with consecutive de- velopment of infection, it is desirable with bactericidal effect on the implant surface and a firm fixation of the prosthesis. This could be obtained by local and sustained release of active agents from the implant surface that promotes more dense bone formation around the pros- thesis and/or has a bactericidal effect. It has been shown that the number of early failures associated with poor implant fixation have been reduced when osteoconduc- tive coatings have been deposited on the implant surfaces [11,12]. Hydroxyapatite, a calcium phosphate material resembling the mineral phase found in bone [13], is the most studied and applied osteoconductive material, and earlier in vitro studies have proven the feasibility of biomimetic hydroxyapatite (BHA) as carrier of various active agents such as antibiotics [14], bisphosphonates (BIS) [15] and bone morphogenetic proteins (BMP) [16] for targeted administration at the site of implant. In the present study, the possibility of incorporating various agents to a BHA coating by a rapid on-site loading method was evaluated in vivo. The aim is to provide su r- geons with an option to choose on-site if and what type of drug should be incorporated into the coating. In the design of the study, BIS and BMP were chosen, as they affect the bone formation surrounding the implant, which easily can be analyzed. The effect of antibiotics would be on the inhibition of bacteria entering the incision wound and such study would be more complicated to perform and more difficult to defend from an ethical standpoint as it would involve a subs tantial risk for the animals. 2. Materials and Methods 2.1. Implant Preparations Cylindrical implants of titanium grade 2 with 8.0 mm length and 3.5 mm diameter were used in this compara- tive study, see Figure 1. The implants were delivered with a mounting device used to screw th e implants in the right position, this part was subsequently removed after fixation of the implants. Three different surface modifi- cations of the implants were evaluated and compared to a native titanium surface, see Table 1. Out of the total 20 implants that were prepared, 5 were left non-coated and 15 were coated with a layer of BHA. Preparation of the BHA coatings was performed as descried in earlier work [17]. Briefly, a layer of anatase TiO2 was deposited on the titanium cylinders using physical vapor deposition (PVD) before the samples were immersed in 40ml Dul- becco’s phosphate buffered saline (Dulbecco’s PBS, Sigma-Aldrich) each for 4 days at 60℃. The samples were subsequently retrieved and analyzed with scanning Figure 1. Photograph of implant where the cylindrical pin constitutes the actual sample, the screw at the top was used to fixate the implant in the cortical bone. Table 1. Names and corresponding surface characteristics of the different sample. Sample typeNo. of samplesSurface Control 5 Native titanium Test 1 5 BHA coating Test 2 5 BHA coating loaded with BMP-2 Test 3 5 BHA coating loaded with BIS electron microscopy (SEM) to examine the precipitated layer of BHA. It was observed that the BHA-layer was not fully covering the cylinders and therefore the im- plants were once more immersed in PBS for additional 7 days at 37℃. BHA-coatings prepared in a similar way have been described earlier [17-19] and have been shown to be highly porous and calcium deficient compared with stoichiometric HA, something that promotes incorpora- tion of bioactive molecules [14,19]. After deposition of the BHA-layer, the implants were rinsed in deionized water, dried in air and sterilized by autoclaving (30 min, 120℃). In the operating room, 5 BHA-coated implants were placed in individual sterile plastic tubes containing 0.5 ml of an autoclaved aqueous solution of the bisphos- phonatePamindronate (0.5 mg/ml, Sigma-Aldrich). An- other 5 BHA-coated implants were soaked in a 0.5 ml recombinant human BMP-2 solution (rhBMP-2, 0.15 mg/ ml , I n d u ct O S ®, Wyeth Europe) in formulation buffer containing 2.5% glycine, 0.5% sucrose, 0.01% Polysor- bate 80, 5 mM sodium chloride and 5 mM L-glutamic acid. The implants were immersed in the solutions for 10 - 30 minutes before implantation. 2.2. Animal Study The in vivo study was designed for intra-osseous implan- tation in sheep for 6 weeks. Five 24-month-old female Grivette sheep, weighing from 51 to 63 kg, were used in the study. Prior to the surgical procedure, the animals were fasted overnight. At the time of implantation, pre-medication and anesthesia was performed by intra- venous injection of a thiopental-pentobarbital mixture (Nesdonal®, Merial; and Pentobarbital Sodique, CEVA Santé animale) and atropine (AtropinumSulfuricum, C opyright © 2011 SciRes. JBNB  152 In Vivo Evaluation of Functionalized Biomimetic Hydroxyapatite for Local Delivery of Active Agents Aguettant) followed by the inhalation of an O2-isoflurane (1% - 4%) mixture (Afrane®, Baxter). Each animal re- ceived a first analgesic (Flunixine, Meflosyl®, Fort Dodge Santé Animale) and as a prophylactic measure, perioperative antibiotics penicillin procaine and penicil- lin benzathine (Duplocilline®, Intervet) were given. In addition, each animal received a second analgesic (Bu- prenorphine, Temgesic®, Schering Plough) preoperat ively. Bone defects were created bilaterally in the distal femoral epiphysis and the sites were implanted with samples. Each animal was implanted with 4 samples, one of each type. An orthopedic drill was used to create cy- lindrical defects with approximately 2.3 mm in diameter by < 9 mm deep in the distal femoral epiphysis. Drilling was performed under constant irrigation with saline solu- tion (NaCl 0.9%) and suction. Intra-osseous implantation was performed as recommended by the ISO 10993-6 standard (2007) with the cylindrical parts of the samples inserted in the cancellous bone. Once implanted, the samples were in contact with bone along the sides while the bottom was facing a cavity in the bone. This enabled a study of how bone is formed around the implan ts under different conditions. The animals were treated and kept according to the conditions conformed by the EU re- quirements of farm animals (EC Directive 86/609). Dur- ing the observation period, penicillin procaine and peni- cillin benzathine (Duplocilline®, Intervet) were continued on days 3, 6 and 9 following surgery. The analgesic (flunixine, Meflosyl®, Fort Dodge Santé Animale) was administered every other day for one week following surgery. After a 6-week implantation period, the animals were sacrificed by lethal injection of a barbiturate (DolethalND, Vetoquinol) and implanted sites were sam- pled. The protocol for the study was approved by BIOMATECH (France) Ethical Committee on October 14, 2008. 2.3. Sample Preparation The retrieved samples were dehydrated in ethanol solu- tions of increasing concentration, cleared in xylene, and embedded in poly-methyl methacrylate (PMMA). One longitudinal section in the long axis of the implant was obtained per specimen where one half of the sample was sectioned by a microcutting and grinding technique adapted from Donath et al [20]. The remaining half from each specimen was sputtered with a thin gold/palladium layer to enable SEM analysis. 2.4. Histology Sections were stained with modified Paragon for semi- quantitative and qualitative analyses. Histological analy- ses were performed using a Nikon microscope coupled with a digital camera (magnifications of x4, x10, x20 and x40). Semi-quantitative evaluation of local tolerance was performed according to ISO 10993-6 standard. 2.5. Bone/Implant Contact and Adjacent Bone Density The samples prepared for SEM analysis were analyzed in backscatter mode using 15 keV acceleration voltage (Leo 1550 FEG Gemini, Zeiss). The bone/implant contact was calculated from the images by measuring the contact length along the periphery of the sides and bottom of the implants, see example in Figure 2. Quantitative analysis was performed on the SEM images using a software (LeicaQwin) to calculate the bone density within the nearest 200 m of tissue surrounding the implants. Areas of soft tissue and bone along the sides and bottom of im- plants were determined from the difference in contrast between the two areas. The bone/soft tissue ratio was calculated from these measurements. 3. Results and Discussion No local signs of necrosis were observed macroscopi- cally with either the test or control samples. Moderate signs of hemorrhage were observed at the level of the soft tissues surrounding the type 1 and type 2 samples. No signs of exudates were observed with either the test or control samples with exception of one animal, which showed the presence of a subcutaneous liquid pocket in the right and left femur graded as marked exudates and increasing the mean grade of exudation similarly in all groups. No local intolerance signs were observed for any of the samples except for one of the test 3 samples, where a marked infiltration of lymphocytes without significant implication on the bone healing was observed. Marked grade of newly formed bone showing characteristics of a mixture of lamellar and woven bone with osseous con- densation were seen along the surface for all sample types. In terms of healing performance, the histology analysis demonstrated that the control samples and the Figure 2. SEM-image of implanted sample (test 3). The im- age shows bone around the e ntire sample. C opyright © 2011 SciRes. JBNB 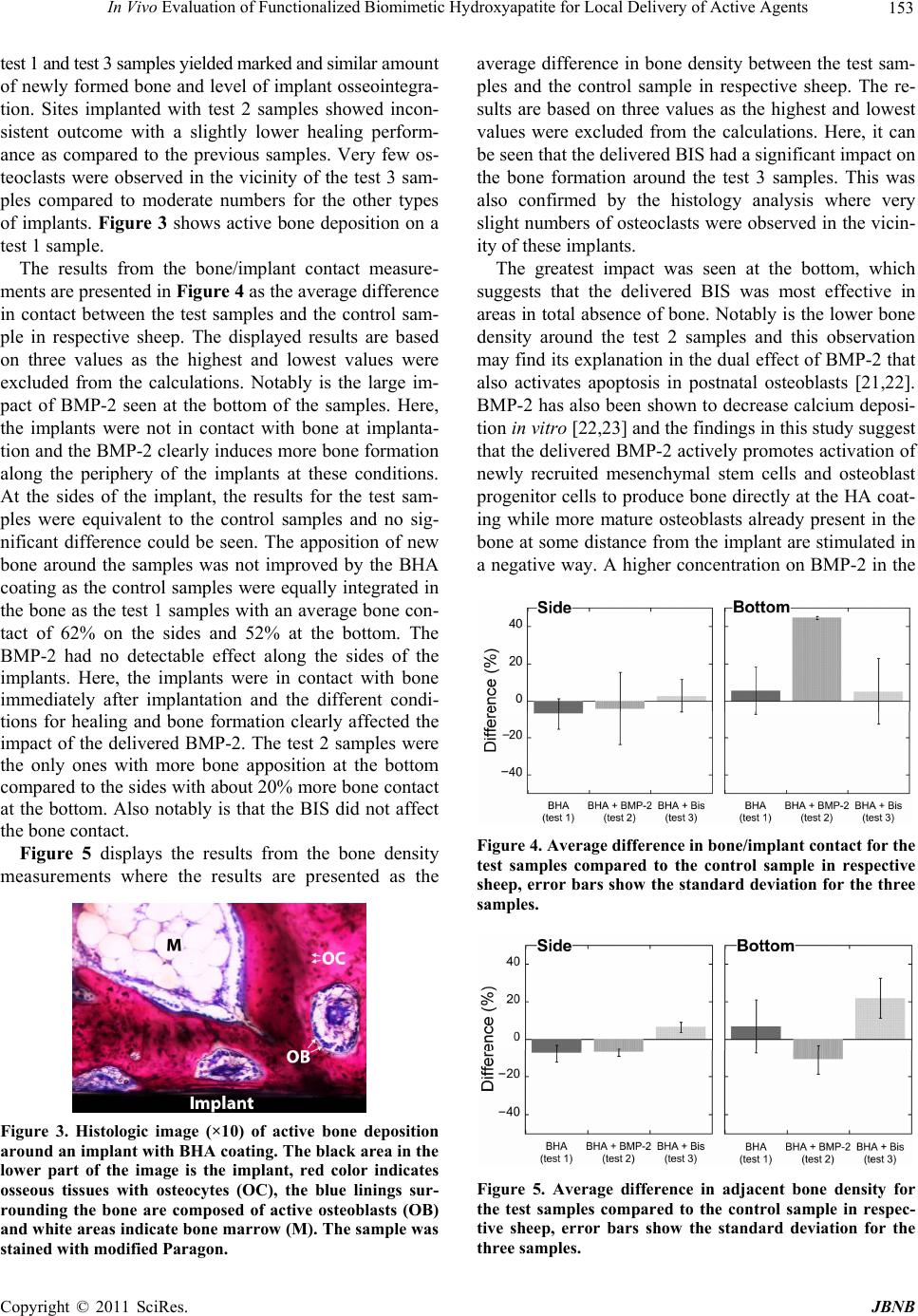 In Vivo Evaluation of Functionalized Biomimetic Hydroxyapatite for Local Delivery of Active Agents 153 te s t 1 a n d t e s t 3 s a mp l e s y i e l d ed m a r k e d a n d s i mi l a r amount of newly formed bone and level of implant osseointegra- tion. Sites implanted with test 2 samples showed incon- sistent outcome with a slightly lower healing perform- ance as compared to the previous samples. Very few os- teoclasts were observed in the vicinity of the test 3 sam- ples compared to moderate numbers for the other types of implants. Figure 3 shows active bone deposition on a test 1 sample. The results from the bone/implant contact measure- ments are presented in Figure 4 as the average difference in contact between the test samples and the control sam- ple in respective sheep. The displayed results are based on three values as the highest and lowest values were excluded from the calculations. Notably is the large im- pact of BMP-2 seen at the bottom of the samples. Here, the implants were not in contact with bone at implanta- tion and the BMP-2 clearly induces more bone formation along the periphery of the implants at these conditions. At the sides of the implant, the results for the test sam- ples were equivalent to the control samples and no sig- nificant difference could be seen. The apposition of new bone around the samples was not improved by the BHA coating as the control samples were equally integrated in the bone a s the test 1 samp les with an aver age bone con- tact of 62% on the sides and 52% at the bottom. The BMP-2 had no detectable effect along the sides of the implants. Here, the implants were in contact with bone immediately after implantation and the different condi- tions for healing and bone formation clearly affected the impact of the delivered BMP-2. The test 2 samples were the only ones with more bone apposition at the bottom compared to the sides with about 20% more bone contact at the bottom. Also notably is that the BIS did not affect the bone contact. Figure 5 displays the results from the bone density measurements where the results are presented as the Figure 3. Histologic image (×10) of active bone deposition around an implant with BHA coating. The black area in the lower part of the image is the implant, red color indicates osseous tissues with osteocytes (OC), the blue linings sur- rounding the bone are composed of active osteoblasts (OB) and white areas indicate bone marrow (M). The sample was stained with modified Paragon. average difference in bone density between the test sam- ples and the control sample in respective sheep. The re- sults are based on three values as the highest and lowest values were excluded from the calculations. Here, it can be seen that the delivered BIS had a significant impact o n the bone formation around the test 3 samples. This was also confirmed by the histology analysis where very slight numbers of osteoclasts were observed in the vicin- ity of these implan ts. The greatest impact was seen at the bottom, which suggests that the delivered BIS was most effective in areas in total absence of bone. Notably is the lower bone density around the test 2 samples and this observation may find its explanation in the dual effect of BMP-2 that also activates apoptosis in postnatal osteoblasts [21,22]. BMP-2 has also been shown to decrease calcium deposi- tion in vitro [22,23] and the findings in this study suggest that the delivered BMP-2 actively promotes activation of newly recruited mesenchymal stem cells and osteoblast progenitor cells to produce bone directly at the HA co at- ing while more mature osteoblasts already present in the bone at some distance from the implant are stimulated in a negative way. A higher concentration on BMP-2 in the Figure 4. Average difference in bone/implant contact for the test samples compared to the control sample in respective sheep, error bars show the standard deviation for the three samples. Figure 5. Average difference in adjacent bone density for the test samples compared to the control sample in respec- tive sheep, error bars show the standard deviation for the three samples. C opyright © 2011 SciRes. JBNB  154 In Vivo Evaluation of Functionalized Biomimetic Hydroxyapatite for Local Delivery of Active Agents loading solution might have rendered a more effective functionalization of the BHA coating, resulting in a higher amount of BMP-2 delivered to the tissues. This could have caused another biological response if the ef- fect is dose-dependent. The BHA coating alone was not observed to have any impact on the bone formation around the implants after 6 weeks of implantation, which is in line with earlier studies [24]. Yet, it is in con trary to the positive effects of biomimetic HA on bone formation observed by Barrère et al [25]. The findings in this study prove that it is possible to obtain a positive effect on the bon e formation in vivo via local delivery of active agents from implant coatings comprised of BHA. In the future, this strategy of incor- porating active molecules to BHA coatings in the oper- ating room immediately prior to implant insertion is suggested to be employed to deliver single drugs or com- binations of drugs including, e.g., antibiotics from the surfaces to reduce the incident rate of surgery-related infections. 4. Conclusions The study demonstrates an effective method to function- alize implants immediately prior to surgery. The func- tionalization procedure in the operating room did not lead to any infections in the animals and the active molecules delivered from the coatings were shown to have an impact on the bone formation. BMP-2 was shown to promote more bone formation directly along the periphery of the implants in areas without bone and BIS promoted more dense bone formation. The proposed strategy is also suggested to be used for local release of, e.g., antibiotics in the future to reduce the incident rate of surgery-related infections. 5. Acknowledgements The Swedish Research Council is acknowledged for funding this study. REFERENCES [1] M. Dobzyniak, T. K. Fehring and S. Odum, “Early Failure in Total Hip Arthroplasty,” Clinical Orthopaedics and Related Research, Vol. 477, 2006, pp. 76-78. doi:10.1097/01.blo.0000203484.90711.52 [2] P. F. Sharkey, W. J. Hoza ck , R. H. Rothman, S. Sha stri and S. M. Jacoby, “Why are Total Knee Arthroplasties Failing To- day,” Clinical Orthopaedics and Related Research, Vol. 404, 2002, pp. 7-13. doi:10.1097 /00003086-200211000-00003 [3] S. Kurtz, K. Ong, E. Lau, F. Mowat and M. Halpern, “Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030,” Journal of Bone and Joint Surgery(American Volume), Vol. 89A, No. 4, 2007, pp. 780-785. doi:10.2106/JBJS.F.00222 [4] K. A. Krackow and D. S. Hungerford, “Revision and Primary Hip and Knee Arthroplasty—A Cost-Analysis,” Clinical Orthopaedics and Related Research, Vol. 311, 1995, pp. 136-141. [5] T. K. Fehring, S. Odum, W. L. Griffin, J. B. Mason and M. Nadaud, “Early Failures in Total Knee Arthroplasty,” Clinical Orthopaedics and Related Research, Vol. 392, 2001, pp. 315-318 . doi:10.1097 /00003086-20 0111000-0 0041 [6] A. J. Man gra m, T. C. H or an, M. L. Pea r son , L. C. Si lve r a nd W. R. Jarvis, “Guideline for Prevention of Surgical Site Infection, 1999,” Infection Control and Hospital Epidemi- ology, Vol. 20, N o. 4, 1999 , pp. 25 0-278. doi:10.1086/501620 [7] L. Y. Carreon, R. M. Puno, J. R. Dimar, S. D. Glassman and J. R. Johnson, “Perioperative Complications of Posterior Lumbar de Compression and Arthrodesis in Older Adults,” Journal of Bone and Joint Surgery(American Volume), Vol. 85A, No. 11, 200 3, pp. 2089-2092 . [8] L. Pulido, E. Ghanem, A. Joshi, J. J. Purtill and J. Parvizi, “Periprosthetic Joint Infection: The Incidence, Timing, and Predisposing Factors,” Clinical Orthopaedics and Related Research, Vol. 466, No. 7, 2008, pp. 1710-1715. doi:10.1007/s11999-008-0209-4 [9] A. G. Gristina, “Implant Failure and the Immune-Incom petent Fibro-Inflammatory Zone,” Clinical Orthopaedics and Related Research, Vol. 298, 1994, pp. 106-118. [10] D. Campo cci a, L. M on tan ar o an d C. R . Ar ci ola “T he S ig ni fi- cance of Infection Related to Orthopedic Devices and Issues of Antibiotic Resistance,” Biomaterials, Vol. 27, No. 11, 2006, pp. 2331-2 339. do i:10.1016/j.biomaterials.2005.11 .044 [11] I. Landor, P. Vavrik, A. Sosna, D. Jahoda, H. Ha hn and M. Daniel, “Hydroxyapatite Porous Coating and the Osteointe- gration of the Total Hip Replacement,” Archives of Rtho- paedic and Trauma Surgery, Vol. 127, No. 2, 2007, pp. 81-89. doi:10.1007/s00402-006-0235-1 [12] R. G. T. Geesink, “Osteoconductive Coatings for Total Joint Arthroplasty,” Clinical Orthopaedics and Related Research, Vol. 395, 2002, pp. 53-65. doi:10.1097/00003086-200202000-00007 [13] R. Z. LeGeros, J. P. LeGeros Hydroxyapatite and T. Kokubo, “Bioceramics and Their Clinical Applications,” Woodhead Publishing Limited, Sawston, 2008. [14] U. Brohede, J. Forsgren, S. Roos, A. Mihranyan, H. Engqvist and M. Strømme, “Multifunctional Implant Coatings Providing Possibilities for Fast Antibiotics Loading with Subsequent Slow Release,” Journal of Materials Science—Material s in Medicine, Vol. 20, No. 9, 2009, pp. 1859-1867. doi:10.1007/s10856-009-3749-6 [15] J. Åberg, U. Brohede, A. Mihranyan, M. Strømme and H. Engqvist, “Bisphosphonate Incorporation in Surgical Im- plant Coatings by Fast Loading and Co-Precipitation at Low Drug Concentrations,” Journal of Materials Science: Materials in Medicine, Vol. 20, No. 10, 2009, pp. 2053- 2061. [16] S. Piskounova, J. Forsgren, U. Brohede, H. Engqvist and M. Strømme, “In Vitro Characterization of Bioactive Titanium Dioxide/Hydroxyapatite Surfaces Functionalized with BMP-2,” Journal of Biomedical Materials Research Part B—Applied Biomaterials, Vol. 91B, No. 2, 2009, pp. 780- 787. doi:10.1002/jbm.b.31456 C opyright © 2011 SciRes. JBNB  In Vivo Evaluation of Functionalized Biomimetic Hydroxyapatite for Local Delivery of Active Agents Copyright © 2011 SciRes. JBNB 155 [17] U. Brohede, S. X. Zhao, F. Lindberg, A. Mihranyan, J. Forsgren, M. Strømme, et al., “A Novel Graded Bioactive High Adhesion I mplant Coating,” Applied Su rface Science, Vol. 255, No. 17, 2009, pp. 7723-7728. doi:10.1016/j.apsusc.2009.04.149 [18] J. Forsgren, F. Svahn, T. Jarmar and H. Engqvist, “Forma- tionand Adhesion of Biomimetic Hydroxyapatite Deposited on Titanium Substrates,” Acta Biomaterialia, Vol. 3, No. 6, 2007, pp. 980-984. doi:10.1016/j.actbio.20 07.03.006 [19] A. Mihranyan, J. Forsgren, M. Strømme and H. Engqvist, “Assessing Surface Area Evolution during Biomimetic Growth of Hydroxyapatite Coatings,” Langmuir, Vol. 25, No. 3, 2009, pp. 1292-1295. doi:10.1021/la803520k [20] K. Donath and G. Breuner, “A Method for the Study of Undecalcified Bones and Teeth with Attached Soft-Tiss ues-the Sage-Schliff (Sawing and Grinding) Technique,” Journal of Oral Pathology and Medicine, Vol. 11, No. 4, 1982, pp. 318-32 6. doi:10.11 11/j.1600-071 4.1982 .tb00172 .x [21] E. Hay, J. Lemonnier, O. Fromigue and P. J. Marie, “Bone Morphogenetic Protein-2 Promotes Osteoblast Apoptosis through a Smad-Independent, Protein Kinase C-Dependent Signaling Pathway,” Journal of Biological Chemistry, Vol. 276, No. 31, 200 1, pp. 290 28-29036 . doi:10.1074/jbc.M0112652 00 [22] E. Hay, J. Lemonnier, O. Fromigue, H. Guenou and P. J. Marie, “Bone Morphogenetic Protein Receptor IB Signaling Mediates Apoptosis Independently of Differen- tiation in Osteoblastic Cells,” Journal of Biological Chemistry, Vol. 279, No. 3, 2004, pp. 1650-1658. [23] C. A. Luppen, E. Smith, L. Spevak, A. L. Boskey and B. Frenkel, “Bone Morphogenetic Protein-2 Restores Minera- lization in Glucocorticoid-Inhibited MC3T3-E1 Os- teoblast Cultures,” Journal of Bone and Mineral Research, Vol. 18, No. 7, 2003, pp . 1186- 1197. doi:10.1359/jbmr .2003.1 8.7.11 86 [24] F. M. He, G. L. Yang, X. X. Wang and S. F. Zhao, “Bone Responses to Rough Titanium Implants Coated with Biomimetic Ca-P in Rabbit Tibia,” Journal of Biomedical Materials Research Part B—Applied Biomaterials, Vol. 90B, No. 2, 2009, pp. 857-863. doi:10.1002/jbm.b.31355 [25] F. Barrere , C . M. van der V alk , G . M eijer , R . A. J. D alm eijer , K. de Groot and P. Layrolle, “Osteointegration of Bio- mimetic Apatite Coating Applied onto Dense and Porous Metal Implants in Femurs of Goats,” Journal of Biomedical Materials Research Part B-Applied Biomaterials, Vol. 67B, No. 1 , 2 00 3, p p . 6 5 5- 66 5 . doi:10.1002/jbm.b.10057 |

